Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
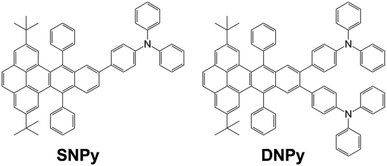
Synthesis and physical properties of triphenylamine-functionalized twistacenes: blue-emitting fluorophores†
Jingdan Duana,
Huahang Pana,
Changting Weib,
Jinchong Xiao *a,
Guixia Zhaia and
Wenming Su*b
*a,
Guixia Zhaia and
Wenming Su*b
aCollege of Chemistry and Environmental Science, Key Laboratory of Chemical Biology of Hebei Province, Hebei University, Baoding 071002, P. R. China. E-mail: jcxiaoicas@163.com
bPrintable Electronics Research Centre, Suzhou Institute of Nanotech and Nano-bionics, Chinese Academy of Sciences, Suzhou 215123, P. R. China. E-mail: wmsu2008@sinano.ac.cn
First published on 8th February 2017
Abstract
Two novel triphenylamine-decorated twistacenes SNPy and DNPy have been synthesized and characterized. Both of them display bright blue luminescence with high quantum yields in dichloromethane and as thin films. Based on the TGA analyses, they present high thermal stability up to 385 °C and 450 °C, respectively. The fabricated electroluminescent devices using the resulting twistacenes as emitters show blue luminescence of 2856 cd m−2 for SNPy and 4116 cd m−2 for DNPy.
Introduction
As one of the most appealing branches of organic electronics, organic light emitting diodes (OLEDs) are a subject of considerable investigation in recent decades due to their potential applications in full color flat panel displays and solid state lighting.1–5 Compared with their inorganic counterparts, organic π-conjugated compounds display many advantages. In general, these organic molecules consist of many kinds of interesting molecular structures, which is beneficial for rationally and precisely tuning their physical properties.6 In some cases, this can provide more room to approach high electroluminescent performance in molecular level and supramolecular level.7–12 In addition, detailing intensively the optoelectronic behaviors can uncover some new phenomena. For example, the recent reported thermally activated delayed fluorescence (TADF) has received much attention because the exceptional high theoretical internal quantum efficiency of 100% might be fully used in OLEDs.13,14 By using an aggregation-induced emission (AIE) concept, highly fluorescent materials could be prepared through modifying the AIE moiety with other functional groups, where the as-formed novel molecules were utilized as active layers in nondoped OLEDs and fluorescent sensors for biomolecules with high specificity.15–18 Thus, the preparation of efficient luminescent materials has received a growing interest in scientific and technological opinions at present. Indeed, since stable blue emitters play a crucial role in next-generation displays, how to design and synthesize these prototypes is still an urgent issue and a challenge.Twistacenes, as a family of polycyclic aromatic hydrocarbons, usually refer to sterically encumbered acenes, which can be tracked back to last century.19–22 In 1987, Pascal and co-workers synthesized several linear polycyclic aromatic hydrocarbons showing exceptional nonplanar deformations.23 These molecules were subsequently named as twisted acenes that exhibited highly stable and antioxidation than unsubstituted acenes. Wudl, Zhang and Pérez groups prepared in succession a series of symmetric and unsymmetric twistacenes containing pyrene unit in the terminal.24–31 More interesting, the resulting compounds can suppress the stacking interaction in the solid state to a certain extent, which were utilized as emitters in electroluminescent devices. Recently, in our group, some substituted twistacenes consisting of heterocycles and five-membered all-carbon rings replacing six-membered rings were designed and synthesized that displayed appealing electroluminescent performance and nonlinear optical properties.32–40
In the continuous work, herein we have synthesized two novel triphenylamine-modified twistacenes SNPy and DNPy (Scheme 1). Triphenylamine presenting three-dimensional architecture could be decorated with different π-conjugated systems. The as-resulting compounds showed isotropic optical and charge-transport properties and could be used in electroluminescent materials, organic field effect transistor and photovoltaic conversion.41–44 As expected, the motif in the molecular design might improve the hole transporting behavior, decrease the stacking interaction and fluorescence quenching to some extent. Molecules SNPy and DNPy emitted strong blue light in dichloromethane and solid state. The fabricated electronic devices based on them exhibited fascinating electroluminescent performance.
Results and discussion
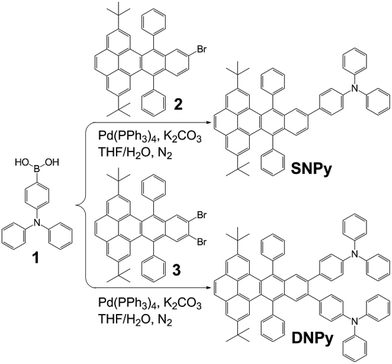
The synthetic procedure of SNPy and DNPy is depicted in Scheme 2. Molecules 2 and 3 were obtained according to the reported methods in our laboratory.32,34 Compounds SNPy (91%) and DNPy (86%) were successfully synthesized by a straightforward Suzuki coupling reactions between 4-(diphenylamino)phenylboronic acid (1) and 2/3 with Pd(PPh3)4 as catalyst. The formed molecules were characterized by melting points, FT-IR, 1H NMR, 13C NMR spectroscopy and MALDI-TOF mass spectroscopy. In addition, both of them can be dissolved in the common solvents such as dichloromethane, chloroform, toluene and THF. Thermogravimetric analysis (TGA) guaranteed their thermal stability (5% weight loss) to 385 °C for SNPy and 450 °C for DNPy, respectively, in nitrogen atmosphere (Fig. S1†).The optical properties of compounds SNPy and DNPy were investigated in dilute dichloromethane solution (1 × 10−5 M) and in thin films. As shown in Fig. 1a and Table 1, molecules SNPy and DNPy in dichloromethane presented similar profile with broad featureless absorption bands. The maximum absorption peaks at 357 nm originated from the π–π* transitions of the skeleton. Note that SNPy exhibited a shoulder peak at 400 nm and DNPy presented the shoulder peak at 390 nm. When excited at 357 nm, both of them emitted strong blue light centered at 479 nm with the quantum yields (Φf) of 0.66 for SNPy and 0.36 for DNPy, respectively, using 9,10-diphenylanthracene (Φf = 0.95) as a standard.32 Additionally, the optical energy gaps (Eg) calculated from the absorption edges in solution state are almost the same, which are 2.94 and 2.87 eV, respectively. The spectra of SNPy and DNPy in solid state also showed similar broad absorption, which might be assigned to the interaction between single molecules in the densely packed film. Their corresponding emission spectra exhibited negligible changes compared with those in dichloromethane. It should be pointed out that the full width at half maxima of SNPy/DNPy in thin films are smaller than those in dichloromethane, inferring that such twisted structure was beneficial for suppressing the intermolecular interaction in large part.
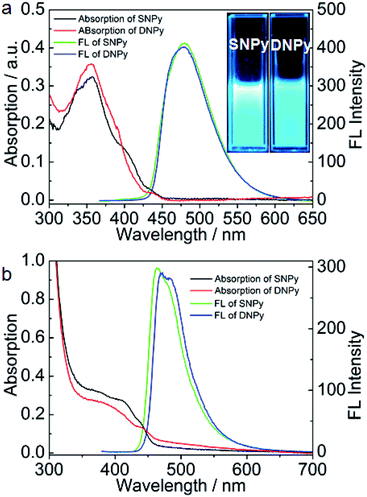 | ||
| Fig. 1 UV-Vis absorption and fluorescence spectra of SNPy and DNPy in dichloromethane (a) and in thin film (b). Inset: the fluorescence image. | ||
The electrochemical behaviors of compounds SNPy and DNPy were studied in a three-electrode electrochemical cell with tetrabutyl ammonium hexafluorophosphate (Bu4NPF6, 0.1 M) as electrode and the results are shown in Fig. 2 and Table 1. Note that the potentials are corrected against Fc/Fc+. Compounds SNPy and DNPy exhibit two reversible oxidation potentials (Eoxonset) at 0.46 V/0.83 V for SNPy and 0.45 V/0.90 V for DNPy, respectively. These data mean that the first oxidation process results from the oxidation of amine units and the second belongs to the oxidation of the parent building blocks, which compared with the reported results.32,34–36 Notably, molecule DNPy displays slightly negatively oxidation waves compared to SNPy, being indicative of the stronger electron-donating ability. Based on the first oxidation potentials, the highest occupied molecular orbital (HOMO) energy levels were estimated to be −5.26 eV for SNPy and −5.25 eV for DNPy, respectively. Furthermore, the lowest-unoccupied molecular orbital (LUMO) energy levels of SNPy and DNPy were calculated to be −2.32 and −2.38 eV, respectively, from the HOMO levels and Eg values in solution. Accordingly, both SNPy and DNPy could be expected to be suitable candidates for blue emitters.
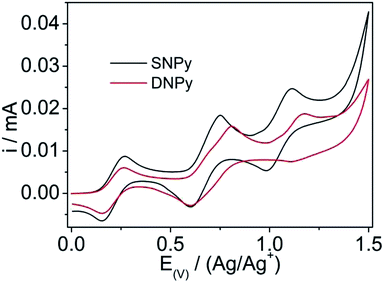 | ||
| Fig. 2 Cyclic voltammogram of SNPy and DNPy in anhydrous dichloromethane containing tetrabutylammonium hexafluorophosphate (TBAPF6). Scan rate: 50 mV s−1. | ||
The organic light-emitting diodes (OLED) were fabricated to examine the electroluminescence performance for compounds SNPy and DNPy. The devices contain the following structures: ITO/TAPC (20 nm)/CBP: emitter (30 nm, x wt%)/TPBi (50 nm)/Liq (2 nm)/Al, where TAPC = 4,4′-cyclohexylidenebis[N,N-di(p-tolyl)aniline], CBP = 4,4′-di(9H-carbazol-9-yl)-1,1′-biphenyl, TPBi = 1,3,5-tris(2-N-phenylbenzimidazolyl)benzene. All the related chemical structures, device configurations, and the energy level diagrams are shown in Fig. 3. The concentrations of compounds SNPy/DNPy were tuned from 9% to 12%. Further increase of the doping concentration was helpless to enhance the electroluminescent performance.
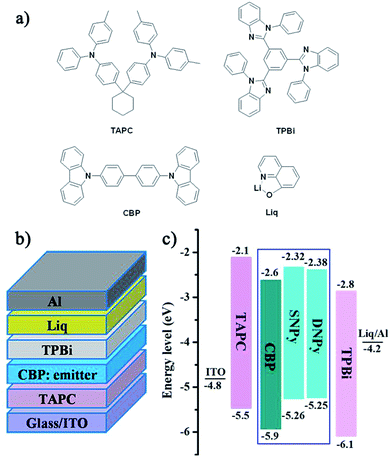 | ||
| Fig. 3 (a) Chemical structures of the related materials, (b) schematic configurations and, (c) energy-level diagram. | ||
Current density–voltage–luminance (J–V–L), current efficiency–luminance–power efficiency (CE–L–PE) and electroluminance spectra of the resulting devices are shown in Fig. 4. Device SNPy turned on the low voltages (1.0 cd m−2) at 5.80 V for 9% and 6.51 V for 12%, respectively, which was smaller than those of device DNPy (the low voltages at 6.70 V for 9% and 7.23 V for 12%). All of the devices emitted strong blue light with the maximum brightness of 2856 cd m−2 at 14.6 V for SNPy (9%) and 4116 cd m−2 at 16.4 V for DNPy (9%). These data gained the advantage over that of 11,12-difluoro-9,14-diphenyl-dibenzo[de,qr]tetracene reported in our group.35 However, when the doping concentrations were 12%, the brightness reduced to 2414 cd m−2 at 14.6 V for SNPy and was similar to that of DNPy at 16.4 V. The current density of SNPy is slightly higher than that of device DNPy, being indicative of a better charge-transport ability. Interestingly, the current efficiencies exhibited a negligible change (2.57 cd A−1 for SNPy and 3.05 cd A−1 for DNPy) as the dye concentration increased, inferring no obvious concentration quenching effect. Devices SNPy and DNPy displayed the power efficiency of 0.90 lm W−1 and 0.99 lm W−1 at 100 cd m−2, respectively. In addition, the electroluminescent peaks of SNPy and DNPy were at 460 nm, which was obviously hypsochromic shift compared with those in solution and in thin film as presented in Fig. 3e and f. Considering the fluorescence quantum yields of SNPy and DNPy, device DNPy exhibited good hole/electron charge carrier balance. On the basis of all the experimental analyses, the doping concentration of 9% presented optimal electroluminescent performance.
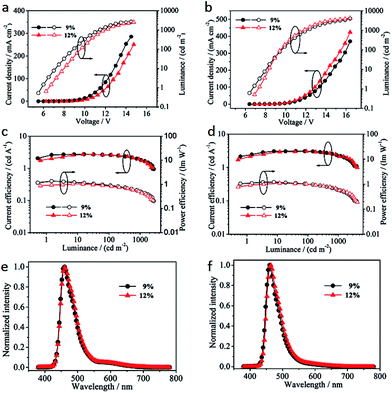 | ||
| Fig. 4 J–V–L of device SNPy (a) and device DNPy (b). CE–L–PE of device SNPy (c) and device DNPy (d). EL spectra for device SNPy (e) and device DNPy (f). | ||
Conclusions
In summary, two novel triphenylamine-modified twistacenes SNPy and DNPy were synthesized through one-step concise Suzuki coupling reaction. The effect on their optoelectronic and thermal properties have been carefully examined in a comparative manner. The electroluminescent devices using the formed twistacenes as functional layers emitted blue light with a perfect luminance. This study also demonstrates that functionalized twistacenes can be employed as an active candidates in OLED devices.Experimental section
Device fabrication and measurements
Prior to use, the indium tin oxide (ITO) glass substrates were thoroughly cleaned with solvent and treated with oxygen plasma for 3 min. On the top of the substrates, the other layers were deposited in succession by the vacuum thermal evaporation. All of the organic materials and metal anode were deposited layer by layer under a vacuum of 5 × 10−4 Pa. The related organic materials were used as purchased from commercial companies. The EL performances of the devices were measured with a Spectra Scan PR655 and a computer controlled Keithley 2400 Sourcemeter. The active device area was 4 mm2, and only the luminance in the forward direction was measured. The fabricated devices were performed under ambient conditions without further encapsulation.Synthesis route
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mL) at 85 °C under nitrogen for 48 h. The cooled mixture was extracted with dichloromethane and then the collected organic phase was washed with brine, dried over Na2SO4. After removing the solvent, the formed residue was purified by column chromatography (silica gel, petroleum ether) to give compound SNPy as a yellow solid (736 mg, 91%). Mp: 312.0–312.7 °C. FT-IR (KBr): 3051, 2954, 2866, 1585, 1487, 1282, 696 cm−1. 1H NMR (600 MHz, CDCl3, 298 K): δ = 8.18 (d, J = 7.2 Hz, 2H), 8.08 (s, 1H), 7.92 (d, J = 9.0 Hz, 1H), 7.86 (d, 4H), 7.72 (d, J = 9.0 Hz, 1H), 7.66 (t, 4H), 7.59–7.56 (m, 4H), 7.54 (d, J = 8.4 Hz, 2H), 7.50–7.46 (m, 2H), 7.30 (t, 4H), 7.16–7.14 (m, 6H), 7.07 (t, 2H), 1.13 (s, 18H). 13C NMR (150 MHz, CDCl3, 298 K): δ = 147.7, 147.3, 147.26, 147.23, 142.7, 137.2, 136.3, 136.0, 134.7, 132.5, 132.4, 131.1, 130.33, 130.32, 130.1, 129.7, 129.6, 129.5, 129.3, 127.9, 127.7, 127.6, 127.56, 127.5, 127.4, 126.9, 124.9, 124.6, 123.9, 123.88, 123.8, 123.7, 123.1, 122.2, 122.17, 34.8, 31.4. MS (MALDI-TOF): calc. for C62H51N: [m/z] 809.4, found: [m/z] 809.0.
4 mL) at 85 °C under nitrogen for 48 h. The cooled mixture was extracted with dichloromethane and then the collected organic phase was washed with brine, dried over Na2SO4. After removing the solvent, the formed residue was purified by column chromatography (silica gel, petroleum ether) to give compound SNPy as a yellow solid (736 mg, 91%). Mp: 312.0–312.7 °C. FT-IR (KBr): 3051, 2954, 2866, 1585, 1487, 1282, 696 cm−1. 1H NMR (600 MHz, CDCl3, 298 K): δ = 8.18 (d, J = 7.2 Hz, 2H), 8.08 (s, 1H), 7.92 (d, J = 9.0 Hz, 1H), 7.86 (d, 4H), 7.72 (d, J = 9.0 Hz, 1H), 7.66 (t, 4H), 7.59–7.56 (m, 4H), 7.54 (d, J = 8.4 Hz, 2H), 7.50–7.46 (m, 2H), 7.30 (t, 4H), 7.16–7.14 (m, 6H), 7.07 (t, 2H), 1.13 (s, 18H). 13C NMR (150 MHz, CDCl3, 298 K): δ = 147.7, 147.3, 147.26, 147.23, 142.7, 137.2, 136.3, 136.0, 134.7, 132.5, 132.4, 131.1, 130.33, 130.32, 130.1, 129.7, 129.6, 129.5, 129.3, 127.9, 127.7, 127.6, 127.56, 127.5, 127.4, 126.9, 124.9, 124.6, 123.9, 123.88, 123.8, 123.7, 123.1, 122.2, 122.17, 34.8, 31.4. MS (MALDI-TOF): calc. for C62H51N: [m/z] 809.4, found: [m/z] 809.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mL) at 85 °C under nitrogen for 48 h. After cooling to room temperature, brine was added to the mixture. The formed solution was extracted with dichloromethane. The collected organic phase was dried over Na2SO4. After removing the solvent, the residue was purified by column chromatography (silica gel, petroleum ether) to give compound DNPy as a yellow solid (450 mg, 86%). Mp: 206.0–206.9 °C. FT-IR (KBr): 3034, 2959, 2867, 1593, 1493, 1275, 806, 697 cm−1. 1H NMR (600 MHz, CDCl3, 298 K): δ = 8.14 (s, 2H), 7.93 (s, 2H), 7.85 (d, J = 5.4 Hz, 4H), 7.69 (d, J = 7.2 Hz, 4H), 7.57 (t, 1J = 7.8 Hz, 2J = 7.2 Hz, 4H), 7.46 (t, J = 7.2 Hz, 2H), 7.25 (t, 1J = 7.8 Hz, 2J = 7.2 Hz, 8H), 7.10 (d, J = 7.8 Hz, 12H), 7.02 (T, J = 7.2 Hz, 4H), 6.96 (d, J = 8.4 Hz, 4H), 1.13 (s, 18H). 13C NMR (150 MHz, CDCl3, 298 K): δ = 147.7, 147.3, 146.3, 142.6, 138.4, 135.9, 135.8, 132.5, 131.3, 130.8, 130.3, 130.1, 129.7, 129.3, 129.27, 127.8, 127.6, 126.9, 124.4, 123.9, 122.9, 122.8, 122.2, 34.8, 31.4. MS (MALDI-TOF): calc. for C80H64N2: [m/z] 1052.5, found: [m/z] 1051.9.
4 mL) at 85 °C under nitrogen for 48 h. After cooling to room temperature, brine was added to the mixture. The formed solution was extracted with dichloromethane. The collected organic phase was dried over Na2SO4. After removing the solvent, the residue was purified by column chromatography (silica gel, petroleum ether) to give compound DNPy as a yellow solid (450 mg, 86%). Mp: 206.0–206.9 °C. FT-IR (KBr): 3034, 2959, 2867, 1593, 1493, 1275, 806, 697 cm−1. 1H NMR (600 MHz, CDCl3, 298 K): δ = 8.14 (s, 2H), 7.93 (s, 2H), 7.85 (d, J = 5.4 Hz, 4H), 7.69 (d, J = 7.2 Hz, 4H), 7.57 (t, 1J = 7.8 Hz, 2J = 7.2 Hz, 4H), 7.46 (t, J = 7.2 Hz, 2H), 7.25 (t, 1J = 7.8 Hz, 2J = 7.2 Hz, 8H), 7.10 (d, J = 7.8 Hz, 12H), 7.02 (T, J = 7.2 Hz, 4H), 6.96 (d, J = 8.4 Hz, 4H), 1.13 (s, 18H). 13C NMR (150 MHz, CDCl3, 298 K): δ = 147.7, 147.3, 146.3, 142.6, 138.4, 135.9, 135.8, 132.5, 131.3, 130.8, 130.3, 130.1, 129.7, 129.3, 129.27, 127.8, 127.6, 126.9, 124.4, 123.9, 122.9, 122.8, 122.2, 34.8, 31.4. MS (MALDI-TOF): calc. for C80H64N2: [m/z] 1052.5, found: [m/z] 1051.9.Acknowledgements
J. C. Acknowledges the financial support from the National Natural Science Foundation of China (21102031, 21442010 and 21672051), the Natural Science Foundation of Hebei Province for Distinguished Young Scholar (Cultivation Project, B2015201183), the Natural Science Foundation of Hebei Province (B2014201007), and the Natural Science Foundation of Hebei University (2015JQY02).Notes and references
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature, 1990, 347, 539–541 CrossRef CAS.
- C. W. Tang and S. A. Vanslyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- J. Kido, M. Kimura and K. Nagai, Science, 1995, 267, 1322–1324 CrossRef PubMed.
- M. Romain, S. Thiery, A. Shirinskaya, C. Declairieux, D. Tondelier, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, Angew. Chem., Int. Ed., 2015, 54, 1176–1180 CrossRef CAS PubMed.
- M. Shimizu and T. Hiyama, Chem.–Asian J., 2010, 5, 1516–1531 CrossRef CAS PubMed.
- M. Shimizu, Y. Takeda, M. Higashi and T. Hiyama, Angew. Chem., Int. Ed., 2009, 48, 3653–3656 CrossRef CAS PubMed.
- Z. Zhao, S. Chen, C. Y. K. Chan, J. W. Y. Lam, C. K. W. Jim, P. Lu, Z. Chang, H. S. Kwok, H. Qiu and B. Tang, Chem.–Asian J., 2012, 7, 484–488 CrossRef CAS PubMed.
- U. H. F. Bunz, J. U. Engelhart, B. D. Lindner and M. Schaffroth, Angew. Chem., Int. Ed., 2013, 52, 3810–3821 CrossRef CAS PubMed.
- J. T. Markiewicz and F. Wudl, ACS Appl. Mater. Interfaces, 2015, 7, 28063–28085 CAS.
- W. Jiang, Y. Li and Z. Wang, Chem. Soc. Rev., 2013, 42, 6113–6127 RSC.
- J. Xiao, B. Yang, J. I. Wong, Y. Liu, F. Wei, K. Tan, X. Teng, Y. Wu, L. Huang, C. Kloc, F. Boey, J. Ma, H. Zhang, H. Yang and Q. Zhang, Org. Lett., 2011, 13, 3004–3007 CrossRef CAS PubMed.
- J. Xiao, H. Yang, Z. Yin, J. Guo, F. Boey, H. Zhang and Q. Zhang, J. Mater. Chem., 2011, 21, 1423–1427 RSC.
- S. Hirata, Y. Sakai, K. Masui, H. Tanaka, S. Y. Lee, H. Nomura, N. Nakamura, M. Yasumatsu, H. Nakanotani, Q. Zhang, K. Shizu, H. Miyazaki and C. Adachi, Nat. Mater., 2015, 14, 330–336 CrossRef CAS PubMed.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS.
- X. Zhan, Z. Wu, Y. Lin, Y. Xie, Q. Peng, Q. Li, D. Ma and Z. Li, Chem. Sci., 2016, 7, 4355–4363 RSC.
- L. Chen, G. Lin, H. Peng, S. Ding, W. Luo, R. Hu, S. Chen, F. Huang, A. Qin, Z. Zhao and B. Tang, Mater. Chem. Front., 2017, 1, 176–180 RSC.
- D. Ding, K. Li, B. Liu and B. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- Y. Liu, C. Deng, L. Tang, A. Qin, R. Hu, J. Sun and B. Tang, J. Am. Chem. Soc., 2011, 133, 660–663 CrossRef CAS PubMed.
- R. A. Pascal Jr, Chem. Rev., 2006, 106, 4809–4819 CrossRef PubMed.
- J. Lu, D. M. Ho, N. J. Vogelaar, C. M. Kraml, S. Bernhard, N. Byrne, L. R. Kim and R. A. Pascal Jr, J. Am. Chem. Soc., 2006, 128, 17043–17050 CrossRef CAS PubMed.
- J. Lu, D. M. Ho, N. J. Vogelaar, C. M. Kraml and R. A. Pascal Jr, J. Am. Chem. Soc., 2004, 126, 11168–11169 CrossRef CAS PubMed.
- X. Qiao, D. M. Ho and R. A. Pascal Jr, Angew. Chem., Int. Ed., 1997, 36, 1531–1532 CrossRef CAS.
- R. A. Pascal Jr, W. D. McMillan, D. V. Engen and R. G. Eason, J. Am. Chem. Soc., 1987, 109, 4660–4665 CrossRef.
- J. Xiao, H. M. Duong, Y. Liu, W. Shi, L. Ji, G. Li, S. Li, X. Liu, J. Ma, F. Wudl and Q. Zhang, Angew. Chem., Int. Ed., 2012, 51, 6094–6098 CrossRef CAS PubMed.
- J. Xiao, C. D. Malliakas, Y. Liu, F. Zhou, G. Li, H. Su, M. G. Kanatzidis, F. Wudl and Q. Zhang, Chem.–Asian J., 2012, 7, 672–675 CrossRef CAS PubMed.
- J. Xiao, S. Liu, Y. Liu, L. Ji, X. Liu, H. Zhang, X. Sun and Q. Zhang, Chem.–Asian J., 2012, 7, 561–564 CrossRef CAS PubMed.
- J. Xiao, Y. Divayana, Q. Zhang, H. M. Doung, H. Zhang, F. Boey, X. Sun and F. Wudl, J. Mater. Chem., 2010, 20, 8167–8170 RSC.
- H. M. Duong, M. Bendikov, D. Steiger, Q. Zhang, G. Sonmez, J. Yamada and F. Wudl, Org. Lett., 2003, 5, 4433–4436 CrossRef CAS PubMed.
- Q. Xu, H. M. Duong, F. Wudl and Y. Yang, Appl. Phys. Lett., 2004, 85, 3357–3359 CrossRef CAS.
- J. Li, Y. Zhao, J. Lu, G. Li, J. Zhang, Y. Zhao, X. Sun and Q. Zhang, J. Org. Chem., 2015, 80, 109–113 CrossRef CAS PubMed.
- D. Rodríguez-Lojo, D. Pérez, D. Pena and E. Guitián, Chem. Commun., 2015, 51, 5418–5420 RSC.
- Z. Liu, J. Xiao, Q. Fu, H. Feng, X. Zhang, T. Ren, S. Wang, D. Ma, X. Wang and H. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 11136–11141 CAS.
- X. Zhang, H. Song, J. Xiao, T. Ren, S. Wang, Z. Liu, X. Ba and Y. Wu, Aust. J. Chem., 2015, 68, 505–512 CrossRef CAS.
- B. Lv, X. Shen, J. Xiao, J. Duan, X. Wang and Y. Yi, Chem.–Asian J., 2015, 10, 2677–2682 CrossRef CAS PubMed.
- J. Xiao, Z. Liu, X. Zhang, W. Wu, T. Ren, B. Lv, L. Jiang, X. Wang, H. Chen, W. Su and J. Zhao, Dyes Pigm., 2015, 112, 176–182 CrossRef CAS.
- Z. Liu, W. Wang, W. Xu, H. Chen, X. Zhang, T. Ren, X. Wang, J. Zhao and J. Xiao, Dyes Pigm., 2015, 115, 143–148 CrossRef CAS.
- X. Zhang, S. Li, Z. Liu, S. Wang and J. Xiao, NPG Asia Mater., 2015, 7, e230 CrossRef CAS.
- S. Chen, J. Xiao, X. Zhang, X. Shen, X. Liu, F. Shen, Y. Yi and Y. Song, Dyes Pigm., 2016, 134, 9–18 CrossRef CAS.
- B. Lv, J. Xiao, J. Zhou, X. Zhang, J. Duan, W. Su and J. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 18998–19003 CAS.
- X. Wu, J. Xiao, R. Sun, T. Jin, J. Yang, G. Shi, Y. Wang, X. Zhang and Y. Song, Adv. Opt. Mater., 2016 DOI:10.1002/adom.201600712.
- J. Wang, K. Liu, L. Ma and X. Zhan, Chem. Rev., 2016, 116, 14675–14725 CrossRef CAS PubMed.
- Y. Shirota, J. Mater. Chem., 2005, 15, 75–93 RSC.
- M. Sonntag, K. Kreger, D. Hanft and P. Strohiegl, Chem. Mater., 2005, 17, 3031–3039 CrossRef CAS.
- S. Roquet, A. Cravino, P. Leriche, O. Alévêque, P. Frère and J. Roncali, J. Am. Chem. Soc., 2006, 128, 3459–3466 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TGA data, additional copies of FT-IR, 1H, 13C NMR spectra and mass spectroscopy. See DOI: 10.1039/c6ra28814f |
| This journal is © The Royal Society of Chemistry 2017 |