Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of star-glycopolymers by Cu(0)-mediated radical polymerisation in the absence and presence of oxygen†
Hui Xue‡
a,
Lun Peng‡b,
Yishi Donga,
Yuqing Zhengb,
Yafei Luana,
Xiang Hua,
Gaojian Chen *ab and
Hong Chen
*ab and
Hong Chen *a
*a
aState and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren'ai Road, Suzhou 215123, P. R. China. E-mail: chenh@suda.edu.cn; Fax: +86 512 65880827; Tel: +86 512 65880827
bCenter for Soft Condensed Matter Physics and Interdisciplinary Research, Soochow University, Suzhou 215006, P. R. China. E-mail: gchen@suda.edu.cn; Tel: +86 512 65884406
First published on 26th January 2017
Abstract
In this paper, novel star glycopolymers were synthesized via Cu(0)-mediated radical polymerisation at ambient temperature. The reaction was fast with little star–star coupling. Moreover, star glycopolymers can be obtained without removing oxygen from the polymerisation mixture. The effects of solvent and the ratio of initiator/catalyst/ligand on polymerisation were investigated, and the optimal conditions for the synthesis of star glycopolymer were determined. In addition, the binding ability between synthesised glycopolymers and concanavalin A (ConA) was studied using a turbidity test and quartz crystal microbalance-dissipation (QCM-D). Compared with linear glycopolymers, star glycopolymers showed higher binding ability to specific lectins and the strongest binding was obtained when the molecular weight was medium.
1. Introduction
Carbohydrates are key molecules in life. They are not only the main energy source, but also the basis for cell identification and intercellular signalling, due to the specific interaction with proteins.1–4 A number of diseases, such as cancer and immune disorders, are closely related to abnormal interactions between sugar and protein.5–7 Although the interaction between individual sugar molecules and protein is weak, it can be greatly enhanced by the combination of a plurality of sugar molecules, resulting in what is called a ‘glycoclusters effect’.8,9 In order to study the interactions between carbohydrates and proteins, glycopolymers with multiple pendant sugar units have been synthesized and applied in many fields such as histological engineering, drug controlled release, biosensors, proteome analysis and so on.10–16 It has been shown that dendritic sugar molecules and derivatives possess high protein binding ability due to higher local concentration of sugar units.17–19 Many reports demonstrated the potential of star-polymers in medicine due to the unique structure, special hydrodynamics and multisite modification.20–23 Compared with their linear counterparts, star polymers possess additional properties thanks to their compact structure and high arm density.24 Therefore, it is of significance to develop robust and convenient synthetic methods for obtaining well-defined star glycopolymers.Star glycopolymers have been successfully synthesized via Reversible Addition–Fragmentation Chain Transfer Polymerisation (RAFT)25–27 and Atom Transfer Radical Polymerisation (ATRP).28,29 However, there are disadvantages such as easily coupling between star molecules, producing linear by-product and needing high reaction temperature. Cu(0)-mediated Living Radical Polymerisation (LRP) possesses many advantages,30–35 although the mechanism is under debate. Glycopolymers with narrow molecular weight distributions have been successfully prepared in a well-controlled manner via room temperature Cu(0)-mediated living radical polymerisation.36,37 Cyclodextrin-based glycoclusters/star glycopolymers were synthesized by Haddleton and coworkers via the combination of CuAAC Huisgen coupling and copper-mediated living radical polymerisation.38 More recently, Davis and Haddleton showed that well-defined PDMAEA stars with narrow molecular weight distributions can be obtained by copper(0) mediated living radical polymerisation.39 In aqueous media, copper(0)-mediated radical polymerisation wherein reactive Cu(0) and Cu(II)X2 species, obtained by disproportionation of Cu(I)X species in the presence of a diversity of N-containing ligands, mediate an ultrafast living radical polymerisation of acrylates at room temperature or below. As being highlighted by Zhang et al., it is able to rapidly achieve high molecular weights with excellent control of molecular weight distribution, with perfect retention of chain end functionality and low bi-radical termination ratio.37,40,41 Herein we report the synthesis of star glycopolymers via room temperature Cu(0)-mediated radical polymerisation in aqueous media in the absence and presence of oxygen. Star glycopolymers were successfully synthesized with high reaction rate at room temperature without protecting sugar monomers and the polymerisation can still be carried out in the presence of oxygen (Scheme 1). This method provides a promising pathway for the synthesis of star glycopolymers in different situations without degassing. In addition, the specific binding ability of glycopolymers to ConA was measured by turbidity experiment and QCM-D, and it provides in-depth information for designing star glycopolymers with potential application in targeted drug delivery.42,43
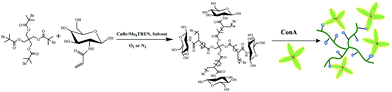 | ||
| Scheme 1 Schematic representation of four arms star glycopolymer via Cu(0)-mediated radical polymerisation and the specific interaction between glycopolymers and ConA. | ||
2. Experimental
2.1 Materials
2-Bromoisobutyryl bromide (Sigma-Aldrich, 98%), 3-(2-amino ethyl methyl) amine (Me6-TREN, Sigma-Aldrich, 97%), concanavalin A (ConA, Sigma-Aldrich), 11-mercaptoundecanoic acid (Sigma-Aldrich, 95%), N-hydroxysuccinimide (Sigma-Aldrich, 98%), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (Sigma-Aldrich, 98%), D-(+)-glucosamine hydrochloride (TCI, 98%) and acryloyl chloride (Aladdin, 96%) were used as received. Pentaerythritol (Aladdin, 98%) was used after dried in vacuum oven. Deuterated chloroform (CDCl3, 99.8%) and deuterium oxide (D2O, 99.9%) were purchased from Cambridge Isotope Laboratories, Inc. Cuprous bromide (CuBr, J&K, 98%) was used after purification (washed with glacial acetic acid and methanol, stirred for 30 min, filtrated and dried under vacuum for 24 h). Triethylamine, anhydrous magnesium sulfate (MgSO4), potassium carbonate (K2CO3), concentrated ammonia solution, hydrogen peroxide (30 vol%), methanol, dimethylformamide (DMF) and other solvents were all purchased from the Sinopharm Chemical Reagent Co., Ltd. (China) and used as received. The dialysis membranes were purchased from Solarbio Life Science, Inc. The monomer acryloyl D-(+)-glucosamine (AGA) was synthesized according to the ref. 44.2.2 Synthesis
2.3 Binding assay of glycopolymer and ConA
Glycopolymer (5 mg) and ConA (1 mg) were dissolved in 1 mL phosphate buffered saline (PBS, pH = 7.4, with 0.1 mM CaCl2 and MnCl2) respectively. Then both of the solutions (0.4 mL) were mixed with each other, and the absorbance data were obtained in 30 minutes by UV-spectrophotometer at 25 °C (λ = 360 nm).The QCM-D chips (AT-cut, 5 MHz, 14 mm in diameter) were washed by ethanol and deionized water iteratively. Both sides of the chips were treated in ozone atmosphere for 30 minutes respectively and then cleaned with ethanol and deionized water. Mixture of concentrated ammonia solution, hydrogen peroxide solution and deionized water, with ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in volume, was used to boil the chips at 75 °C for 10 minutes, followed by rinsing them with abundant deionized water. The chips were dipped in 1 mL ethanol solution of 11-mercaptoun-decanoic acid (0.2 M) overnight and then washed with ethanol. Dried chips were dipped in 1 mL PBS solution of N-hydroxysuccinimide and N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride (0.05 M respectively) and then rinsed with deionized water. The QCM-D chips were placed in the fluid and exposed to the solution. Detection in QCM-D experiments was carried out in flow-through mode with a flow rate of 10 μL min−1 at 25 °C. The baseline was set up by passing the PBS (with 0.1 mM CaCl2 and MnCl2) that mentioned earlier through for about 4.5 hours. Then freshly prepared ConA solutions (PBS with 0.1 mM CaCl2 and MnCl2, 1 mg mL−1) were passed through for about 3 hours to obtain the adsorption plateau. After that, the chips were washed with the former PBS to remove loosely attached protein.
5 in volume, was used to boil the chips at 75 °C for 10 minutes, followed by rinsing them with abundant deionized water. The chips were dipped in 1 mL ethanol solution of 11-mercaptoun-decanoic acid (0.2 M) overnight and then washed with ethanol. Dried chips were dipped in 1 mL PBS solution of N-hydroxysuccinimide and N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride (0.05 M respectively) and then rinsed with deionized water. The QCM-D chips were placed in the fluid and exposed to the solution. Detection in QCM-D experiments was carried out in flow-through mode with a flow rate of 10 μL min−1 at 25 °C. The baseline was set up by passing the PBS (with 0.1 mM CaCl2 and MnCl2) that mentioned earlier through for about 4.5 hours. Then freshly prepared ConA solutions (PBS with 0.1 mM CaCl2 and MnCl2, 1 mg mL−1) were passed through for about 3 hours to obtain the adsorption plateau. After that, the chips were washed with the former PBS to remove loosely attached protein.
2.4 Characterization
1H NMR spectra of the polymers were recorded on an Agilent 400 MHz nuclear magnetic resonance (NMR) instrument, using D2O as the solvent. 13C NMR spectra of the polymers were recorded on an INOVA 300 MHz nuclear magnetic resonance (NMR) instrument using D2O as solvent. The molecular weights and molecular weight distributions of the polymers were measured on a waters 1515 gel permeation chromatography (GPC) system equipped with a PL aquagel-OH MIXED-M column using PEG as the standard sample. A mixture of millipore water containing 70% of 0.2 M NaNO3, 0.1 M NaH2PO4 with a pH of 7 and 30% methanol was used as the mobile phase and was delivered at a flow rate of 1 mL min−1. GPC traces were acquired by an Agilent PL-GPC 50 gel permeation chromatograph system equipped with a refractive index detector and using a 5 μm Guard, 5 μm MIXED-D column. PMMA was used as the standard samples and DMF containing 0.05 mol L−1 lithium bromide was used as the eluent at a flow rate of 1 mL min−1. The measurement was operated at 50 °C. The binding ability of glycopolymers and ConA was measured by Ultraviolet spectrophotometer on a Shimadzu (Kyoto, Japan) UV-3600. The quartz crystal microbalance-dissipation (QCM-D, HRBio, Suzhou, China) measurements primed with water or PBS at a speed of 10 μL min−1 and 25 °C were conducted using a Q-Sense-E4instrument (Q-Sense, Sweden) with control software (Resonant Probes GmbH, Goslar, Germany) to analyse the data.3. Results and discussion
3.1 Effect of solvent in the polymerisation
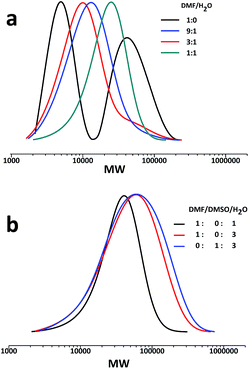
Cu(0)-mediated LRP is a robust technique for polymer synthesis. As previously reported the complex Cu(I)/Me6-TREN possess the best disproportionation efficiency in H2O, and efficiently mediated the polymerisation process.45,46 Considering the solubility of the initiator and disproportionation efficiency of catalyst, the experiments were carried out in the mixed solvents of DMF/H2O or DMSO/H2O. In order to determine the optimal condition for the synthesis of four-arm star glycopolymers, polymerisations with different solvent compositions and ratios of bromine/cuprous bromide/ligand (Br/Cu/L) ratios were conducted in the present work.It can be seen from Table 1 that for the same polymerisation time, the conversion increases with increasing water content of the solvent, indicating that Cu(0) is formed more efficiently in water and then enhances the initiation of the polymerisation. For example, when DMF/H2O is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, polymer with molecular weight of 18
1, polymer with molecular weight of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and PDI = 1.45 was obtained after 6 h. Meanwhile, with the increasing of water content in the mixed solvent, the polymer's GPC trace shows that the shoulder peaks at high molecular weight portion taper and disappear in Fig. 1a. It seems that couplings between star molecules are significantly inhibited, which offer an effective solution to the termination problem in the synthesis of star glycopolymers. However, when the water content increases to 75%, the polymer molecular weight is much higher than theoretical value with lower conversion rate within the same reaction time, no matter in the mixture of H2O with DMF or DMSO. Moreover, the PDIs become high (>2.2). It is possibly due to the poor solubility of the initiator in water. At the very beginning, few initiator molecules dissolve in the solvent, and more initiators dissolve with the progress of polymerisation. Since the propagation time of each initiator is different, tails appear at the low molecular weight part of GPC curves, and PDIs broaden. From this we can draw a conclusion: when the volume ratio of DMF/H2O is 1
700 and PDI = 1.45 was obtained after 6 h. Meanwhile, with the increasing of water content in the mixed solvent, the polymer's GPC trace shows that the shoulder peaks at high molecular weight portion taper and disappear in Fig. 1a. It seems that couplings between star molecules are significantly inhibited, which offer an effective solution to the termination problem in the synthesis of star glycopolymers. However, when the water content increases to 75%, the polymer molecular weight is much higher than theoretical value with lower conversion rate within the same reaction time, no matter in the mixture of H2O with DMF or DMSO. Moreover, the PDIs become high (>2.2). It is possibly due to the poor solubility of the initiator in water. At the very beginning, few initiator molecules dissolve in the solvent, and more initiators dissolve with the progress of polymerisation. Since the propagation time of each initiator is different, tails appear at the low molecular weight part of GPC curves, and PDIs broaden. From this we can draw a conclusion: when the volume ratio of DMF/H2O is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the solvent is suitable for the synthesis of star glycopolymers (Fig. 2).
1, the solvent is suitable for the synthesis of star glycopolymers (Fig. 2).
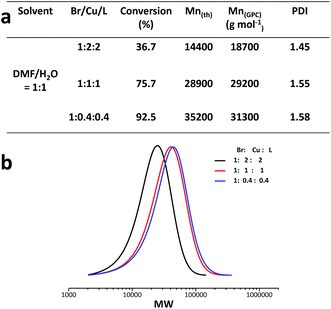
| Solvent | Solvent ratios (V) | Time (h) | [M]/[I]a | Br/Cu/Lb | Conversion (%) | Mn(th) | Mnc (g mol−1) | PDIc |
|---|---|---|---|---|---|---|---|---|
| a Monomer/initiator. The initial concentration of monomer [M(AGA)]0 is 0.33 mmol mL−1.b (Initiator × 4)/catalyst/ligand.c Obtained by aqueous GPC. | ||||||||
| DMF/H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
6 | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
21.0 | 8500 | Bimodal | |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 40 | 29.1 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
9000 | 1.85 | ||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 40 | 34.2 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.67 | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 40 | 36.7 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.45 | ||
| DMF/H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75.7 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.55 |
| DMF/H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 40 | 11.0 | 4800 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2.20 | |
| DMSO/H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 40 | 13.5 | 5800 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.33 | |
3.2 Influence of ratios of initiator/catalyst/ligand
Br/Cu/L ratio is a key parameter in the activation/deactivation process, and thus has an effect on the polymerisation rate and controllability. To make sure full complexation of Cu(0) with Me6-TREN, the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Cu/L) was used. Experiments with different ratios of initiator/catalyst were carried out to investigate the effect of catalytic ratio on the synthesis of acrylamide star glycopolymers. Since a four-arm initiator was used, Br/Cu here refers to the initial molar ratios of (initiator × 4)/catalyst. It can be seen that the polymerisation rate increases and PDI broadens slightly when the ratio of Br/Cu changes from 1
1 (Cu/L) was used. Experiments with different ratios of initiator/catalyst were carried out to investigate the effect of catalytic ratio on the synthesis of acrylamide star glycopolymers. Since a four-arm initiator was used, Br/Cu here refers to the initial molar ratios of (initiator × 4)/catalyst. It can be seen that the polymerisation rate increases and PDI broadens slightly when the ratio of Br/Cu changes from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4. Low Br/Cu ratio is in favour of polymerisation regulation, while high Br/Cu ratio helps to accelerate the polymerisation. There's no shoulder in all of the three GPC curves, which indicates that this polymerisation system can reduce star–star coupling.
0.4. Low Br/Cu ratio is in favour of polymerisation regulation, while high Br/Cu ratio helps to accelerate the polymerisation. There's no shoulder in all of the three GPC curves, which indicates that this polymerisation system can reduce star–star coupling.
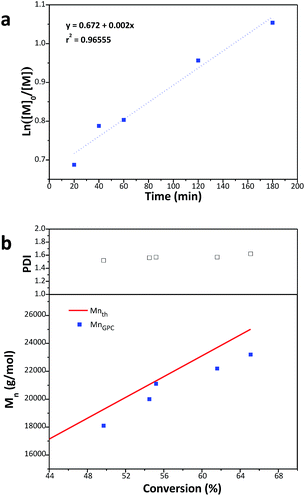
3.3 Kinetic of the polymerisation of AGA
Polymerisation system with Br/Cu/L (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the solvent of DMF/H2O (1
1) in the solvent of DMF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was chosen for kinetic investigation, which possesses moderate polymerisation rate and controllability. ln([M0]/[M]) shows a linear relationship with time in (Fig. 3a), and the first order kinetics indicate that the radical concentration is constant. Molecular weight of the polymer increases linearly with the conversion and slightly diverges (especially at higher conversion) from the theoretical molecular weight. The reason is that the hydrodynamic radii of star molecules are smaller than linear ones with the same molecular weight in solution. As linear PMMA is used as standard samples in DMF GPC, the measured molecular weights by GPC are lower than the theoretical molecular weights. We found that the measured molecular weights by NMR are slightly higher than the GPC values and are close to the theoretical molecular weights (Table S1 and Fig. S5†). During the whole polymerisation, the polydispersity index remains at about 1.5.
1) was chosen for kinetic investigation, which possesses moderate polymerisation rate and controllability. ln([M0]/[M]) shows a linear relationship with time in (Fig. 3a), and the first order kinetics indicate that the radical concentration is constant. Molecular weight of the polymer increases linearly with the conversion and slightly diverges (especially at higher conversion) from the theoretical molecular weight. The reason is that the hydrodynamic radii of star molecules are smaller than linear ones with the same molecular weight in solution. As linear PMMA is used as standard samples in DMF GPC, the measured molecular weights by GPC are lower than the theoretical molecular weights. We found that the measured molecular weights by NMR are slightly higher than the GPC values and are close to the theoretical molecular weights (Table S1 and Fig. S5†). During the whole polymerisation, the polydispersity index remains at about 1.5.
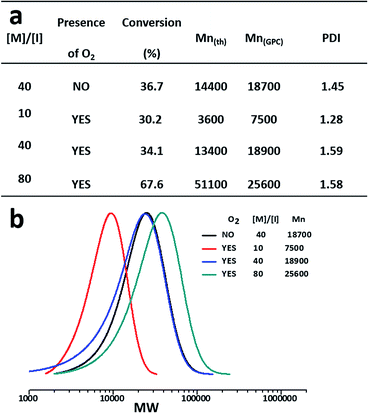
3.4 Synthesis of star glycopolymers in the presence of O2
For the synthesis of sugar macromolecules in living organism, the presence of oxygen is unavoidable. However, removing oxygen from the polymerisation mixture is required for the traditional free radical polymerisation or living radical polymerisation systems, which is bound to affect the researches on in situ synthesis of sugar macromolecules in biological systems. Previous studies have shown that AGET, ATRP and other polymerisation systems containing reducing components have a certain ability to endure oxygen.47–49 Cu(0) is a good reductant which will react with oxygen, so we wonder whether the present system can still proceed in the presence of oxygen, since much Cu(0) will be produced in the disproportionation process.To ensure the amount of Cu(0) is enough to catalyse the system in the presence of oxygen, the ratio of Br/Cu/L (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was chosen. As shown in Fig. 4, the polymerisation can still move on with a high conversion in the presence of oxygen. Polymers with different molecular weights (from 7500 to 25
2) was chosen. As shown in Fig. 4, the polymerisation can still move on with a high conversion in the presence of oxygen. Polymers with different molecular weights (from 7500 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were easily obtained by changing the monomer/initiator (M/I) ratios, and the molecular weight distributions were kept reasonably narrow. Previous reports of the molecular weight distributions of star glycopolymers synthesized by RAFT polymerisation could reach to 1.9.25 Relatively narrow molecular weight distributions of star glycopolymers ranging from 1.1 to 1.5 can be obtained via ATRP and the combination of SET-LRP/click chemistry, whose polymerisations were carried out for 15 to 24 hours in the absence of oxygen.29,38 The tolerance to oxygen and shorter polymerisation time guarantee our approach more possibilities in complex systems.
000) were easily obtained by changing the monomer/initiator (M/I) ratios, and the molecular weight distributions were kept reasonably narrow. Previous reports of the molecular weight distributions of star glycopolymers synthesized by RAFT polymerisation could reach to 1.9.25 Relatively narrow molecular weight distributions of star glycopolymers ranging from 1.1 to 1.5 can be obtained via ATRP and the combination of SET-LRP/click chemistry, whose polymerisations were carried out for 15 to 24 hours in the absence of oxygen.29,38 The tolerance to oxygen and shorter polymerisation time guarantee our approach more possibilities in complex systems.
3.5 Effects of different glycopolymers on specific binding to lectin
The specific interaction between sugar and lectin is the basis for intercellular signal transduction, and plays a vital role in many life activities.11 The strength of binding between multiple ligands and lectin depends on ligand structure, molecular skeleton freedom,50 binding-site density51,52 and molecular weight.53,54The binding abilities between synthetic star glycopolymers and lectins were first investigated through detecting changes of the mixed solution's turbidity, and the experimental results are shown in Fig. 5. The figure shows that the absorbance of star glycopolymer solution increases obviously, on the contrary, no change appears in linear molecules with a similar molecular weight and concentration, which indicates that the star glycopolymers possess a better specific binding ability with lectin. Chances are that star molecules tend to form crosslinkings with different lectins, and thereby more particles precipitate from the solution, leading to more obvious turbidity change. Furthermore, the star glycopolymer with Mn = 4700 shows the highest binding rate to ConA, and with the further increasing of molecular weight, the changing rate of turbidity gradually decreases, which coincides with the results from previous studies.9,27 Both results of the present work and other reports show that higher molecular weights do not always enhance binding with proteins, and molecular weight optimization is necessary. To obtain more information about the interactions between glycopolymer and ConA, QCM-D data (Fig. 6) were used. For better comparison, we calculated the relative binding (the frequency change after polymer injection divided by the frequency change before polymer injection) of different polymers. It shows that star glycopolymers show higher binding potency to ConA than linear ones. And the star glycopolymers with Mn = 4700 and 9000 present higher relative binding value than the star glycopolymer with Mn = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700, indicating that medium molecular weight is better for lectin binding.
700, indicating that medium molecular weight is better for lectin binding.
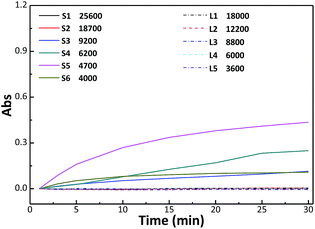 | ||
| Fig. 5 Turbidity measurement of glycopolymers with ConA. S means star glycopolymers and L means linear glycopolymers. The number behind means the molecular weight. | ||
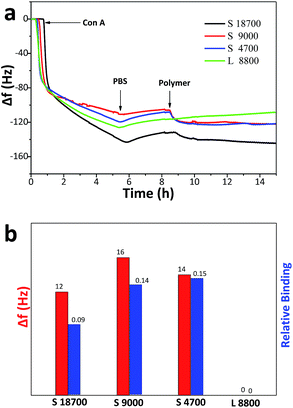 | ||
| Fig. 6 (a) Plots of the frequency changes and (b) protein absorption of QCM in response to ConA (0.1 mg mL−1), PBS, and glycopolymers (1 mg mL−1). | ||
4. Conclusion
A series of star glycopolymers were synthesized via Cu(0)-mediated radical polymerisation. The effects of solvent composition and ratio of initiator/catalyst/ligand were investigated and the optimized condition was determined. When a mixed solvent of DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is used, both good dissolution of the oil-soluble initiator and efficient disproportionation of Cu(I) can be achieved. A fast polymerisation rate and relatively good controllability can be obtained when the ratio of Br/Cu/L is 1
1 is used, both good dissolution of the oil-soluble initiator and efficient disproportionation of Cu(I) can be achieved. A fast polymerisation rate and relatively good controllability can be obtained when the ratio of Br/Cu/L is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In the present work, we find that the polymerisation can still proceed and star glycopolymers with different molecular weights are able to be obtained in the presence of oxygen. The turbidity and QCM-D results show that star glycopolymers possess better specific binding ability to lectin than linear ones, and the star glycopolymers with medium molecular weights show higher specific binding potency. The star glycopolymers conveniently synthesized can find applications in the delivery of drugs, the study of the biomolecular interactions between carbohydrates and proteins, and for the fabrication of other sugar-based functional materials. The approach to prepare glycopolymers in the presence of oxygen provides a facile way for in situ polymerisations that can guarantee more possibilities in complex biological systems.
1. In the present work, we find that the polymerisation can still proceed and star glycopolymers with different molecular weights are able to be obtained in the presence of oxygen. The turbidity and QCM-D results show that star glycopolymers possess better specific binding ability to lectin than linear ones, and the star glycopolymers with medium molecular weights show higher specific binding potency. The star glycopolymers conveniently synthesized can find applications in the delivery of drugs, the study of the biomolecular interactions between carbohydrates and proteins, and for the fabrication of other sugar-based functional materials. The approach to prepare glycopolymers in the presence of oxygen provides a facile way for in situ polymerisations that can guarantee more possibilities in complex biological systems.
Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21474071, 21374069), Jiangsu Clinical Research Center for Cardiovascular Surgery and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for financial support.Notes and references
- L. L. Kiessling and J. C. Grim, Chem. Soc. Rev., 2013, 42, 4476–4491 RSC.
- V. Ladmiral, E. Melia and D. M. Haddleton, Eur. Polym. J., 2004, 40, 431–449 CrossRef CAS.
- S. R. S. Ting, G. Chen and M. H. Stenzel, Polym. Chem., 2010, 1, 1392–1412 RSC.
- A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzler, Glycans in Acquired Human Diseases, 2009 Search PubMed.
- T. M. Allen, Nat. Rev. Cancer, 2002, 2, 750–763 CrossRef CAS PubMed.
- R. Duncan, Nat. Rev. Cancer, 2006, 6, 688–701 CrossRef CAS PubMed.
- R. Sunasee and R. Narain, Macromol. Biosci., 2013, 13, 9–27 CrossRef CAS PubMed.
- J. J. Lundquist and E. J. Toone, Chem. Rev., 2002, 102, 555–578 CrossRef CAS PubMed.
- J. E. Gestwicki, C. W. Cairo, L. E. Strong, K. A. Oetjen and L. L. Kiessling, J. Am. Chem. Soc., 2002, 124, 14922–14933 CrossRef CAS PubMed.
- Y. Terada, W. Hashimoto, T. Endo, H. Seto, T. Murakami, H. Hisamoto, Y. Hoshino and Y. Miura, J. Mater. Chem. B, 2014, 2, 3324–3332 RSC.
- Y. Miura, Polym. J., 2012, 44, 679–689 CrossRef CAS.
- R. Narain, Engineered carbohydrate-based materials for biomedical applications: polymers, surfaces, dendrimers, nanoparticles, and hydrogels, John Wiley & Sons, 2011 Search PubMed.
- K. Chen, M. Bao, A. Muñoz Bonilla, W. Zhang and G. Chen, Polym. Chem., 2016, 7, 2565–2572 RSC.
- K. Ferji, C. Nouvel, J. Babin, M.-H. Li, C. Gaillard, E. Nicol, C. Chassenieux and J.-L. Six, ACS Macro Lett., 2015, 4, 1119–1122 CrossRef CAS.
- F. Li, D. Pei, Q. Huang, T. Shi and G. Zhang, Carbohydr. Polym., 2014, 99, 728–735 CrossRef CAS PubMed.
- C. Schatz and S. Lecommandoux, Macromol. Rapid Commun., 2010, 31, 1664–1684 CrossRef CAS PubMed.
- Y. M. Chabre and R. Roy, Curr. Top. Med. Chem., 2008, 8, 1237–1285 CrossRef CAS PubMed.
- S. Zhang, Q. Xiao, S. E. Sherman, A. Muncan, A. D. Ramos Vicente, Z. Wang, D. A. Hammer, D. Williams, Y. Chen and D. J. Pochan, J. Am. Chem. Soc., 2015, 137, 13334–13344 CrossRef CAS PubMed.
- S. Vandewalle, S. Wallyn, S. Chattopadhyay, C. R. Becer and F. Du Prez, Eur. Polym. J., 2015, 69, 490–498 CrossRef CAS.
- Z. Li, E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 306, 98–101 CrossRef CAS PubMed.
- D. J. Cameron and M. P. Shaver, Chem. Soc. Rev., 2011, 40, 1761–1776 RSC.
- S. J. Lam, N. M. O'Brien-Simpson, N. Pantarat, A. Sulistio, E. H. Wong, Y. Y. Chen, J. C. Lenzo, J. A. Holden, A. Blencowe, E. C. Reynolds and G. G. Qiao, Nat. Microbiol., 2016, 1, 16162 CrossRef PubMed.
- A. Sulistio, J. Lowenthal, A. Blencowe, M. N. Bongiovanni, L. Ong, S. L. Gras, X. Zhang and G. G. Qiao, Biomacromolecules, 2011, 12, 3469–3477 CrossRef CAS PubMed.
- W. Wu, W. Wang and J. Li, Prog. Polym. Sci., 2015, 46, 55–85 CrossRef CAS.
- A. Ghadban and L. Albertin, Polymers, 2013, 5, 431–526 CrossRef.
- J. Bernard, X. Hao, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Biomacromolecules, 2006, 7, 232–238 CrossRef CAS PubMed.
- Y. Chen, G. Chen and M. H. Stenzel, Macromolecules, 2010, 43, 8109–8114 CrossRef CAS.
- X. H. Dai and C. M. Dong, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 817–829 CrossRef CAS.
- S. Qiu, H. Huang, X. H. Dai, W. Zhou and C. M. Dong, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2009–2023 CrossRef CAS.
- Q. Zhang, A. Anastasaki, G.-Z. Li, A. J. Haddleton, P. Wilson and D. M. Haddleton, Polym. Chem., 2014, 5, 3876–3883 RSC.
- B. M. Rosen and V. Percec, Chem. Rev., 2009, 109, 5069–5119 CrossRef CAS PubMed.
- R. Aksakal, M. Resmini and C. R. Becer, Polym. Chem., 2016, 7, 171–175 RSC.
- N. P. Truong, W. Gu, I. Prasadam, Z. Jia, R. Crawford, Y. Xiao and M. J. Monteiro, Nat. Commun., 2013, 4, 1902 CrossRef PubMed.
- D. Konkolewicz, Y. Wang, P. Krys, M. Zhong, A. A. Isse, A. Gennaro and K. Matyjaszewski, Polym. Chem., 2014, 5, 4396–4417 RSC.
- A. Anastasaki, V. Nikolaou, G. Nurumbetov, P. Wilson, K. Kempe, J. F. Quinn, T. P. Davis, M. R. Whittaker and D. M. Haddleton, Chem. Rev., 2016, 116, 835–877 CrossRef CAS PubMed.
- J. S. Basuki, L. Esser, H. T. T. Duong, Q. Zhang, P. Wilson, M. R. Whittaker, D. M. Haddleton, C. Boyer and T. P. Davis, Chem. Sci., 2014, 5, 715–726 RSC.
- Q. Zhang, J. Collins, A. Anastasaki, R. Wallis, D. A. Mitchell, C. R. Becer and D. M. Haddleton, Angew. Chem., Int. Ed., 2013, 52, 4435–4439 CrossRef CAS PubMed.
- Q. Zhang, L. Su, J. Collins, G. Chen, R. Wallis, D. A. Mitchell, D. M. Haddleton and C. R. Becer, J. Am. Chem. Soc., 2014, 136, 4325–4332 CrossRef CAS PubMed.
- R. Whitfield, A. Anastasaki, N. P. Truong, P. Wilson, K. Kempe, J. A. Burns, T. P. Davis and D. M. Haddleton, Macromolecules, 2016, 49, 8914–8924 CrossRef CAS.
- Q. Zhang, P. Wilson, Z. Li, R. McHale, J. Godfrey, A. Anastasaki, C. Waldron and D. M. Haddleton, J. Am. Chem. Soc., 2013, 135, 7355–7363 CrossRef CAS PubMed.
- Q. Zhang, P. Wilson, A. Anastasaki, R. McHale and D. M. Haddleton, ACS Macro Lett., 2014, 3, 491–495 CrossRef CAS.
- M. Mahkam, Drug Delivery, 2007, 14, 147–153 CrossRef CAS PubMed.
- F. Suriano, R. Pratt, J. P. Tan, N. Wiradharma, A. Nelson, Y. Y. Yang, P. Dubois and J. L. Hedrick, Biomaterials, 2010, 31, 2637–2645 CrossRef CAS PubMed.
- J. Lu, C. Fu, S. Wang, L. Tao, L. Yan, D. M. Haddleton, G. Chen and Y. Wei, Macromolecules, 2014, 47, 4676–4683 CrossRef CAS.
- F. Alsubaie, A. Anastasaki, V. Nikolaou, A. Simula, G. Nurumbetov, P. Wilson, K. Kempe and D. M. Haddleton, Macromolecules, 2015, 48, 5517–5525 CrossRef CAS.
- F. Alsubaie, A. Anastasaki, V. Nikolaou, A. Simula, G. Nurumbetov, P. Wilson, K. Kempe and D. M. Haddleton, Macromolecules, 2015, 48, 6421–6432 CrossRef CAS.
- K. Min, W. Jakubowski and K. Matyjaszewski, Macromol. Rapid Commun., 2006, 27, 594–598 CrossRef CAS.
- L. Zhang, Z. Cheng, S. Shi, Q. Li and X. Zhu, Polymers, 2008, 49, 3054–3059 CrossRef CAS.
- M. Tao, L. Zhang, H. Jiang, Z. Zhang, J. Zhu, Z. Cheng and X. Zhu, Macromol. Chem. Phys., 2011, 212, 1481–1488 CrossRef CAS.
- T. Hasegawa, S. Kondoh, A. Kazunori Matsuura and K. Kobayashi, Macromolecules, 1999, 32, 6595–6603 CrossRef CAS.
- C. W. Cairo, J. E. Gestwicki, M. Kanai and L. L. Kiessling, J. Am. Chem. Soc., 2002, 124, 1615–1619 CrossRef CAS PubMed.
- C. R. Becer, M. I. Gibson, J. Geng, R. Ilyas, R. Wallis, D. A. Mitchell and D. M. Haddleton, J. Am. Chem. Soc., 2010, 132, 15130–15132 CrossRef CAS PubMed.
- M. Kanai, K. H. Mortell and L. L. Kiessling, J. Am. Chem. Soc., 1997, 119, 9931–9932 CrossRef CAS.
- Y. Chen, M. S. Lord, A. Piloni and M. H. Stenzel, Macromolecules, 2015, 48, 346–357 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR of 4-arm initiator, AGA and typical 4-arm star glycopolymer. See DOI: 10.1039/c6ra28763h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |