Open Access Article
Open Access ArticleImmobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption
S. S. Shen abc,
J. J. Yangabc,
C. X. Liud and
R. B. Bai*abc
abc,
J. J. Yangabc,
C. X. Liud and
R. B. Bai*abc
aCenter for Separation and Purification Materials & Technologies, Suzhou University of Science and Technology, Suzhou 215009, P. R. China. E-mail: ceebairb@live.com
bSuzhou Key Lab of Separation and Purification Materials & Technologies, Suzhou 215009, P. R. China
cJiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, Suzhou 215009, P. R. China
dIsola Group, Singapore 629586
First published on 7th February 2017
Abstract
An affinity membrane was prepared by immobilizing copper ions (Cu2+) on chitosan (CS) and cellulose acetate (CA) blend hollow fibers, and the affinity membrane (denoted as CS/CA-Cu) was evaluated for its performance in the adsorption of a model protein, Bovine Serum Albumin (BSA). The analysis indicated that the amount of copper ions coupled on CS/CA-Cu reached around 3.9 mg per g-membrane. The experimental results showed that CS/CA-Cu achieved a high adsorption capacity of the 69 mg-BSA per g-membrane, under the typical solution condition of pH at 7.4 and ionic strength at 0.12 M, with the nonspecific adsorption being as low as less than 15%. The adsorption isotherm followed the Langmuir model and the adsorption kinetics exhibited both transport-limited and attachment-limited behaviors. CS/CA-Cu also performed favorably in the neutral pH range and at low ionic strength.
1. Introduction
Immobilized metal ion affinity membrane (IMAM) has been used increasingly as an effective means for bio-separations.1–5 A major challenge in this technique is the preparation of the available IMAM from suitable polymeric membrane substrate.6 The desired membrane substrate should have high hydrophilicity, microporous structure, narrow pore size distribution, high density of functional groups (such as –OH, –COOH, –SH, –NH2, –N+R3, –SO3−, etc.), good mechanical, chemical, and thermal stability.7The common practice in preparing IMAM is usually through surface modification of the commercially available membranes. Generally, four steps are involved in the preparation of an IMAM from those membrane substrates, including (1) preparation of the base membrane; (2) activation of the base membrane by introducing reactive groups (epoxy groups, hydroxyl groups or others); (3) coupling of metal ion ligand chelators and (4) immobilization of metal ion ligands on the membrane via chelators.
Research efforts have been made to prepare the substrate membranes from polymers with reactive functional groups, mostly from regenerated cellulose or cellulose derivatives.8 This approach can eliminate the necessity for a second step in the common method, and thus simplify the preparation procedures. However, the major functional groups (usually –OH) in the cellulose related polymer substrates have only limited reactivity and they still require additional chelating agents to facilitate the coupling of the metal ion ligands. For example, glycidyl methacrylate (GMA) has been used to attach a chelator, iminodiacetic acid (IDA), to fabricate the copper coupled IMAM from cellulose acetate (CA) membrane.9
In the past 1–2 decades, chitosan (CS, poly-β-(1,4)-2-amino-2-deoxy-D-glucose), which is one of the most abundant polysaccharides on the earth, has attracted significant research and application interest because of its non-toxic, biodegradable and highly biocompatible properties. CS is also abundant in the reactive functional group of –NH2 that can be used as a possible metal ion ligand chelator.10 However, due to the poor mechanical strength of CS, most studies have either coated CS on a base membrane11,12 or blended it with some matrix polymer, such as cellulose.13,14 The coated or blended materials have been found to exhibit enhanced adsorption capacity,15 which was attributed to the large number of functional groups from CS, particularly the amino groups that can act as the active sites for the attachment or adsorption of transition metal ions as well as other organic compounds, such as dyes, and proteins.16
Liu et al. reported the preparation of a blend hollow fiber membrane from CS and CA, with CA as the matrix polymer and CS as the functional polymer.17,18 The prepared blend membranes were found to be highly hydrophilic and porous, have good mechanical strength, and can effectively adsorb transition metal ions, including copper ions.19 In the literature, it was reported that copper(II) ions show effective antibacterial action, either alone or in the form of complexes.9,20 There are also a few reports on CS-modified CA membrane that exhibited antibacterial properties.21,22
In this study, an IMAM was prepared by immobilizing copper(II) ions as the metal ion ligand onto the CS/CA blend hollow fiber membrane. The prepared IMAM (denoted as CS/CA-Cu) was examined for its amount of coupled copper ion ligand, the capacity of adsorption for a model protein (Bovine Serum Albumin, or BSA), and the extent of the specific and non-specific adsorption involved, as well as the utilization rate of the coupled copper ion ligand.
2. Materials and methods
2.1 Materials
CS was obtained from Aldrich, and was indicated as a low molecular weight product. The molecular weight (MW) and deacetylation degree of the CS were determined by GPC (Gel Permeation Chromatography) to be at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and by titration at 73.5%, respectively.23 CA was purchased from Fluka and the acetyl content and MW were 40% and 37
000 g mol−1 and by titration at 73.5%, respectively.23 CA was purchased from Fluka and the acetyl content and MW were 40% and 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 according to the supplier, respectively. Formic acid (98–100 wt%) from Fluka was used as the co-solvent for dissolving both CS and CA together to prepare the blend hollow fiber membrane. Copper sulphate (CuSO4·5H2O, Naealai Tesque Co., Tokyo) was used in the coupling of copper ion ligands on the prepared membrane. Bovine Serum Albumin (BSA), from Sigma and with MW of 67 kDa, was used to prepare the protein solution for the adsorption experiments. Other common chemicals as needed were purchased from various suppliers and used without further purification.
000 g mol−1 according to the supplier, respectively. Formic acid (98–100 wt%) from Fluka was used as the co-solvent for dissolving both CS and CA together to prepare the blend hollow fiber membrane. Copper sulphate (CuSO4·5H2O, Naealai Tesque Co., Tokyo) was used in the coupling of copper ion ligands on the prepared membrane. Bovine Serum Albumin (BSA), from Sigma and with MW of 67 kDa, was used to prepare the protein solution for the adsorption experiments. Other common chemicals as needed were purchased from various suppliers and used without further purification.
2.2 Preparation of CS/CA-Cu
The base CS/CA blend hollow fiber membrane was prepared in a similar method described in detail in the literature.19 The characteristic structure and morphology of the blend hollow fiber membrane was obtained by scanning electron microscopy (SEM, JEOL JSM-5600, JEOL Ltd., Japan) or field emission scanning electron microscopy (FESEM, JEOL JSM-6700F, JEOL Ltd., Japan). The dimensions of the prepared hollow fiber membrane were also measured using the software supplied with SEM/FESEM.The CS/CA blend hollow fiber membrane was then immobilized with copper ion ligands. To prepare the IMAM, a 4.4 g amount of the dry CS/CA was added to 200 mL of the copper sulphate solution with an initial concentration of 1 g L−1 (in terms of Cu2+) and an initial pH of 5. The mixture in the flask was stirred on a magnetic stirrer at 150 rpm and room temperature (22–23 °C) for 2 h to allow the Cu2+ ions to react with the amine groups (–NH2) of the CS/CA membrane to form complexes. The copper ion-coupled hollow fiber membrane was washed thoroughly first with DI water and then with 10 mM phosphate buffer solution (containing 0.12 M NaCl concentration and having pH = 7.4). The finally obtained IMAM is denoted as CS/CA-Cu.
The amount of copper ions coupled onto CS/CA-Cu was estimated by stripping it with 500 mL of 50 mM EDTA solution and then analyzing the concentration of copper ions in the stripping solution using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Perkin-Elmer Optima 3000DV).
2.3 Protein adsorption experiments
The adsorption of BSA on the porous hollow fiber membranes (CS/CA-Cu or the control CS/CA) was conducted in batch mode. For the isotherm adsorption study, 1.1 g amount (dry weight) of the CS/CA-Cu or CS/CA was placed into a 50 mL of a BSA solution with an initial concentration in the range of 0.4 to 4 g L−1. The initial pH and NaCl concentration in all solutions were kept constant at 7.4 and 0.12 M, respectively. The adsorption mixture was shaken at 150 rpm and room temperature in a water bath shaker for 24 h to ensure the adsorption equilibrium being fully reached. The BSA concentrations in the solution before and after the adsorption test were determined with a UV-Vis spectrometer at 278 nm. The adsorption amounts of BSA on the CS/CA-Cu or CS/CA membrane were calculated by mass balance analysis (adsorption equilibrium data at two other specific pH values of 4.9 and 6.1 were also obtained under the similar test conditions to pH 7.4 described above).The effects of the solution pH and ionic strength on BSA adsorption performance by CS/CA and CS/CA-Cu were also investigated. A 1.1 g sample (dry weight) of CS/CA-Cu or CS/CA was placed into a 50 mL BSA solution with an initial concentration of 2.5 g L−1. For the effect of the solution pH, the pH in the solutions was adjusted to a range of 4.9–8.0 using different buffer solutions (10 mM NaAc/HAc for pH 4–6 and 10 mM NaH2PO4/Na2HPO4 for pH 6–8). Owing to the possibility of CS dissolution in acidic solutions and CA degradation under basic conditions, experiments for pH below 4 and above 8.0 were not conducted. For the effect of the ionic strength, the ionic concentration in the solutions was varied in the range of 0–1.8 M by adding sodium chloride (NaCl) to the solutions. The ionic strength in the experiments for the pH effect was fixed at 0.12 M, and the pH in the experiments for examining the ionic strength effect was fixed at 7.4.
For the adsorption kinetic study, a 1.1 g amount (dry weight) of CS/CA-Cu was added to a flask containing 150 mL BSA solution at an initial concentration of 2.5 g L−1. The solution pH was controlled at 7.4 and the ionic strength in the solution was set to 0.12 M. The mixture was shaken at 150 rpm in a water bath shaker at room temperature. At various desired time intervals, a 3 mL amount of the solution was sampled from the flask to determine the BSA concentration in the solution.
3. Results and discussion
3.1 Characteristics of CS/CA-Cu
The obtained CS/CA blend hollow fiber membrane had an O.D. and I.D. of 1.13 and 0.67 mm, respectively (wall thickness at around 230 μm). The surface and cross-section morphologies of the hollow fiber membrane are shown in Fig. 1. The hollow fiber membrane generally had a symmetric and spongy structure, and the cross-section was full of highly interconnected pores. This type of porous structure is in fact desirable for affinity membrane because it can provide high process flow rates at low operation pressures as well as large specific surface areas for high binding capacities. The blend hollow fiber membrane had a relatively denser outer surface, compared to the more porous inner surface, but the pore size (about 70 nm) was large enough to allow the free passage of BSA molecules (<14 × 4 × 4 nm) into the internal binding sites of the membrane. The analyses showed that the hollow fiber membrane had a porosity of 79.1%, a specific surface area of 14.1 m2 g−1, and a CS content of 12% by weight.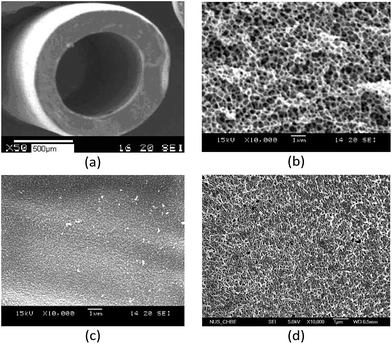 | ||
Fig. 1 Morphology of CS/CA blend hollow fiber membrane. (a) Overall view ×50, (b) cross-section ×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, (c) outer surface ×10 000, (c) outer surface ×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and (d) inner surface ×10 000 and (d) inner surface ×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. | ||
The amount of metal ions coupled on the membrane can be one of the crucial factors affecting the capacity of protein adsorption on IMAMs. In this study, the amount of copper ions coupled on CS/CA-Cu blend hollow fiber membrane was measured and determined to be 3.9 mg per g-membrane (or 32.5 mg Cu2+ per g-CS).19
3.2 BSA adsorption on CS/CA-Cu
Nonspecific binding is undesirable for affinity separation because it may reduce the separation resolution. The experimental results for the amounts of BSA adsorbed on CS/CA and CS/CA-Cu under different initial BSA concentrations are shown in Fig. 2. The amounts of BSA adsorbed on CS/CA were low, displaying a slight increase with increasing BSA concentrations and achieved a maximum amount of about 9 mg per g-membrane under the conditions examined (BSA concentrations up to 4 g L−1). The adsorption of BSA on the control CS/CA membrane may be due to the results of nonspecific interactions, such as electrostatic and hydrophobic interactions between BSA and the CS/CA membrane.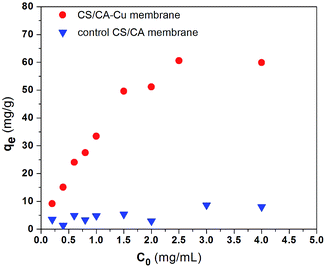 | ||
| Fig. 2 Adsorption of BSA on the control CS/CA and CS/CA-Cu at pH 7.4 (C0 – initial BSA concentration, qe – equilibrium BSA adsorption amount). | ||
To estimate the specific binding of BSA on CS/CA-Cu (i.e., adsorption of BSA via immobilized copper ions), one may take the amount of 9 mg-BSA per g-membrane as a maximum estimation of the possible nonspecific binding of BSA on CS/CA-Cu. Clearly, from the experimental results in Fig. 2, the amounts of BSA adsorbed on CS/CA-Cu were much greater than those on the control CS/CA, and the difference became more significant when the initial BSA concentration in the solution was increased from 0.2 to 2.5 g L−1.
The adsorption amounts appeared to approach a plateau with further increases in the BSA concentrations from 2.5 up to 4 g L−1. The maximum adsorption amount on CS/CA-Cu at the plateau level was about 60 mg-BSA per g-membrane (or 13.8 mg mL−1) in this case (see Fig. 2). Therefore, the specific binding of BSA on CS/CA-Cu (due to the metal ion ligand) can be deduced as 51 mg g−1 (or 11.7 mg mL−1). Hence it can be considered that at least 85% of the BSA adsorbed on CS/CA-Cu was through the specific binding with the immobilized copper ion ligands. This result of highly specific adsorption should not be surprising if one considers the possible formation of complexes between the numerous imidazole groups of BSA with the copper ion ligands on CS/CA-Cu. In the literature, a regenerated cellulose membrane immobilized with copper ions as the ligand was reported to have an adsorption amount of 6.25 mg mL−1 for BSA.24
Interestingly, the utilization of the coupled copper ions in this study appears to be much higher than that reported in the literature. The utilization of metal ion ligands has been defined as the amount of protein bond by per unit mass of metal ion ligand.24 In this study, the copper ion utilization was calculated to be 15.4 mg-BSA per mg-Cu2+ (or 0.24 μM-BSA per mg-Cu2+). This is in contrast to 1.5 mg-BSA per mg-Cu2+ (or 0.0226 μM-BSA per mg-Cu2+) on microporous polyethylene substrate membrane in ref. 25 or 1.26 mg-BSA per mg-Cu2+ (or 0.019 μM-BSA per mg-Cu2+) on microporous cellulose substrate membrane in ref. 24.
It has been generally known that a copper ion can form 6 coordination bonds with surrounding chelators. The participation number of amine groups on CS polymer with a copper ion has been suggested to be in the range of 1–3, with other coordination sites being occupied by OH− or H2O.26 Therefore, in general, there are 5 to 3 binding sites (those occupied by OH− or H2O) available for a coupled copper ion on CS/CA-Cu to bind BSA or other proteins. However, for mostly used multidentate chelators, such as iminodiacetic acid (IDA) and nitrilotriacetic acid (NTA) for IMAMs, they are known to provide 3 or 4 binding arms with a metal ion ligand. Therefore, a metal ion ligand on those IMAMs with the conventional chelators can probably have only 3 or 2 free binding sites for protein adsorption. This might explain the high utilization of copper ion ligands on CS/CA-Cu achieved in this study.
3.3 Effect of pH on BSA adsorption
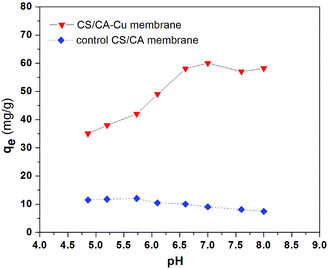
In general, the adsorption of protein in immobilized metal ion affinity chromatography is usually governed by the coordination of the metal ion ligand on the IMAMs with the electron-donor atoms or groups exposed on the surface of the proteins to be separated.27,28 Hence, the pH of the liquid phase can have an effect on the binding capacity of IMAMs for protein.Fig. 3 shows the effects of solution pH on the adsorption uptake of BSA on CS/CA-Cu and CS/CA obtained from the experiments (ionic strength at 0.12 M NaCl). The amount of BSA adsorbed on the CS/CA base membrane decreased slightly with increasing pH. This may be expected because the electrostatic interaction between CS/CA and BSA molecules became less favorable when BSA was negatively charged at pH > 4.7 and the amine groups of CS shifted from positively charged at pH < 6.3 to neutral or even negatively charged at pH > 6.3. However, the amount of BSA binding on CS/CA-Cu increased remarkably from 35 mg g−1 to 58 mg g−1 with the increase in solution pH from 4.9 to 6.5, and then stayed at a plateau level with further increases in pH. Therefore, it appears that the adsorption of BSA on CS/CA-Cu was favored under neutral conditions. This condition may be in fact desirable in practical protein separation processes as it can often minimize the need for pH adjustment.
The pH dependency of BSA binding onto CS/CA-Cu may be explained by the nature of the interactions between the copper ion ligands and the BSA molecules. The affinity interaction between copper ions (electron acceptors) and imidazole nitrogen (electron donors) of BSA would dominate the specific binding. The imidazole nitrogen (pKa = 6.95) on BSA is protonated under acidic conditions. Hence, the adsorption uptake amount at pH 4.9 was low because of the weakened electron donor–acceptor interactions due to the existence of a strong electrostatic repulsion force that possibly hindered the approach of BSA to the copper ion ligands on CS/CA-Cu.
With increasing pH from 4.9 to 7, the imidazole nitrogen atoms became less protonated and thus the transport of BSA in solution to the copper ions of CS/CA-Cu was favored (due to lower electrostatic repulsion force). This has led to the observed increase in BSA adsorption on CS/CA-Cu with the increase in solution pH. However, with the further increase in the solution pH above 7, most imidazole nitrogen atoms were at their deprotonated state, which provided the most favorable condition for BSA adsorption on CS/CA-Cu. On the other hand, more OH– in the solution led to an increased number of OH– from the solution being coordinated with the copper ion ligands on CS/CA-Cu, which did not favor BSA binding uptake on CS/CA-Cu through affinity interaction. These two counteractions appeared to compensate for each other and the net adsorption amount was maintained at the plateau level in the pH range from 7 to 8.
3.4 Effect of ionic strength on BSA adsorption
The dependence of BSA adsorption by CS/CA and CS/CA-Cu on the ionic strength of the solution was examined under the condition of pH 7.4 and initial BSA concentration of 2.5 g L−1, and the results are shown in Fig. 4. The adsorption uptake amounts of BSA on CS/CA-Cu generally decreased with increasing ionic strength (in terms of NaCl concentration). Although the changes in the uptake amounts of BSA (about 58 mg g−1) were not apparent in the low ionic strength range from 0 to 0.09 M, the adsorption uptake amounts of BSA on CS/CA-Cu decreased significantly with the further increase of the ionic strength from 0.09 to 1.8 M (changed from about 60 mg g−1 at 0.09 M to only about 24.5 mg g−1 at 1.8 M; see Fig. 4).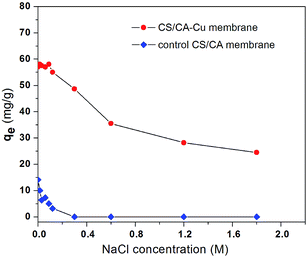 | ||
| Fig. 4 Effect of ionic strength on BSA adsorption on the control CS/CA and the CS/CA-Cu membranes at pH 7.4 and C0 = 2.5 g L−1. | ||
The adsorption uptake amounts of BSA on the control CS/CA membrane also decreased with increasing ionic strength. At an ionic strength of 0.3 M and above, the adsorption uptake amounts of BSA on CS/CA actually decreased to zero. If one takes the amount of BSA adsorbed on CS/CA as the indication of the nonspecific adsorption, the increase in ionic strength in the solution appears to effectively suppress or eliminate the nonspecific binding of BSA on the control CS/CA membrane and thus on CS/CA-Cu.
Therefore, the decrease in BSA binding on CS/CA-Cu with increasing ionic strength may be due to two facts. First, the decrease in nonspecific adsorption (or electrostatic interaction) on the CS/CA substrate led to the decreased adsorption of BSA on CS/CA-Cu. On the other hand, the high NaCl concentration in solution would enhance the competition of the coordination sites of the copper ion ligands on CS/CA-Cu by the Cl− anions, which can reduce the extent of the electron donor–acceptor interaction between the copper ion ligands on CS/CA-Cu and the BSA molecules to be adsorbed.29 Higher adsorption amounts at lower ionic strengths may be another process advantage for CS/CA-Cu as an IMAM because this makes it unnecessary, as in several other situations, to increase the ionic strength in the solution and enhance the adsorption performance.
3.5 Adsorption isotherms results
The two most common adsorption isotherm models are the Langmuir and Freundlich models, which can be given in the forms of eqn (1) and (2), respectively, as below.
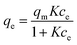 | (1) |
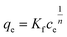 | (2) |
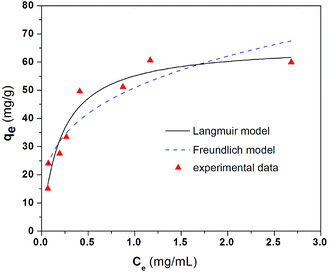
Eqn (1) and (2) are used to fit the experimental results in Fig. 2 and the fitted results for the adsorption isotherm data of BSA on CS/CA-Cu are shown in Fig. 5. Fitting analysis was conducted by applying the non-linear regression method for qe versus Ce through Origin Software 6.0. The parameter values determined from the fitting analysis are given in Table 1. It can be found that the Langmuir model fits the experimental data well; however, the Freundlich model is much less satisfactory. The results therefore indicate that BSA adsorption on CS/CA-Cu was dominated by chemical interaction and probably in a monolayer pattern. This is expected as, in the early discussion, 85% of the adsorbed BSA on CS/CA-Cu was deduced to be through specific binding (i.e., chemical interaction). BSA has an isoelectrical point at pH 4.9. Under the experimental condition of pH 7.4, BSA was negatively charged. Hence, multi-layer adsorption may be unlikely due to the electric repulsion between the adsorbed BSA and BSA in the solution to be further adsorbed. The maximum adsorption amount (qm) and the adsorption equilibrium constant K obtained from the Langmuir model fitting calculation were 66.35 mg g−1 and 4.89 mL mg−1, respectively. The predicted adsorption capacity of 66.35 mg g−1 is very close to the experimental maximum value of 60 mg g−1 shown in Fig. 2.
| Model | pH | Adsorption isotherm parameter | ||
|---|---|---|---|---|
| R2 | qm (mg g−1) | K (mL mg−1) | ||
| Langmuir | 4.9 | 0.98 | 39.78 | 3.32 |
| 6.1 | 0.98 | 53.10 | 4.71 | |
| 7.4 | 0.93 | 66.35 | 4.89 | |
| Model | pH | Adsorption isotherm parameter | ||
|---|---|---|---|---|
| R2 | Kf (mg g−1) (mL mg−1)−1/n | 1/n | ||
| Freundlich | 4.9 | 0.86 | 28.12 | 0.28 |
| 6.1 | 0.92 | 40.45 | 0.28 | |
| 7.4 | 0.85 | 50.91 | 0.27 | |
The adsorption isotherm data were also evaluated at two other pH values of 4.9 and 6.1; the results are shown in Fig. 6. The parameter values obtained by the non-linear regression of eqn (1) and (2) to the experimental data are also included in Table 1. Again, these results also showed that the Langmuir model had a better fit to the experimental data than the Freundlich model. The predicted maximum adsorption amounts, qm, from the Langmuir model are 39.78 mg g−1 for pH 4.9 and 53.10 mg g−1 for pH 6.1 in comparison to the 66.35 mg g−1 for pH 7.4 mentioned early. These predicted maximum adsorption amounts indicate a similar change trend in the adsorption performance with the effect of solution pH discussed in Section 3.3.
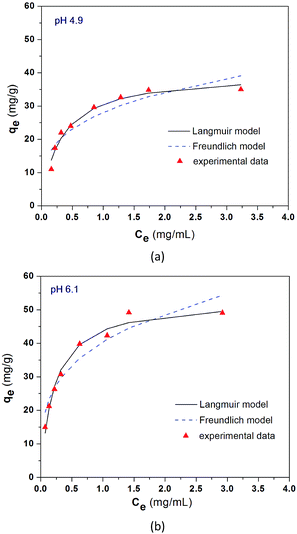 | ||
| Fig. 6 Equilibrium adsorption results of BSA on CS/CA-Cu at pH 4.9 (a) and pH 6.1 (b) under room temperature. | ||
3.6 Adsorption kinetic behaviour
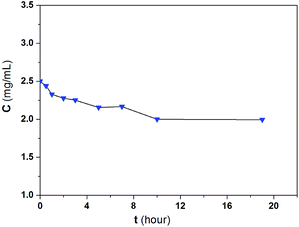
The kinetic changes in the BSA concentrations in the bulk solution with adsorption time from a typical experiment are presented in Fig. 7. The adsorption took about 10 h to reach equilibrium. Two processes may be involved in the adsorption of BSA on CS/CA-Cu: (a) the transport of BSA from the bulk solution to the surface of CS/CA-Cu and (b) the attachment of BSA on CS/CA-Cu. In the initial stage, the surface of CS/CA-Cu was relatively free of BSA and the BSA molecules that arrived at the surface of CS/CA-Cu may attach instantly to it. Hence, the adsorption rate may be dominated by the diffusion of BSA from the bulk solution to the surface of CS/CA-Cu in this case.Based on the Fickian diffusion law, the amount of adsorption by diffusion-controlled dynamics as a function of time may be given as follows:30
| qt = kdt0.5 | (3) |
Fig. 8a shows a plot of q(t) versus t0.5 for the experimental results in Fig. 7. A linear relationship of q(t) against t0.5 is indeed observed in the initial period of time. The results suggest the existence and the importance of diffusion-controlled transport mechanisms in BSA adsorption on CS/CA-Cu, at least in the beginning of the process.
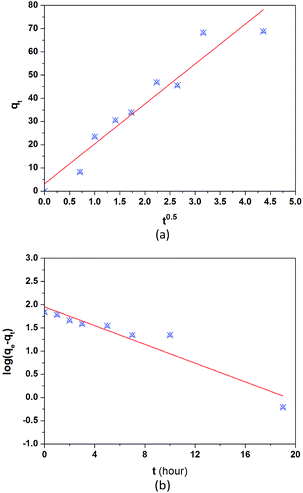 | ||
| Fig. 8 Fitted results of typical adsorption kinetic models: (a) diffusion-controlled adsorption kinetics model and (b) attachment-controlled adsorption kinetic model. | ||
On the other hand, the adsorption rate may also be controlled by the attachment of BSA on CS/CA-Cu, particularly in the later stages when CS/CA-Cu may be gradually saturated with previously adsorbed BSA. For attachment controlled adsorption, the amount of adsorption may be given by the Lagergren model as follows:31
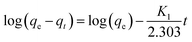 | (4) |
The plot of log(qe − qt) vs. t in terms of eqn (4) for the experimental results shown in Fig. 7 is presented in Fig. 8b. An approximate linear relationship indicated in eqn (4) is observed for almost the entire adsorption process, suggesting that the attachment process was indeed very important in the adsorption kinetics of BSA on CS/CA-Cu, particularly when the kinetic study was conducted at a relatively high BSA concentration in this case.
4. Conclusion
The CS/CA blend hollow fiber membrane can be used directly for the immobilization of metal ion ligands to prepare IMAM. The obtained CS/CA blend hollow fiber membrane achieved an immobilized amount of copper ions of 3.9 mg g−1 membrane. The prepared CS/CA-Cu showed an adsorption capacity of 69 mg-BSA per g membrane at pH 7.4 and a NaCl concentration of 0.12 M under room temperature. At least 85% of the adsorbed BSA was probably through specific interactions by the copper ion ligands on CS/CA-Cu with the BSA molecules. The utilization of the coupled copper ion ligand reached as high as 15.4 mg-BSA per g-Cu2+. The adsorption isotherm was well described by the Langmuir isotherm model. The adsorption kinetics appeared to be affected by the transport-controlled mechanism in the initial stage but attachment-controlled mechanism throughout the entire process. In the pH range of 4.9–8.0 and ionic strength of 0.0–1.8 M examined, BSA adsorption on the prepared CS/CA-Cu IMAM showed the best performance at around neutral pH values (6.5–8) and at low ionic strength (<0.09 M), attributed to the reduced repulsive electrostatic interaction or the reduced effect of competing anions, which favored the affinity interaction of BSA with the copper ion ligands. The study shows the potential prospects of using CS/CA hollow fiber membrane to prepare IMAMs for protein separation. Further investigation will be done in the enhancement of the adsorption amount and in the establishment of the dynamic separation performance.Acknowledgements
The financial support from the National Natural Science Foundation of China (No. 51478282), Suzhou Key Lab of Separation and Purification Materials & Technologies (SZS201512), and The Open Project of Jiangsu Key Laboratory of Environmental Science and Engineering (No. ZD131206) are greatly appreciated.References
- J. Zhu and G. Sun, Facile fabrication of hydrophilic nanofibrous membranes with an immobilized metal–chelate affinity complex for selective protein separation, ACS Appl. Mater. Interfaces, 2014, 6(2), 925–932 CAS.
- G. Bayramoglu and M. Y. Arica, Poly(methyl methacrylate-glycidiyl methacrylate) film with immobilized iminodiacetic acid and Cu(II) ion: for protein adsorption, Fibers Polym., 2012, 13(10), 1225–1232 CrossRef CAS.
- S. Bhattacharjee, J. L. Dong, Y. D. Ma, S. Hovde, J. H. Geiger, G. L. Baker and M. L. Bruening, Formation of high-capacity protein-adsorbing membranes through simple adsorption of poly(acrylic acid)-containing films at low pH, Langmuir, 2012, 28(17), 6885–6892 CrossRef CAS PubMed.
- C. I. Chen, Y. M. Ko, W. L. Lien, Y. H. Lin, I. T. Li, C. H. Chen, C. J. Shieh and Y. C. Liu, Development of the reversible PGA immobilization by using the immobilized metal ion affinity membrane, J. Membr. Sci., 2012, 401, 33–39 CrossRef.
- C. I. Chen, Y. M. C. J. Shieh and Y. C. Liu, Direct penicillin G acylase immobilization by using the self-prepared immobilized metal affinity membrane, J. Membr. Sci., 2011, 380, 34–40 CrossRef CAS.
- J. H. Wu, Z. Wang, W. T. Yan, Y. Wang, J. X. Wang and S. C. Wang, Improving the hydrophilicity and fouling resistance of RO membranes by surface immobilization of PVP based on a metal-polyphenol precursor layer, J. Membr. Sci., 2015, 496, 58–69 CrossRef CAS.
- H. Nagami, H. Umakoshi, T. Kitaura, G. L. Thompson, T. Shimanouchi and R. Kuboi, Development of metal affinity-immobilized liposome chromatography and its basic characteristics, Biochem. Eng. J., 2014, 84, 66–73 CrossRef CAS.
- Y. M. Ke, C. I. Chen, P. M. Kao, H. B. Chen, H. C. Huang, C. J. Yao and Y. C. Liu, Preparation of the immobilized metal affinity membrane with high amount of metal ions and protein adsorption efficiencies, Process Biochem., 2010, 45(4), 500–506 CrossRef CAS.
- S. Asapu, S. Pant, C. L. Gruden and I. C. Escobar, An investigation of low biofouling copper-charged membranes for desalination, Desalination, 2014, 338, 17–25 CrossRef CAS.
- V. K. Thakur and S. I. Voicu, Recent advances in cellulose and chitosan based membranes for water purification: a concise review, Carbohydr. Polym., 2016, 146, 148–165 CrossRef CAS PubMed.
- T. Chakrabarty and V. K. Shahi, Modified chitosan-based, pH-responsive membrane for protein separation, RSC Adv., 2014, 4(95), 53245–53252 RSC.
- K. Liu, L. H. Chen, L. L. Huang and Y. N. Lai, Evaluation of ethylenediamine-modified nanofibrillated cellulose/chitosan composites on adsorption of cationic and anionic dyes from aqueous solution, Carbohydr. Polym., 2016, 151, 1115–1119 CrossRef CAS PubMed.
- H. P. S. A. Khalil, C. K. Saurabh, A. S. Adnan, M. R. Nurul Fazita, M. I. Syakir, Y. Davoudpour, M. Rafatullah, C. K. Abdullah, M. K. M. Haafiz and R. Dungani, A review on chitosan–cellulose blends and nanocellulose reinforced chitosan biocomposites: properties and their applications, Carbohydr. Polym., 2016, 150, 216–226 CrossRef PubMed.
- H. Celebi and A. Kurt, Effects of processing on the properties of chitosan/cellulose nanocrystal films, Carbohydr. Polym., 2015, 133, 284–293 CrossRef CAS PubMed.
- E. Salehi, P. Daraei and A. A. Shamsabadi, A review on chitosan-based adsorptive membranes, Carbohydr. Polym., 2016, 152, 419–432 CrossRef CAS PubMed.
- A. Li, R. J. Lin, C. Lin, B. Y. He, T. T. Zheng, L. B. Lu and Y. Cao, An environment-friendly and multi-functional absorbent from chitosan for organic pollutants and heavy metal ion, Carbohydr. Polym., 2016, 148, 272–280 CrossRef CAS PubMed.
- C. X. Liu and R. B. Bai, Preparation of chitosan/cellulose acetate blend hollow fibers for adsorptive performance, J. Membr. Sci., 2005, 267, 68–77 CrossRef CAS.
- C. X. Liu and R. B. Bai, Preparing highly porous chitosan/cellulose acetate blend hollow fibers as adsorptive membranes: effect of polymer concentrations and coagulant compositions, J. Membr. Sci., 2006, 279, 336–346 CrossRef CAS.
- C. X. Liu and R. B. Bai, Adsorptive removal of copper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes, J. Membr. Sci., 2006, 284, 313–322 CrossRef CAS.
- G. Mary, S. K. Bajpai and N. Chand, Copper(II) ions and copper nanoparticles-loaded chemically modified cotton cellulose fibers with fair antibacterial properties, J. Appl. Polym. Sci., 2009, 113, 757–766 CrossRef CAS.
- S. Waheed, A. Ahmad, S. M. Khan, S. Gul, T. Jamil, A. Islam and T. Hussain, Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan, Desalination, 2014, 351, 59–69 CrossRef CAS.
- A. G. Boricha and Z. V. P. Murthy, Preparation of N,O-carboxymethyl chitosan/cellulose acetate blend nanofiltration membrane and testing its performance in treating industrial wastewater, Chem. Eng. J., 2010, 157, 393–400 CrossRef CAS.
- X. Jiang, L. Chen and W. Zhong, A new linear potentiometric titration method for the determination of deacetylation degree of chitosan, Carbohydr. Polym., 2003, 54, 457–463 CrossRef CAS.
- C. Y. Wu, S. Y. Suen, S. C. Chen and J. H. Tzeng, Analysis of protein adsorption on regenerated cellulose-based immobilized copper ion affinity membranes, J. Chromatogr., 2003, 996, 53–70 CrossRef CAS PubMed.
- H. Iwata, K. Saito, S. Furusaki, T. Sugo and J. Okamato, Adsorption characteristics of an immobilized metal affinity membrane, Biotechnol. Prog., 1991, 7, 412–418 CrossRef CAS PubMed.
- M. Rhazia, J. Desbrières, A. Tolaimate, M. Rinaudob, P. Votterod and A. Alagui, Contribution to the study of the complexation of copper by chitosan and oligomers, Polymer, 2002, 43, 1267–1276 CrossRef.
- E. K. M. Ueda, P. W. Gout and L. Morganti, Current and prospective applications of metal ion-protein binding, J. Chromatogr., 2003, 988, 1–23 CrossRef CAS PubMed.
- R. S. Pasquinelli, R. E. Shepherd, R. R. Koepsel, A. Zhao and M. M. Ataai, Design of affinity tags for one-Step protein purification from immobilized zinc columns, Biotechnol. Prog., 2000, 16, 86–91 CrossRef CAS PubMed.
- N. Li and R. B. Bai, Copper adsorption on chitosan–cellulose hydrogel beads: behaviors and mechanisms, Sep. Purif. Technol., 2005, 42, 237–247 CrossRef CAS.
- M. Alkan, M. Dogan, Y. Turhan, O. Demirbas and P. Turan, Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solutions, Chem. Eng. J., 2008, 139, 213–223 CrossRef CAS.
- X. Zhang and R. B. Bai, Mechanisms and kinetics of humic acid adsorption to chitosan-coated granules, J. Colloid Interface Sci., 2003, 264, 30–38 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |