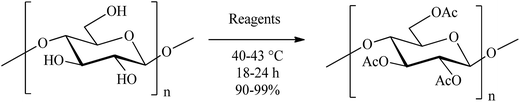
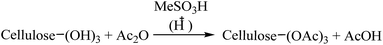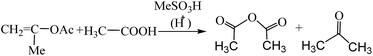Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Value-additive utilization of agro-biomass: preparation of cellulose triacetate directly from rice straw as well as other cellulosic materials†
Amita Sharma‡
a,
Santosh Kumar Giri‡b,
K. P. Ravindranathan Kartha *b and
Rajender S. Sangwan*a
*b and
Rajender S. Sangwan*a
aCenter of Innovative and Applied Bioprocessing (CIAB), C-127, Phase-8, S.A.S. Nagar, Mohali-160071, Punjab, India. E-mail: sangwan@ciab.res.in; Tel: +91-172-2292028
bDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, S.A.S Nagar, Punjab-160062, India. E-mail: rkartha@niper.ac.in; Fax: +91-172-2214692; Tel: +91-172-2292028
First published on 23rd February 2017
Abstract
Non-forage crop residues like rice straw are among the most important and pertinent renewable biomass available for utilization for producing biofuels, specialty chemicals, aromatics etc. Recovery of cellulose from them and its processing for products are two key steps of value addition to these materials that are available in abundance. Of these, rice straw is perhaps the most recalcitrant of all. In this study, production of cellulose triacetate (CTA) from cellulose by three different (alternate) practical methods are reported. It has been demonstrated that cellulose in the form of microcrystalline cellulose (MCC), other celluloses commercially available from chemical suppliers, cotton linters, and rice straw as such (directly after powdering) are suitable as substrates for the reaction. The methods make use of either acetic acid, acetyl chloride or isopropenyl acetate (IPA) as the acetyl donor in the presence of methane sulfonic acid (a cheap and green reagent) as the acid catalyst. The conventional acetyl donor agent, acetic anhydride is also a suitable alternative reagent for the purpose. The post-reaction work-up procedure involves neutralization and filtration as the only two simple successive steps. The processes reported thus eliminate the need for using restricted/toxic chemicals (acetic anhydride and pyridine, respectively) and provide cellulose tri-O-acetate of high purity in excellent yields. Preparation of CTA directly from rice straw has been achieved in >70% yield, representing acetylation of the straw cellulose into its triacetate to an extent of 90%. With IPA as the acyl donor, acetone was a valuable and recoverable co-product. Although not demonstrated in this study, the residue from the acetylation reaction can be utilized for the recovery of value-added materials such as fine silica.
Introduction
Carbohydrates, namely, cellulose, hemicelluloses, starch and sucrose together constitute approximately 75% of plant biomass in which cellulose forms the major component amounting to more than 40% of the total biomass composition.1,2 Although cellulose is organic and biodegradable, is non-toxic and is built of anhydroglucose units, its unique macromolecular structure makes it insoluble in water as well as organic solvents. The fact that it undergoes decomposition before melting also makes it less amenable to industrial processing. To a great extent this problem is usually addressed by its conversion to soluble derivatives. Derivatized cellulosics find many important applications in the fiber, paper, optical film, membrane, coating, and paint industries.3 Among the important cellulose derivatives are the esters (acetate, sulfonate, etc.) and the ethers (alkyl, hydroxyalkyl, carboxyalkyl, etc.)4 that serve well in the textile and various polymer industries.5–9 Cellulose triacetate (CTA) is one of the oldest man made cellulose esters that have been used widely in the industry and is generally prepared by treating cellulose with acetic anhydride in acetic acid in the presence of H2SO4/HClO4 as catalyst; and often suffers from the limitations of low degree of substitution (DS) and poor yields for various reasons.10 Use of H2SO4 often also leads to residual sulfur (from sulfate ester substitution) in the final product. Liebert and Heinze reported that treatment of cellulose with vinyl acetate in DMSO/TBAF leads to the formation of CTA but is accompanied by side reactions such as oxidation.11 Homogeneous reaction conditions for the purpose of smooth reactions are also reportedly achieved by using various ionic liquids12,13 such as 1-allyl-3-methylimidazolium chloride,14 1-butyl-3-methylimidazolium chloride,15,16 those based on choline chloride and ZnCl2, as in [ChCl][ZnCl2]2,17 etc. Moreover, for the synthesis of CTA the Ac2O–I2 system originally proposed for simple sugars18 has also been reported either at 100 °C under mechanical stirring19 or under a microwave.20,21 Biswas and associates22 have also reported an acetic anhydride-based process of obtaining CTA directly from rice hull (i.e. rice grain husk) but the yields were far lower than those with isolated cellulose from the feedstock. Also, rice husk is a minor (rice grain to husk ratio of only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) by-product most of which is being profitably used in the milling industry itself for boiler operations and direct power generation. It is in this context that we like to now report our findings that CTA can be conveniently prepared by reacting cellulose [in the form of microcrystalline cellulose (MCC), cellulose or rice straw] with an acyl donor reagent such as isopropenyl acetate or acetyl chloride or glacial acetic acid in the presence of methanesulfonic acid (MeSO3H, MeSA).23 The method does not require use of Ac2O, the acetyl donor whose supply is restricted by law, and is simple, economical, and easy to operate and green. The reaction produces no toxic waste and is suitable for scale up. On using IPA as the acyl donor, the by-product, namely, acetone formed can be collected fully by simple distillation.
0.2) by-product most of which is being profitably used in the milling industry itself for boiler operations and direct power generation. It is in this context that we like to now report our findings that CTA can be conveniently prepared by reacting cellulose [in the form of microcrystalline cellulose (MCC), cellulose or rice straw] with an acyl donor reagent such as isopropenyl acetate or acetyl chloride or glacial acetic acid in the presence of methanesulfonic acid (MeSO3H, MeSA).23 The method does not require use of Ac2O, the acetyl donor whose supply is restricted by law, and is simple, economical, and easy to operate and green. The reaction produces no toxic waste and is suitable for scale up. On using IPA as the acyl donor, the by-product, namely, acetone formed can be collected fully by simple distillation.
Results and discussion
We recently had noted that MeSA is an excellent green catalyst for the acetylation of hydroxyl and sulfhydryl group-containing substrates such as alcohols and thiols. This prompted us to investigate its applicability in the acetylation of polymeric and difficult-to-acetylate carbohydrates such as cellulose. The much investigated and most widely used acetyl donor, Ac2O itself was chosen as the reagent of choice for the initial experiments employing MCC as the substrate for acetylation; and this must also serve as control for comparison of the results of the experiments to follow. Thus, when MCC (1 g) was treated with Ac2O (10 mL) in the presence of MeSA (1 mL) for 24 h at 42 °C, a near-colourless solid product (1.65 g, 93% based on the weight expected for CTA; entry 1, Table 1) was indeed obtained. The overall reaction can be depicted as shown in Scheme 1. The product was freely soluble in organic solvents such as EtOAc, CH2Cl2, CHCl3, etc. and, on removal of the solvent by evaporation, it formed a transparent and clear film indicating it to be the acetate. The acetylated product thus obtained when analysed by IR did not show any hydroxyl absorption bands in its IR spectrum (data not shown) and the signals observed in the 1H NMR spectra (see p. S2 of the ESI†) were in accordance with what was expected for a per-O-acetylated (1,4)-β-linked glucan, confirming its identity as the tri-O-acetate from MCC. Moreover, the product did not show presence of sulfur when subjected to elemental analysis for sulfur. It may also be pointed out that although MeSA–Ac2O system can arguably be considered capable of doing acetolytic cleavage of interglycosidic bonds in carbohydrates, their stability to acetolysis in MeSA–Ac2O system in the case of di- and trisaccharides as well as β-cyclodextrin has already been demonstrated recently.| Entry | Substrate | Reagents (mL) | Reactionb time (h) | Yieldc (%) | ||
|---|---|---|---|---|---|---|
| a Substrate (1 g) was treated with reagents at 42 °C.b Each experiment was repeated twice for checking the reproducibility.c Isolated yield is reported in all the cases.d Based on the percent weight gain in the estimated cellulose content of rice straw. | ||||||
| 1 | MCC | Ac2O (10) | — | MeSA (1) | 24 | 93 |
| 2 | IPA (10) | — | MeSA (1) | 48 | 6 | |
| 3 | IPA (20) | — | MeSA (1) | 72 | 51 | |
| 4 | IPA (10) | AcOH (10) | MeSA (1) | 18 | 99 | |
| 5 | IPA (10) | AcOH (10) | MeSA (0.5) | 20 | 95 | |
| 6 | AcOH (20) | — | MeSA (1) | 18 | 98 | |
| 7 | AcOH (10) | — | MeSA (1) | 20 | 42 | |
| 8 | AcOH (10) | — | MeSA (0.5) | 24 | 20 | |
| 9 | AcOH (20) | AcCl (5) | — | 18 | 90 | |
| 10 | AcOH (20) | AcCl (2.5) | — | 18 | 85 | |
| 11 | AcOH (10) | AcCl (2.5) | — | 18 | 80 | |
| 12 | Cellulose | IPA (10) | AcOH (10) | MeSA (1) | 18 | 98 |
| 13 | AcOH (10) | — | MeSA (1) | 18 | 60 | |
| 14 | AcOH (20) | AcCl (5) | — | 18 | 55 | |
| 15 | Rice straw | IPA (10) | AcOH (10) | MeSA (1) | 24 | 62d |
| 16 | AcOH (10) | — | MeSA (1) | 24 | 60d | |
| 17 | AcOH (20) | — | MeSA (1) | 24 | 73d | |
| 18 | AcOH (20) | AcCl (5) | — | 24 | 40d | |
| 19 | Ac2O (10) | AcOH (10) | MeSA (1) | 24 | 35d | |
| 20 | Ac2O (20) | — | MeSA (1) | 24 | 40d | |
A possible limitation of this method, as is the case with all those that necessitate the use of Ac2O, however, is that it involved use of a substance (Ac2O) that is a somewhat restricted chemical commodity. As an alternative, therefore, the use of isopropenyl acetate (IPA) as the acetylating agent was explored simultaneously (Table 1). Acetylation of a variety of hydroxy compounds (alcohols, enols and phenols) as well as amines using IPA has been described in a patent24 and was followed by detailed publications of the work subsequently by Hull and Hagemeyer25 as well as others;26–30 and the catalysts employed include sulfuric acid and p-toluene sulfonic acid.25 The reactions were found to proceed smoothly and resulted in very high yields of the acetylated product under neat conditions and in the case of some of the amines, it was obtained rather spontaneously (without the aid of an added catalyst). Thus, the process was proved to be useful for the chemoselective acetylation of an amino group in hydroxyalkyl-substituted amino compounds, as for example, in the preparation of N-(2-hydroxyethyl)-acetamide from monoethanolamine. A prominent feature of this work25 in the context of green chemistry is that the desired acetate/acetamide and the by-product of the reaction (acetone) could be separated efficiently by distillation, making good use of the differences in their boiling points. A similar work on the acetylation of simple alcohols and amines using IPA was again reported recently by Pelagalli and co-workers.31 In view of the findings by Hull and co-workers24 for simple alcohols and amines therefore, when MCC (1 g) was treated with IPA (neat, 10–20 mL) in the presence of MeSA (1 mL) for up to 3 days, CTA could be isolated after an aqueous work up and filtration in a yield of up to 51% (entries 2 and 3, Table 1). In the absence of the acidic catalyst no product was obtained (results not listed in the table). Nevertheless, the above results were not satisfactory so far as the product yield and the reaction time were concerned. It may be recalled26,27 that the reaction of IPA with AcOH in the presence of strong acids can generate Ac2O in situ (along with the by-product, acetone) as depicted in Scheme 2. Further, in view of the fact that AcOH can serve as an excellent solvent for CTA, the foregoing reaction of cellulose with IPA was also tried in the presence of added AcOH (entry 4, Table 1). Quite satisfyingly, under these conditions CTA could be isolated in near-quantitative yields (entry 4, Table 1) after 18 h of stirring. Reducing the catalyst concentration to half the above was found to marginally affect the CTA yields (95% yield, entry 5, Table 1). The NMR spectra obtained for the CTA prepared from MCC by the MeSA–IPA–AcOH method as discussed above (Fig. 1 and 2) clearly demonstrated that the product obtained was exclusively the triacetate (CTA). The absence of any hydroxyl absorption signal in the IR spectra and the absence of sulfur on elemental analyses, etc. were also in accordance with the expected results.
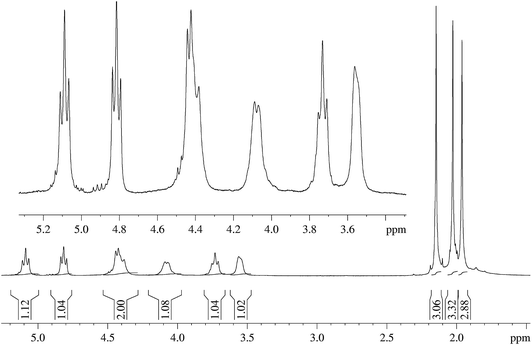 | ||
| Fig. 1 1H NMR spectrum of CTA prepared from MCC by the MeSA–IPA–AcOH method and the expanded 3.5–5.2 ppm region of the spectrum. | ||
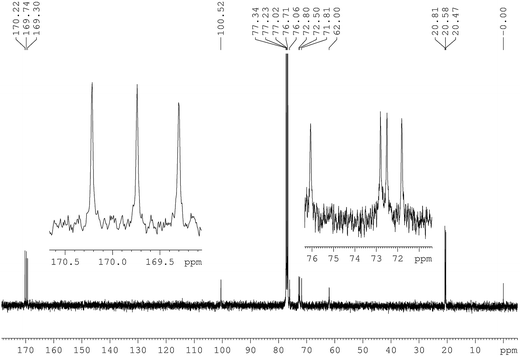 | ||
| Fig. 2 13C NMR spectra of the triacetate (CTA) prepared from MCC using MeSA–IPA–AcOH and the region 70–76 ppm and the region 169–170.5 ppm expanded. | ||
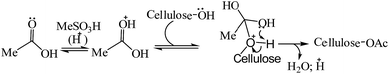
Further, in the light of our earlier finding that MeSA was capable of transferring the acyl group from AcOH to simple alcohols to yield the corresponding acetate esters,23 this reaction was also recruited to test its feasibility for the acetylation of macromolecules like cellulose. When the reaction of MCC was carried out in neat AcOH (without any added IPA) in the presence of MeSA, the desired product CTA could be isolated in similar yields (98%, entry 6, Table 1) with reaction proceeding as per Scheme 3 shown below.
Here again, reducing the quantity of AcOH employed for the reaction to half significantly affected the yield of CTA (42% only, entry 7, Table 1); and when this was also accompanied by the use of a reduced quantity of the catalyst (MeSA) in the reaction mixture, it led to further depletion of yields of CTA (entry 8, Table 1). Thus, it was observed that for a quantitative conversion of the polysaccharide substrate in gram-scale reactions, use of approximately 20 mL AcOH per gram MCC was best suited.
On the basis of the argument that reaction of AcCl with AcOH can also generate Ac2O in situ (eqn (1) below), a set of reactions employing a mixture of AcOH and AcCl was also performed (entries 9–11, Table 1). The results clearly showed that excellent yields (up to 90%) of CTA can be obtained using AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcCl in a ratio of 4
AcCl in a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 20 mL AcOH/g MCC. It can be seen from Table 1 that yields were reduced to 80–85% on reducing the quantum of AcCl or AcOH used for the reaction (entries 10 and 11, Table 1).
1 with 20 mL AcOH/g MCC. It can be seen from Table 1 that yields were reduced to 80–85% on reducing the quantum of AcCl or AcOH used for the reaction (entries 10 and 11, Table 1).
| AcOH + AcCl ⇌ Ac2O + HCl | (1) |
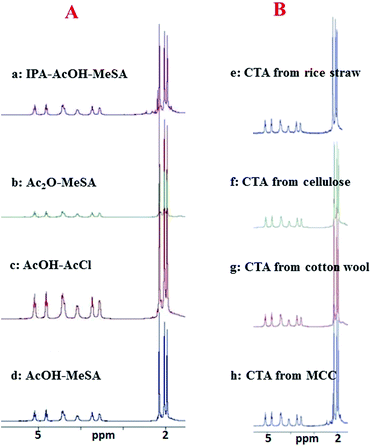
HCl, the by-product formed in the reaction, served as an in situ catalyst for the acyl transfer reaction. This conclusion was forthcoming from the fact that presence of both AcOH and AcCl was essential for the smooth and significant progress of the reaction. Experiments conducted by exclusion of either of them did not produce the desired result, even when the reaction was allowed to go up to 4 days, (data not shown in the table). Thus, a combination of AcOH–AcCl also formed an excellent system for efficient acetylation of MCC. However, the fact that AcCl is a corrosive reagent and fuming in air by nature, could prove to be practical limitations of this method in large scale preparations unless addressed by adopting the appropriate reaction kettles and methods of handling such reagents. An overlay of the 1H NMR spectra of the triacetates prepared from MCC by the four different methods enumerated above have been presented in Fig. 3 for enabling a direct comparison of these methods in a facile manner. In all the cases, it can be seen that, the triacetate (CTA) has unambiguously been the reaction product obtained. After optimization of the above methods for acetylation using MCC as the model cellulosic substrate, the three non-acetic anhydride acetylation systems (IPA–AcOH–MeSA, AcOH–MeSA and AcOH–AcCl, methods A, B and C, respectively) were separately evaluated against the commercially available cellulose (entries 12–14, Table 1). It was found (i) that the commercially available cellulose could be acetylated highly efficiently and with ease under these conditions affording the ester as a tri-O-acetate (see Fig. 3 for the NMR spectra) in all the three cases and, (ii) that the best results in terms of the yield of CTA were obtained by the IPA–AcOH–MeSA method.
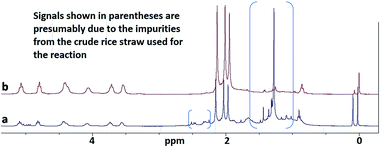
Now, it may be recalled that most of the methods hitherto reported in the literature for the preparation of CTA from crude cellulosics such as straw from a crop, involved a priori alkali extraction step to retrieve cellulose from them. The treatment involves use of a strong alkali such as NaOH; and is followed by the acetylation step by the conventional Ac2O-method. Therefore it could not have perhaps been surprising that when rice grain hull/husk was employed by Biswas and co-workers (2006) directly for acetylation using the Ac2O–H2SO4 route, the yields obtained (13.5%) were significantly poorer than those obtained (25%) from cellulose isolated from the biomass. In view of the above situation, an investigation on the applicability of the above described methods for the preparation of CTA directly from rice straw was felt important. Thus, rice straw obtained from a local agricultural field was pulverized in a mechanical mill and the powder was subjected to acetylation by the different methods described above (entries 15–20, Table 1). Indeed, when the methods were extended to the pulverized rice straw (Fig. 4a) as the substrate, CTA could be obtained in very impressive yields (based on an estimated 40% cellulose content) directly after the workup as noted above; and the methods A and B (IPA–AcOH–MeSA and AcOH–MeSA, respectively) were proved best with respect to the yields obtained. The 1H NMR spectrum of the crude CTA thus obtained (Fig. 5a) showed the product to be the desired tri-O-acetate (CTA) along with some residual contaminants originating from the crude straw used as the substrate for the reaction. It may be recalled that rice straw contains substantial quantities of silica; and silica–OH protons show signals around the 2 ppm region in the solid state 1H NMR32–34 as obtained in the high field region of the spectrum shown in Fig. 5b. Moreover, the product could be made virtually free from these contaminating materials by a simple dissolution of the crude CTA preparation in CH2Cl2 or CHCl3 (whereupon the contaminants coalesced into a solid sediment) followed by filtration. This was evidenced by the new 1H NMR spectrum (reproduced in Fig. 5a) of the CTA thus obtained by the dissolution technique.
 | ||
| Fig. 4 Photomicrographs of (a) rice straw, (b) CTA obtained from MCC by method A, and (c) CTA prepared directly from rice straw by method A. | ||
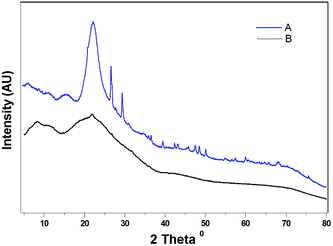
Moreover, the X-ray diffractograms of the triacetate obtained before and after the removal of the chloroform-insolubles show clearly the elimination of the peak at a 2θ of 22.14° typical for the silica (Fig. 6, see also p. S3 of the ESI†) in the latter. Photomicrographs of films of crude CTA preparations obtained (by the evaporation of their respective solutions in CH2Cl2) from rice straw (directly) and from the MCC can be seen in Fig. 4 (Fig. 4c and b, respectively). Likewise the SEM images of the CTA preparations described above revealing their high degree of comparability in terms of their fine structural features can also be confirmed (see p. S4 of the ESI†). It may also be emphasized that the CH2Cl2/CHCl3 insolubles mentioned above are in the process of being subjected to investigations for exploring the possibilities for recovering the rice straw silica possessing great market potential.
A brief review of the methods for the synthesis of CTA from cellulose published in the literature in the past years was felt useful in this context for their due comparison with the methods developed in the current investigation as described above. Table 2 summarises some of the noted methods used for the acetylation of cellulose reported over the past several years. Most of the operations involved in the preparation of CTA by the two classical methods (entries 1 and 2, Table 2), which have been in use for long are carried out at room temperature. The method using H2SO4, however, does not usually lead to the desired triacetate, the maximum DS achieved therein being about 2.8. Therefore, for the CTA preparation, it is required to be subjected to another round of reaction in the presence of HClO4.10 The method utilizing HClO4 for the CTA preparation is, however, carried out in the presence of toluene as a co-solvent in addition to the AcOH used. For the successful acetylations, both these methods require quiet lengthy and careful pre-treatments of the substrates (as for the preparations from cotton linters, etc.) as well, owing to which the actual time required for the reaction could be longer than what are shown in the table. The methods employing the ionic liquids14–17 as well as molecular iodine, on the other hand, require elevated temperatures (80–100 °C) with one (in the latter case) requiring microwave irradiation21 in addition, for high DS of 2.8–3.0 to be obtained. But the iodine-method described by Biswas et al.20,21 is indeed exceptionally short and provided the triacetate in a yield of 82% with isolated cellulose. Likewise, Zhang and co-workers were able to get cellulose acetate of a DS of 2.41 by reaction with Ac2O in the presence of an imidazolium-type acidic ionic liquid at 45 mol% concentration.35 On the other hand Kakuchi et al. reported CTA preparation, again in ionic liquids, but using IPA as the acetyl donor agent with the transesterification having performed at 80 °C.36 The method reported by Liebert and Heinze11 employing TBAF/DMSO in the presence of mono-/di-sodium and potassium hydrogen phosphates at 70 °C makes use of vinyl acetate as the acyl donor and depending upon the excess of the acyl donor used a DS of up to 2.72 is obtained.11 More recent methods reported37–39 make use of the classical basic catalyst pyridine and AcCl in combination with solvents such as CH2Cl2 or dimethyl acetamide, but only partial acetylations (surface acetylation, consequentially leading to relatively low DSs) have been usually achieved (not listed in Table 2).
| Entry | Material used | Reaction condition | % yield & (DS) reported | Ref. | |||
|---|---|---|---|---|---|---|---|
| Reagent(s) | Catalyst(s) | Temp (°C) | Time (h) | ||||
| a Purchased from Aldrich.b Isolated from Caragana korshinskii.c Reaction was done under microwave condition.d Microcrystalline cellulose (MCC) was purchased from SD Fine. | |||||||
| 1 | Cotton linters | Ac2O + AcOH | H2SO4 | rt | 2 | Not given (2.81) | 10 |
| 2 | Cotton linters | Ac2O + AcOH/PhMe | HClO4 | rt | 1 | Not given (triacetate) | |
| 3 | Cellulose | Ac2O | AMIMCl | 80 | 8 | Not given (2.49) | 14 |
| 4 | Cellulose | Ac2O | [ChCl][ZnCl2]2 | 90 | 3 | Not given (47%) | 17 |
| 5 | Cellulosea | Ac2O | I2 | 100 | 10 min | 82 (triacetate) | 20 |
| 6 | (i) MCC | Ac2O | I2 | 100 | 1 | Not given (triacetate) | 19 |
| (ii) Sisal | Ac2O | I2 | 100 | 3 | Not given (2.8) | ||
| 7 | Celluloseb | Ac2O | I2 | 100c | 10 min | 78 (2.8) | 21 |
| 8 | Avicel (Fluka) | Vinyl acetate | DMSO/TBAF | 40 | 70 | Not given (1.04–2.72) | 11 |
| 9 | MCCa | AcCl | [C4mim]+Cl− | 80 | 2 | 86 (triacetate) | 15 |
| 10 | Cellulose | Ac2O | SO3H/PhSO3H–carbon | 80 | 12 | 2–2.94 | 40 |
| 11 | MCCd | AcOH + IPA | MeSA | 40–43 | 18 | 99 (triacetate) | This work |
| 12 | MCCd | AcOH | MeSA | 40–43 | 18 | 98 (triacetate) | |
| 13 | MCCd | AcOH + AcCl | None additional | 40–43 | 18 | 90 (triacetate) | |
| 14 | MCCd | Ac2O | MeSA | 40–43 | 18 | 93 (triacetate) | |
| 15 | Cellulosea | AcOH + IPA | MeSA | 40–43 | 18 | 98 (triacetate) | |
| 16 | Cellulosea | AcOH | MeSA | 40–43 | 18 | 60 (triacetate) | |
| 17 | Cellulosea | AcOH + AcCl | None additional | 40–43 | 18 | 55 (triacetate) | |
While this manuscript was under preparation, a method using Ac2O as the acetylating agent in the presence of sulfonated carbon (SO3H/PhSO3H–carbon) requiring reactions at 80 °C for 12 h was also reported for achieving DSs up to 2.94 (entry 10, Table 2).40 In significant advancement to such methods, the methods reported in this investigation yield cellulose triacetate at 40–43 °C in overnight reactions in a single step (entries 11–17, Table 2). Either neat AcOH or a mixture of AcOH–IPA or AcOH–AcCl, or alternatively, neat Ac2O can be used for the reaction that is catalyzed by MeSA. The cellulose triacetate was invariably obtained in high yields, the best results being obtained in the case of methods using AcOH–MeSA (method B) and IPA–AcOH–MeSA (method A). These latter methods were also therefore performed on straw at 10 g scale without encountering any practical difficulties or affecting the yield of CTA. Further, it may also be noted that the time taken for the reaction can be reduced considerably in the case of AcOH–IPA reaction if the by-product acetone is removed by distillation which requires the reaction to be carried out at temperatures of 60 °C or above. When the acetone produced is removed by distillation slowly, the acetylation can be considered to be complete when the collection of acetone (as distillate) stops. The acetone obtained as by-product can thus be collected and put to use elsewhere.
Experimental
Materials and methods
Cellulose, AcOH, AcCl and IPA (99%) were purchased from Sigma-Aldrich, India. MeSA (98%) was from Alfa-Aesar, John Matthey India. MCC, Ac2O (AR grade), Na2CO3 and NaHCO3 were procured from S. D. Fine-Chem Ltd. Kugelrohr glass oven (B-585) used for drying the samples was from Büchi Labortechnik GmbH. Rice straw was procured directly from a local (Mohali, Punjab) agricultural farm. 1H/13C NMR spectra were recorded in CDCl3 at 400/100 MHz on a Bruker AvanceIII spectrometer. The chemical shifts have been reported in ppm using TMS as the internal standard.Typical procedure for the acetylation of cellulosics
Cellulose (or other cellulosics such as MCC/cotton linters, 1 g) was added to a mixture of: (i) method A: AcOH (10 mL), IPA (10 mL) and MeSA (1 mL) or (ii) method B: AcOH (20 mL) and MeSA (1 mL) or (iii) method C: AcOH (20 mL) and AcCl (5 mL) in a round bottom flask and the mixture was stirred overnight at 40–43 °C. During this period, all the cellulosic material taken initially would have gone into solution and the reaction mixture would have become viscous and jelly. The mixture was then poured in the form of a thin stream into a beaker containing crushed ice and sodium carbonate/sodium bicarbonate while being stirred. The mixture was allowed to stand in the refrigerator for enabling precipitation of the product to complete. The product was then separated at the pump by filtration and after washing with cold water until free of the salts, it was dried in the air followed by drying under vacuum in a Kugelrohr glass oven to constant weight.Typical procedure for the acetylation of rice straw
Coarsely pulverized rice straw (please see Fig. 4 above for a photomicrograph, 1 g) was added to mixture containing AcOH (10 mL), IPA (10 mL) and MeSA (1 mL) and was stirred for overnight at 40–43 °C. All of the rice straw taken had gone into solution by this time and the reaction mixture turned viscous and jelly/swollen. It was poured, while being stirred, on to crushed ice containing sodium carbonate/sodium bicarbonate in a beaker. The mixture was left in the refrigerator for the precipitation to complete. It was then separated by filtration at the pump and was dried under reduced pressure. The residual material thus obtained was then taken up in chloroform and was filtered through a fritted glass filter. The insolubles (containing lignin, hemicellulose, silica, etc.) on the filter were washed thoroughly with chloroform. The combined filtrate (clear and light yellowish brown-coloured) was then concentrated to dryness under a vacuum to obtain the rice straw-derived CTA as a transparent film (0.43 g, 62% based on an estimated cellulose content of 40% in rice straw).The reactions employing the other methods (AcOH–MeSA/AcOH–AcCl/Ac2O–MeSA) can also be performed as described above using the respective reagents as required in each case. For the details on the yields obtained, see Table 1.
Characterization of CTA
The data from FTIR and the 1H and 13C NMR spectral analyses of the synthesized cellulose derivatives were compared with literature data and were found to be in agreement. The powder XRD data (see below) obtained for CTA prepared from rice straw confirmed that the product was not only amorphous but also that it had become free of any silica by dissolution in chloroform and filtration. Elemental analysis of the product confirmed that sulfur was not present in the triacetate prepared.Conclusions
This investigation reports three highly efficient, practical and scalable, alternate methods for high yielding (60 to 70%) production of cellulose triacetate directly from farm crop spare biomass such as rice straw without involving the use of any restricted/toxic chemical ingredients in the process. A typical triacetate preparation implies approximately 30 to 35% of biomass consumption in acetylation. Since rice straw has about 40% cellulose, 60 to 87% acetylation efficiency is thus implied. This is far above the previous reports on rice tissues or their derived cellulose samples. Therefore from a bio refinery perspective, the methods offer advantages of yield and simplicity in the case of acetylation of cellulose (separated from hemi-cellulose and lignin by biomass fractionation) as an aid to integrated processing of biomass. The method uses a cheap and green organic acid catalyst and provides a proof of principle for a way forward to valorise a zero-use and problematic farm residues like rice straw.Acknowledgements
A. S. sincerely thanks CIAB, Mohali & the Department of Biotechnology (DBT), New Delhi for financial assistance and S. K. G. sincerely thanks NIPER, S. A. S. Nagar and the Ministry of Chemicals and Fertilizers, Govt. of India for permission to pursue Ph.D. under its staff development scheme.Notes and references
- Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- R. Rinaldi and F. Schuth, Energy Environ. Sci., 2009, 2, 610–626 CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- I. Cumpstey, ISRN Org. Chem., 2013, 1–27 CrossRef PubMed.
- D. G. Barkalow, R. M. Rowell and R. A. Young, J. Appl. Polym. Sci., 1989, 37, 1009–1018 CrossRef CAS.
- I. A. Wolff, D. W. Olds and G. E. Hilbert, J. Am. Chem. Soc., 1951, 73, 346–349 CrossRef CAS.
- S. Richardson and L. Gorton, Anal. Chim. Acta, 2003, 497, 27–65 CrossRef CAS.
- R. L. Shogren, Carbohydr. Polym., 2003, 52, 319–326 CrossRef CAS.
- K. J. Edgar, C. M. Buchanan, J. S. Debenham, P. A. Rundquist, B. D. Seiler, M. C. Shelton and D. Tindall, Prog. Polym. Sci., 2001, 26, 1605–1688 CrossRef CAS.
- L. J. Tanghe, L. B. Genung, and J. W. Mensch, in Methods in Carbohydrate Chemistry, ed. R. L. Whistler and M. L. Wolfrom, Academic Press, New York, NY, USA, 1963, vol. III, Cellulose, pp. 193–212 Search PubMed.
- T. F. Liebert and T. J. Heinze, Biomacromolecules, 2001, 2, 1124–1132 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- H. L. Ngo, K. LeCompte, L. Hargens and A. B. McEwen, Thermochim. Acta, 2000, 357, 97–102 CrossRef.
- J. Wu, J. Zhang, H. Zhang, J. He, Q. Ren and M. Guo, Biomacromolecules, 2004, 5, 266–268 CrossRef CAS PubMed.
- S. Barthel and T. Heinze, Green Chem., 2006, 8, 301–306 RSC.
- A. Schenzel, A. Hufendiek, C. Barner-Kowollik and M. A. R. Meier, Green Chem., 2014, 16, 3266–3271 RSC.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2005, 7, 705–707 RSC.
- K. P. R. Kartha and R. A. Field, Tetrahedron, 1997, 53, 11753–11766 CrossRef CAS.
- M. P. Paula, T. M. Lacerda and E. Frollini, eXPRESS Polym. Lett., 2008, 2, 423–428 CrossRef.
- A. Biswas, R. L. Shogren and J. L. Willett, Biomacromolecules, 2005, 6, 1843–1845 CrossRef CAS PubMed.
- J. Li, L. P. Zhang, F. Peng, J. Bian, T. Q. Yuan, F. Xu and R. C. Sun, Molecules, 2009, 14, 3551–3566 CrossRef CAS PubMed.
- A. Biswas, B. C. Saha, J. W. Lawton, R. L. Shogren and J. L. Willett, Carbohydr. Polym., 2006, 64, 134–137 CrossRef CAS.
- S. K. Giri and K. P. R. Kartha, RSC Adv., 2015, 5, 11687–11696 RSC.
- D. C. Hull, O. Ridge and A. H. Agett, US Pat., 2 422 016, 1947.
- H. J. Hagemeyer and D. C. Hull, Ind. Eng. Chem., 1949, 41, 2920–2924 CrossRef CAS.
- E. A. Jeffery and D. P. N. Satchell, J. Chem. Soc., 1962, 1876–1887 RSC.
- E. A. Jeffery and D. P. N Satchell, J. Chem. Soc., 1962, 1887–1894 RSC.
- D. P. N. Satchell, J. Chem. Soc., 1962, 1894–1898 RSC.
- D. P. N. Satchell, J. Chem. Soc., 1962, 1899–1905 RSC.
- E. A. Jeffery and D. P. N. Satchell, J. Chem. Soc., 1962, 1913–1917 RSC.
- R. Pelagalli, I. Chiarotto, M. Feroci and S. Vecchio, Green Chem., 2012, 14, 2251–2255 RSC.
- C. E. Bronnimann, R. C. Zeigler and G. E. Maciel, J. Am. Chem. Soc., 1998, 110, 2023–2026 CrossRef.
- H. E. L. Rassy, P. Buisson, B. Bouali, A. Perrard and A. C. Pierre, Langmuir, 2003, 19, 358–363 CrossRef.
- H. E. l. Rassy and A. C. Pierre, J. Non-Cryst. Solids, 2005, 351, 1603–1610 CrossRef.
- X. Zhang, W. Zhang, D. Tian, Z. Zhou and C. Lu, RSC Adv., 2013, 3, 7722–7725 RSC.
- R. Kakuchi, M. Yamaguchi, T. Endo, Y. Shibata, K. Ninomiya, T. Ikai, K. Maeda and K. Takahashi, RSC Adv., 2015, 5, 72071–72074 RSC.
- T. Mukherjee, M. Sani, N. Kao, R. K. Gupta, N. Quazi and S. Bhattacharya, Chem. Eng. Sci., 2013, 101, 655–662 CrossRef CAS.
- F. Hu, N. Lin, P. R. Chang and J. Huang, Carbohydr. Polym., 2015, 129, 208–215 CrossRef CAS PubMed.
- S. A. Ganie, A. Ali and N. Mazumdar, Carbohydr. Polym., 2015, 129, 224–231 CrossRef CAS PubMed.
- L. J. Konwar, P. Mäki-Arvela, A. J. Thakur, N. Kumar and J. Mikkola, RSC Adv., 2016, 6, 8829–8837 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00078b |
| ‡ Amita Sharma and Santosh Kumar Giri are co-first co-authors who contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |