Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel columnar metallomesogens based on cationic platinum(II) complexes without long peripheral chains†
Hao Geng‡
,
KaiJun Luo‡*,
HaoMin Cheng‡,
ShiLin Zhang‡,
HaiLiang Ni‡,
HaiFeng Wang‡,
WenHao Yu‡* and
Quan Li‡*
College of Chemistry and Materials Science, Sichuan Normal University, Sichuan Province, 5 Jingan Road, Jinjiang District, Chengdu, 610066, P. R. China. E-mail: Luo-k-j007@163.com; yuwenhao2010@163.com; liquan6688@163.com
First published on 14th February 2017
Abstract
Phosphorescent cationic platinum(II) complexes were obtained and among them the complexes without any peripheral flexible chains around the platinum(II) center show thermotropic columnar liquid crystal properties.
Recently, emissive metal-containing liquid crystalline complexes (metallomesogens) have caused great interest due to their many potential applications, such as in molecular electronics,1,2 high charge-carrier mobilities3 and polarized luminescence.4 Among the emissive metallomesogens, cyclometalated platinum(II) complexes have become one of the most attractive research fields because of their room temperature phosphorescence, high emission efficiency and square planar geometry which fits for mesomorphism design.5 In general, most metallomesogens always are composed of a metal-centered rigid core and multiple flexible chains around the core. The type of their mesomorphic phase is strongly related to the number, the length and the position of attached flexible chains. The metallomesogens with two flexible chains display smectic phases or nematic phases,6 and with more soft chains it is easier to form columnar phases.7,8 However, there were only a few examples of thermotropic columnar mesogens with non-conventional molecular structures in which liquid crystal molecules are in the absence of peripheral chains.9 Very recently, Swager10 reported a new class of cationic square planar platinum(II) complexes, which were composed of 2-phenylpyridine and 1,4-disubstituted-1H-1,2,3-triazole. By introducing one flexible chain at the triazole, these complexes showed liquid crystalline characteristics with hexagonal columnar phases, which are assigned to strong intermolecular Pt⋯Pt interactions and intensive dispersive forces from the coordination planar twists. Furthermore, these cationic platinum(II) complexes showed mechanochromic properties.
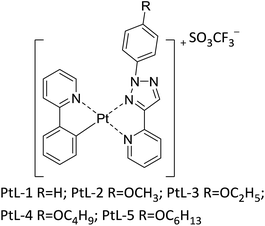
Herein, we report a new type of cationic platinum(II) complexes metallomesogens, for which 2-phenylpyridine acts as cyclometalated ligand and 2-(1-(4-substituted phenyl)-1H-1,2,3-triazole-4-yl) pyridine as ancillary ligand. Unlike the traditional metallomesogens, which generally attached multiple long flexible chains on the ligands, in order to facilitate the formation of liquid crystalline, the complexes only with phenyl or 4-methoxyphenyl connected in 1H-1,2,3-triazole moiety showed thermotropic hexagonal columnar liquid crystalline phases. Although the presented platinum(II) complexes have similar coordination framework to those reported by Swager, this is very rare that the columnar liquid crystal can be formed for these complexes lacking long flexible chains. In addition, all complexes are luminescent in solution at room temperature and at 77 K and the solid state. The cationic platinum(II) complexes were obtained from the platinum(II) μ-chloro-bridged dimers and 1,4-disubstituted-1H-1,2,3-triazole (Fig. 1 and S1†). The platinum(II) μ-chloro-bridged dimers were prepared according to a literature procedure11 and 1,4-disubstituted-1H-1,2,3-triazole were easily obtained by “click chemistry”.12 In synthesis of cationic platinum(II) complexes silver trifluoromethanesulfonate (AgSO3CF3) can accelerate reaction process and SO3CF3− anion serves as counteranion. The cationic platinum(II) complexes should exist two isomers (cis and trans form).10 The 1H NMR revealed that complexes PtL-5 is of a single configuration (trans form), while the complexes PtL-1, PtL-2, PtL-3 and PtL-4 are obtained as a mixture of cis and trans isomers.
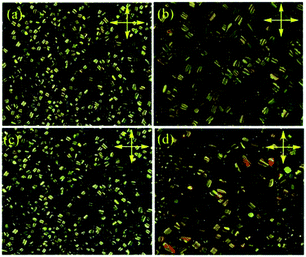
The mesomorphic properties of the complexes were preliminarily investigated by polarized optical microscopy (POM) and differential scanning calorimetry (DSC). Unexpectedly, the complexes with longer side chain, exceeded two alkyl group, melted directly to the isotropic fluid at 278, 244 and 247 °C and no liquid crystalline textures were observed on succeeding cooling process. In contrast, the complexes without side chain (PtL-1) or with only a simple 4-methoxyphenyl connected in 1H-1,2,3-triazole moiety (PtL-2) showed the liquid crystallinity. For the complexes PtL-1 and PtL-2, the columnar textures were observed at 310 and 260 °C by POM on slow cooling from isotropic phase, respectively, and held up to room temperature, suggesting that PtL-1 and PtL-2 can form glass columnar phases (Fig. 2 and S2†). DSC curves were carried out to find transition temperature (Fig. S4†) and data were summarized in Table 1. For the metallomesogens PtL-1, PtL-2, no phase transition was observed when samples cooled from isotropic phase. Second scan profiles showed that PtL-1, PtL-2 are capable of vitrification, glass transition was at 96 °C for PtL-1 and at 101 °C for PtL-2. With further heating, columnar phase melted into isotropic phase at 284 °C for PtL-1 and at 266 °C for PtL-2, respectively. We note that the complex PtL-1 and PtL-2 degraded somewhat in repeated heating and cooling process. While TGA profiles under nitrogen atmosphere showed decomposition temperature (defined as the onset temperature at 5% weight loss) is 330 °C, 320 °C for PtL-1, PtL-2, respectively (Fig. S3†), the holding time TGA analysis indicated 12% weight loss at 300 °C for PtL-1 and 10% weight loss at 260 °C for PtL-2, respectively, when samples were held 10 minutes (Fig. S3†).
| Complexes | Second heating/°C (ΔH [kJ mol−1]) | First cooling/°C (ΔH [kJ mol−1]) |
|---|---|---|
| a Cr, Cr′ and Cr′′: crystal; Colh: hexagonal columnar mesophase; Iso: isotropic phase; G: glass columnar phase. The scan rate is 10 °C min−1 during all cooling and heating process under nitrogen protection. | ||
| PtL-1 | G 96 (0.13) Colh 284 (8.69) Iso | Iso to Colh and G |
| PtL-2 | G 101 (0.18) Colh 266 | Iso to Colh and G |
| PtL-3 | Cr 67 (1.55) Cr′ 278 (16.73) Iso | Iso to Cr |
| PtL-4 | Cr 244 (7.62) Iso | Iso to Cr |
| PtL-5 | Cr 109 (0.94) Cr′ 234 (4.35) Cr′′ 247 (7.64) Iso | Iso to Cr |
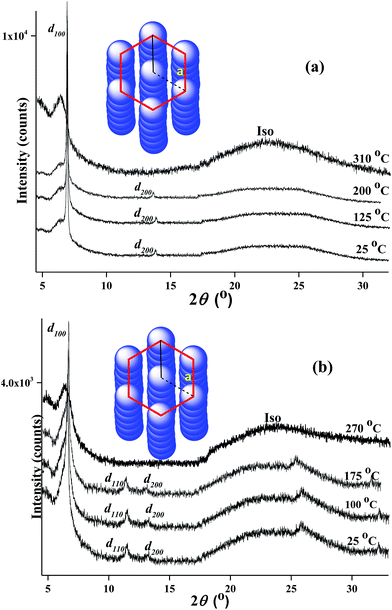
To further illustrate the phase structures clearly, the variable temperature wide angle X-ray diffraction (WAXD) experiments were carried out. The sample was prepared by preheating to beyond clear point and then quenching to room temperature in order to avoid thermal decomposition of samples at high temperature during the period of X-ray diffraction measure and eliminate thermal history.13 Powder WAXD patterns of PtL-1 and PtL-2 at different temperature are shown in Fig. 3. WAXD patterns supported the results obtained from POM observation. The complexes PtL-1 and PtL-2 showed a series of sharp diffraction peaks in the small angle range and diffuse and board scattering halo at wide angle at room temperature (25 °C), indicating formation of glass mesomorphic phase. The diffraction patterns remained in the heating process until melting into the isotropic fluid at 310 °C for PtL-1 and 270 °C for PtL-2, respectively. For the complexes PtL-1, d100 and d200 reflection peaks was observed at 2θ = 6.78° (d = 13.0 Å), 13.58° (d = 6.5 Å), respectively, and reflection peaks are in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2. The distinct diffuse scattering halo was discovered at 2θ = 23.74° (d = 3.7 Å), which corresponds to intermolecular separation between the complexes within each column. For PtL-1 d110 reflection peak was not observed. However, the complex PtL-1 is still deemed to be a liquid crystallinity complex with hexagonal columnar phase, combined with POM and DSC observation as discussed above. For the complexes PtL-2, d100, d110 and d200 reflection peaks were observed in the small-angle region (2θ = 2–12°), corresponding the value of d is 13.4, 7.7, 6.7 Å, respectively, and reflection peaks are about in a ratio of 1
1/2. The distinct diffuse scattering halo was discovered at 2θ = 23.74° (d = 3.7 Å), which corresponds to intermolecular separation between the complexes within each column. For PtL-1 d110 reflection peak was not observed. However, the complex PtL-1 is still deemed to be a liquid crystallinity complex with hexagonal columnar phase, combined with POM and DSC observation as discussed above. For the complexes PtL-2, d100, d110 and d200 reflection peaks were observed in the small-angle region (2θ = 2–12°), corresponding the value of d is 13.4, 7.7, 6.7 Å, respectively, and reflection peaks are about in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2 in their d-spacing, which is consistent with the d value ratio of hexagonal columnar phase. The halo at 22.27° (d = 3.9 Å) are assigned to aromatic–aromatic separation and Pt⋯Pt separation for the complexes PtL-2. Another weak sharp reflection peak was detected at 2θ = 25.78° (d = 3.4 Å) in the wide-angle region are tentatively assigned to short-range correlations of methoxyphenyl connected in 1H-1,2,3-triazole moiety. Moreover, the possible columnar phase stacking structures of PtL-1 and PtL-2 are proposed (Fig. 3), and their lattice parameter value “a” is calculated to be 15.0 and 15.5 Å based on the result of WAXD, respectively.5b Generally, the formation of thermal discotic liquid crystal is balance of rigid central core and “soft” peripheral region. As we well know, in traditional discotic liquid crystal structure, peripheral multiple flexible chains are as the “soft” regions. For non-traditional discotic liquid crystal structure the polarized or polarizable groups such as chlorine, sulfur atoms, cyano and trifluoromethyl act as “soft” regions.9 However, for the presented metallomesogens, PtL-1 and PtL-2, phenyl and methoxyphenyl seem to be as “soft” region. On the other hand, for the complexes PtL-3, PtL-4 and PtL-5 in which peripheral length of alkoxyl group is more than two no liquid crystalline behavior was observed. This reflects subtle relationship of the molecular interactions which control the formation of liquid crystal. The effect of peripheral phenyl and methoxyphenyl on the formation of liquid crystals is in further research.
1/2 in their d-spacing, which is consistent with the d value ratio of hexagonal columnar phase. The halo at 22.27° (d = 3.9 Å) are assigned to aromatic–aromatic separation and Pt⋯Pt separation for the complexes PtL-2. Another weak sharp reflection peak was detected at 2θ = 25.78° (d = 3.4 Å) in the wide-angle region are tentatively assigned to short-range correlations of methoxyphenyl connected in 1H-1,2,3-triazole moiety. Moreover, the possible columnar phase stacking structures of PtL-1 and PtL-2 are proposed (Fig. 3), and their lattice parameter value “a” is calculated to be 15.0 and 15.5 Å based on the result of WAXD, respectively.5b Generally, the formation of thermal discotic liquid crystal is balance of rigid central core and “soft” peripheral region. As we well know, in traditional discotic liquid crystal structure, peripheral multiple flexible chains are as the “soft” regions. For non-traditional discotic liquid crystal structure the polarized or polarizable groups such as chlorine, sulfur atoms, cyano and trifluoromethyl act as “soft” regions.9 However, for the presented metallomesogens, PtL-1 and PtL-2, phenyl and methoxyphenyl seem to be as “soft” region. On the other hand, for the complexes PtL-3, PtL-4 and PtL-5 in which peripheral length of alkoxyl group is more than two no liquid crystalline behavior was observed. This reflects subtle relationship of the molecular interactions which control the formation of liquid crystal. The effect of peripheral phenyl and methoxyphenyl on the formation of liquid crystals is in further research.
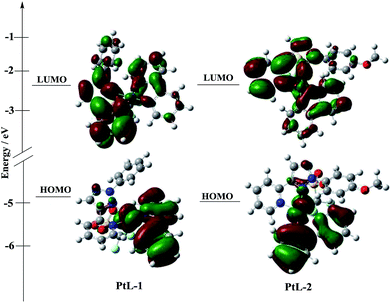
UV-vis absorption and photoluminescence (PL) spectra of all complexes in degassed MTHF solution are shown in Fig. 4, and the relevant data are summarized in Table S1.† UV-vis absorption spectra for the complexes in 10−5 mol L−1 2-methyltetrahydrofuran (MTHF) solution are similar to those of typical cyclometalated platinum(II) complexes, low energy transition bands in the range of 380–410 nm are attributed to ligand-centered 3π–π* transition (3LC) and metal-to-ligand charge transfer (MLCT).14 In order for insight into the photophysical properties of the complexes. B3LYP TD-DFT density functional theory (DFT) calculations were carried out on PtL-1 and PtL-2. The simplified molecular orbitals (MO) diagrams for the investigated complexes are shown in Fig. 5. For PtL-1 and PtL-2, the calculations show that the lowest energy single transition is basically the transition from HOMO to LUMO. The highest occupied molecular orbital (HOMO) is primarily comprised of metal platinum and 2-phenylpyridine, while the lowest unoccupied molecular orbital (LUMO) is originated mainly from the contribution of the triazole ligand and, partly, pyridine ring of 2-phenylpyridine ligand,16 the complexes electronic distribution of the ground and excited state and the calculation results are shown in Table S2.† In addition, the theory UV-vis absorption spectra (Fig. S6†) are basically consistent with the experimental results.
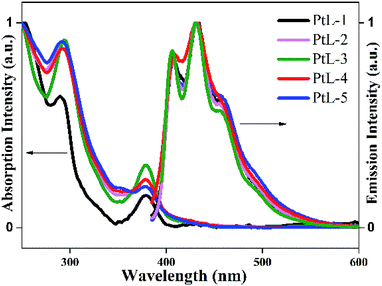 | ||
| Fig. 4 UV-vis absorption and PL emission spectra of the complexes in 10−5 mol L−1 MTHF solution at room temperature. | ||
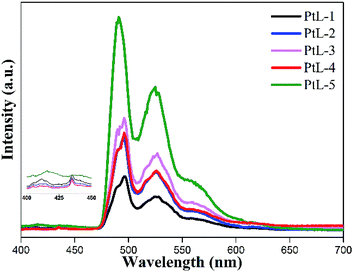
PL spectra of all complexes showed almost identical profiles in range of 410–456 nm and trailed up to 550 nm. The obvious vibronic structural emission spectra with vibronic progressions of about 1400 cm−1, are clearly observed for all complexes at room temperature, indicating that their emissive excited states should contain the 3LC excited state admixed MLCT component. The PL quantum yields (ϕem) of the complexes in degassed MTHF solution are between 0.19 and 0.23 (using Ru[(bpy)3]Cl2·6H2O as a standard in water15). The emission spectra for the complexes at 77 K are distinct different from those at room temperature (Fig. 6). The emission bands in range of 490–560 nm became dominated at the expense of the emission bands in range of 410–450 nm. The solid luminescent spectra at room temperature for the complexes revealed unstructured and red-shifted feature with maximal values in the range of 570–650 nm (Fig. S5 and Table S1†). It is found that with increasing length of side chain the emission bands were gradually red-shifted, suggesting the complexes with longer alkyl side chain facilitate intermolecular π–π interactions and Pt⋯Pt interactions.
In summary, phosphorescent metallomesogens were obtained, for which the molecular shape is non-conventional, even with no peripheral alkyl chains. The formation of liquid crystallinity should be mainly derived from intensive intermolecular interactions. Although some problems, such as effects of SO3CF3− counteranions and 4-methoxyphenyl connected in 1H-1,2,3-triazole moiety on the formation of liquid crystalline, need further study, these novel cationic platinum(II) complexes will bring a new way to design multifunctional metallomesogens.
Acknowledgements
We are grateful for the financial support of the National Natural Science Foundation of China (21172161, 21072147).References
- (a) W.-Y. Wong and C.-L. Ho, Coord. Chem. Rev., 2009, 253, 1709–1758 CrossRef CAS; (b) H. Xiang, J. Cheng, X. Ma, X. Zhou and J. J. Chruma, Chem. Soc. Rev., 2013, 42, 6128–6185 RSC; (c) C. Fan and C. Yang, Chem. Soc. Rev., 2014, 43, 6439–6469 RSC; (d) M. O'Neill and S. M. Kelly, Adv. Mater., 2011, 23, 566–584 CrossRef PubMed.
- (a) Q. Zhao, F. Li and C. Huang, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC; (b) J. A. G. Williams, S. D. Develay, D. L. Rochester and L. Murhy, Coord. Chem. Rev., 2008, 252, 2596–2611 CrossRef; (c) J. Kalinowski, V. Fattori, M. Cocchi and J. A. G. Williams, Coord. Chem. Rev., 2011, 255, 2401–2425 CrossRef CAS; (d) E. Baggaley, J. A. Weinstein and J. A. G. Williams, Coord. Chem. Rev., 2012, 256, 1762–1785 CrossRef CAS.
- (a) X. Feng, V. Marcon, W. Pisula, M. R. Hansen, J. Kirkpatrick, F. Grozema, D. Andrienko, K. Kremer and K. Mìllen, Nat. Mater., 2009, 8, 421–426 CrossRef CAS PubMed; (b) V. W.-W. Yam, V. M. Au and S. Y.-L. Leun, Chem. Rev., 2015, 115, 7589–7728 CrossRef CAS PubMed; (c) Y. F. Wang, Q. Chen, Y. H. Li, Y. Liu, H. Tan, J. T. Yu, M. X. Zhu, H. B. Wu, W. G. Zhu and Y. Cao, J. Phys. Chem. C, 2012, 116, 5908–5914 CrossRef CAS; (d) Y. F. Wang, C. P. Cabry, M. J. Xiao, L. Male, S. J. Cowling, D. W. Bruce, J. W. Shi, W. G. Zhu and E. Baranoff, Chem. – Eur. J., 2016, 22, 1618–1621 CrossRef CAS PubMed.
- (a) Y. F. Wang, Y. Liu, J. Luo, H. R. Qi, X. S. Li, M. J. Ni, M. Liu, M. X. Zhu, W. G. Zhu and Y. Cao, Dalton Trans., 2011, 40, 5046–5051 RSC; (b) B. Y. G. Galyametdinov, A. A. Knyazev, V.-I. Dzhabarov, T. Cardinaels, K. Driesen, C. Görller-Walrand and K. Binnemans, Adv. Mater., 2008, 20, 252–257 CrossRef; (c) S. H. Liu, M. S. Lin, L. Y. Chen, Y. H. Hong, C. H. Tsai, C. C. Wu, A. Poloek, Y. Chi, C. A. Chen, S. H. Chen and H. F. Hsu, Org. Electron., 2011, 12, 15–21 CrossRef CAS.
- (a) S. Sergeyev, W. Pisulab and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC; (b) S. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hgele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel and M. Tosoni, Angew. Chem., Int. Ed., 2007, 46, 4832–4887 CrossRef CAS PubMed; (c) F. Camerel, R. Ziessel, B. Donnio, C. Bourgogne, D. Guillon, M. Schmutz, C. Iacovita and J.-P. Bucher, Angew. Chem. Int. Ed, 2007, 46, 2659–2662 CrossRef CAS PubMed; (d) C. T. Liao, H. H. Chen, H. F. Hsu, A. Poloek, H. H. Yeh, Y. Chi, K. W. Wang, C. H. Lai, G. H. Lee, C. W. Shih and P. T. Chou, Chem. – Eur. J., 2011, 17, 546–556 CrossRef CAS PubMed.
- (a) A. Santoro, A. C. Whitwood, J. A. G. Williams, V. N. Kozhevnikov and D. W. Bruce, Chem. Mater., 2009, 21, 3871–3882 CrossRef CAS; (b) A. Santoro, M. Wegrzyn, A. C. Whitwood, B. Donnio and D. W. Bruce, J. Am. Chem. Soc., 2010, 132, 10689–10691 CrossRef CAS PubMed; (c) M. Soencer, A. Santoro and D. W. Bruce, Dalton Trans., 2012, 41, 14244–142560 RSC; (d) K. M.-C. Wong, L. Wu and V. W.-W. Yam, Chem. – Eur. J., 2010, 16, 6797–6809 CrossRef PubMed; (e) D. Pucci, G. Barberio, A. Crispini, O. Francescangeli, M. Ghedini and M. L. Deda, Eur. J. Inorg. Chem., 2003, 3649–3661 CrossRef CAS.
- (a) E. K. Fleischmann and R. Zentel, Angew. Chem., Int. Ed., 2013, 52, 8810–8827 CrossRef CAS PubMed; (b) V. N. Kozhevnikov, B. Donnio and D. W. Bruce, Angew. Chem., In Ed., 2008, 47, 6286–6289 CrossRef CAS PubMed; (c) B. D. Pate, S. M. Choi, U. Werner-Zwanziger, D. V. Baxter, J. M. Zaleski and M. H. Chisholm, Chem. Mater., 2002, 14, 1930–1936 CrossRef CAS.
- T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed.
- (a) J. Barbera, O. A. Rakitin, M. B. Ros and T. Torroba, Angew. Chem., Int. Ed., 1998, 37, 296–299 CrossRef CAS; (b) S. Basurto, S. Garca, A. G. Neo, T. Torroba, C. F. Marcos, D. Miguel, J. Barbera, M. Blanca Ros and M. R. de la Fuente, Chem. – Eur. J., 2005, 11, 5362–5376 CrossRef CAS PubMed; (c) D. Pucci, I. Aiello, A. Aprea, A. Bellusci, A. Crispini and M. Ghedini, Chem. Commun., 2009, 1550–1552 RSC.
- M. Krikorian, S. Liu and T. M. Swager, J. Am. Chem. Soc., 2014, 136, 2952–2955 CrossRef CAS PubMed.
- J. Brooks, Y. Babayan, S. Lamansky, P. I. Djurovich, I. Tsyba, R. Bau and M. E. Thompson, Inorg. Chem., 2002, 41, 3055–3066 CrossRef CAS PubMed.
- B. Schulze and U. S. Schubert, Chem. Soc. Rev., 2014, 43, 2522–2571 RSC.
- S. H. Chen, J. C. Mastrangelo, T. N. Blantong, A. Bashir-hashemit and K. L. Marshall, Liq. Cryst., 1996, 21, 683–694 CrossRef CAS.
- (a) M. Ebina, A. Kobayashi, T. O. M. Yoshida and M. Kato, Inorg. Chem., 2015, 54, 8878–8880 CrossRef CAS PubMed; (b) D. M. Jenkins and S. Bernhard, Inorg. Chem., 2010, 49, 11297–11308 CrossRef CAS PubMed.
- (a) G. Jones, W. R. Jackson, C. Y. Choi and W. R. Bergmark, J. Phys. Chem., 1985, 89, 294–300 CrossRef CAS; (b) J. DePriest, G. Y. Zheng, N. Goswami, D. M. Eichhorn, C. Woods and D. P. Rillema, Inorg. Chem., 2000, 39, 1955–1963 CrossRef CAS PubMed.
- (a) A. Bossi, A. F. Rausch, M. J. Leitl, R. Czerwieniec, M. T. Whited, P. I. Djurovich, H. Yersin and M. E. Thompson, Inorg. Chem., 2013, 52, 12403–12415 CrossRef CAS PubMed; (b) S. Clam, P. H. Lanoë, V. L. Whittle, M. C. Durrant, J. A. G. Williams and V. N. Kozhevnikov, Inorg. Chem., 2013, 52, 10992–11003 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28767k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |