Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic hydrothermal liquefaction of Euglena sp. microalgae over zeolite catalysts for the production of bio-oil†
Bo Zhangab,
Qisong Lina,
Qinhui Zhangbc,
Kejing Wua,
Weihua Pua,
Mingde Yanga and
Yulong Wu *ad
*ad
aInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, PR China. E-mail: wylong@tsinghua.edu.cn; Fax: +86 10 69771464; Tel: +86 10 89796163
bCollege of Chemical Engineering, China University of Petroleum, Qingdao 266580, PR China
cCollege of Chemistry and Chemical Engineering, Fujian Normal University, Fuzhou 350117, PR China
dBeijing Engineering Research Center for Biofuels, Beijing 100084, PR China
First published on 30th January 2017
Abstract
In this paper, Euglena sp. microalgae with low lipid and high ash contents were successfully converted into bio-oil with/without catalysts through hydrothermal liquefaction (HTL) at 280 °C and a reaction time of 30 min. The introduction of acidic microporous zeolite catalysts (HZSM-22, HZSM-5, H beta, MCM-22, and SAPO-11) with high hydrothermal stability further improved the bio-oil quality in situ. Various methods, including elemental analysis, high heat value (HHV), and gas chromatography (GC)-mass spectrometry (MS), were used to analyze the physicochemical properties of the obtained bio-oil. Results indicated that catalyst addition could enhance C and H contents, reduce O and N contents, and also improve HHV (the maximum value of 37.08 MJ kg−1 was obtained for the H beta catalyst). GC-MS revealed that the bio-oil obtained by direct HTL contained relatively high amounts of N-containing compounds (30.87%) and acid compounds (16.44%). Meanwhile, the catalysts introduced in situ not only lowered the contents of nitrogen and acids to some extent, but also simultaneously increased the hydrocarbon content. This result was most pronounced over the H beta catalyst, which reduced the nitrogen content to 16.68% and decreased the acid content to 9.50%. The hydrocarbon content increased to 43.43%. Ultimately, a reasonable reaction network for Euglena sp. HTL was proposed and provides a basis for the process's further industrialization.
1. Introduction
The global economy is rapidly developing, the global population is growing, traditional fossil fuels are being aggressively depleted, and environmental pollution and climate change are worsening.1 Hence, a new form of energy, which could supplement or replace traditional fossil fuels, must be urgently developed. Among the substitutes, biofuels obtained from biomass, especially from microalgae, have attracted wide attention in recent decades. This interest was motivated by the microalgae's high photosynthetic efficiency, high area-specific yield relative to terrestrial biomass, noncompetition with cultivated land, and capacity to use exhaust gas and wastewater as nutrients.2 In obtaining biofuels, hydrothermal liquefaction (HTL) was identified as an ideally effective conversion method for microalgae with high moisture. Particularly, HTL not only obtains a high liquid conversion rate but also does not require any drying pretreatment.Compared with conventional fossil energy, the bio-oil obtained from HTL contains high oxygen, nitrogen, and sulfur contents, which are strongly affected by feedstock constituents.3 Therefore, contraposing different feedstocks is necessary in choosing or designing different catalysts to improve the bio-oil quality. At present, two main strategies exist to enhance bio-oil quality. The first strategy involves (1) introduction of catalysts to microalgae HTL to directly acquire high-quality bio-oil (named as “one-pot method”). The second strategy involves (2) initial bio-oil acquisition by HTL followed by the use of catalysts to upgrade the bio-oil quality (designated as “two-step method”).4 The one-pot method holds remarkable advantages, such as simple operational procedures and low energy consumption, over the two-step method.5 Currently, the homogeneous catalysts, such as alkali (Na2CO3 and KOH) and organic acids (formic acid and acetic acid), have been used in microalgae HTL.6 Using different catalysts could help extract bio-oil with different properties. For instance, alkali could effectively promote liquefaction, increase bio-oil yield, and suppress char formation,7 whereas organic acids could improve bio-oil flow properties and decrease the boiling point. Heterogeneous catalysts introduced in situ for microalgae HTL are more desirable than homogeneous catalysts because heterogeneous catalysts are easier to separate from reaction products. Duan and Savage8 studied the effect of several heterogeneous catalysts (Pd/C, Pt/C, Ru/C, Ni/SiO2–Al2O3, and CoMo/γ-Al2O3 (sulfided)) on the catalytic HTL of microalga Nannochloropsis sp. The study showed that when Pd/C catalyst was used without hydrogen, the obtained bio-oil yield increased to 57% relative to that (35%) of direct HTL without catalysts. The bio-oil obtained over these catalysts also exhibited a relatively low viscosity and light color compared with the noncatalyzed products. Another transition metal oxide (NiO) catalyst was also introduced in the HTL of Spirulina platensis microalgae.9 Although the NiO catalyst did not increase the bio-oil yield, the catalyst's addition resulted in the increased selectivity for monoaromatic compounds. Biller et al.10 explored the performance of three catalysts (Pt/Al2O3, Ni/Al2O3, and Co/Mo/Al2O3) in the catalytic HTL of the two microalgae Chlorella vulgaris and Nannochloropsis occulta. The study showed that the bio-oil yields increased slightly over these heterogeneous catalysts. Meanwhile, the high heat value (HHV) and the level of deoxygenation increased up to 10%. Chen et al.11 investigated in detail the influence of acid/base catalysts on the catalytic HTL of D. tertiolecta. The acid/base property of the catalyst played different crucial roles in the catalytic HTL process, especially for the KtB base catalyst, which could obtain the maximum conversion rate (94.84%) and bio-oil yield (49.09%).
Despite the fact that the different catalyst types used, such as zeolite catalysts, exhibited excellent catalytic effects during microalgal HTL, no systematic report other than that of Xu et al.12 exists. In the study, the scholars investigated the effect of HZSM-5 and Ce/HZSM-5 catalysts on C. pyrenoidosa HTL. As a common catalyst, zeolite contains various pore structures, numerous active sites, as well as large surface areas, usually acid sites, which facilitate the catalysis of cracking reaction. Furthermore, the strength and concentration of the acid sites could be tailored to specific applications.13 Nonetheless, the zeolite catalysts with cracking characteristics are mostly used in the bio-oil upgrading process. Widayatno et al.14 investigated high-silica zeolite (HSZ-385, 890, 960, and 990) to selectively catalyze bio-oil conversion. The study showed that all of the zeolite materials possess a high deoxygenation ability for the bio-oil by H2O removal from acid sites. The beta-type zeolite of HSZ-960 showed a high selectivity for hydrocarbons. Duan et al.15 investigated the nine-type zeolite with different Si/Al ratios for the catalytic hydrothermal upgrading of pretreated algal bio-oil with H2 addition. All of the zeolite promoted the deoxygenation and denitrification reaction of the pretreated bio-oil because of the presence of acid sites. In brief, modest research is available on the effects of different zeolites on the catalytic HTL of microalgae, especially the studies that systematically compare and analyze results. Hence, drawing a clear conclusion on the basis of the works published by different groups is difficult.
In this paper, the microalga Euglena sp., which holds the advantage of having both animal and plant characteristics and being highly unmutated, was first used as HTL feedstock. Five different zeolite catalysts (HZSM-22, HZSM-5, H beta, MCM-22, and SAPO-11) were also introduced in the HTL of Euglena sp. in a batch-type reactor. The physicochemical properties of the obtained bio-oil with/without catalysts were studied by elemental analysis (EA), HHV, and gas chromatography (GC)-mass spectrometry (MS) in detail. Meanwhile, a reasonable reaction network for Euglena sp. HTL was also proposed.
2. Experimental
2.1 Materials
The feedstock of Euglena sp. microalgae was purchased from Xi'an Victory Biochemical Co. (Xi'an, China). The feedstock was firstly mashed in agate mortar, followed by sieved at 200 mesh sieve, then dried at 105 °C for 12 h prior to use. The analysis of Euglena sp. feedstock was shown in Table 1. The five zeolite catalysts, including HZSM-22 (SiO2/Al2O3 molar ratio of 90), HZSM-5 (SiO2/Al2O3 molar ratio of 60), H beta (SiO2/Al2O3 molar ratio of 30), MCM-22 (SiO2/Al2O3 molar ratio of 50), SAPO-11 (SiO2/P2O5/Al2O3 = 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were provided by Shanghai Novel chemical technology co., LTD. All other chemicals used were of analytical reagent grade without any purification. The water used was deionized water.
1) were provided by Shanghai Novel chemical technology co., LTD. All other chemicals used were of analytical reagent grade without any purification. The water used was deionized water.
| Analyses | Euglena |
|---|---|
| a ar = as received. daf = dry ash free.b Determined by difference.c Average of duplicates. | |
| Proximate analysis (wt%) | |
| Moisture War | 4.1 |
| Volatiles Var | 55.75 |
| Ash Aar | 29.42 |
| Fixed carbon CFar | 10.73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Biochemical composition analysis (wt%)ar | |
| Carbohydrates | 49.5 |
| Protein | 30.4 |
| Lipids | 11.8 |
| Chlorophyll | 0.34 |
| Otherb | 7.96 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Elemental analysis (wt% daf)c | |
| C | 32.76 |
| H | 5.14 |
| N | 3.81 |
| Ob | 28.87 |
| H/C (molar ratio) | 1.88 |
| O/C (molar ratio) | 0.66 |
| HHV (MJ kg−1) | 13.29 |
2.2 Experimental procedures
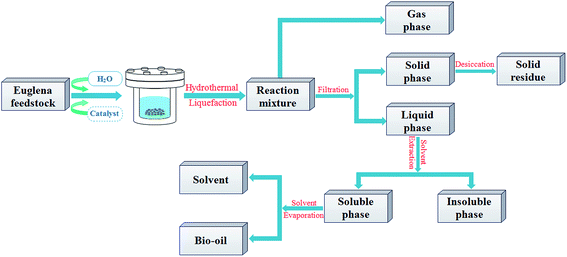
The experiments have been carried out in a non-stirred batch-type stainless steel reactor with a volume capacity of 50 mL and heated by an external electrical furnace, which is designed to a maximum temperature of 500 °C and pressure of 35 MPa. The Euglena sp. processed by HTL were carried out based on 3 g dry microalgae with 30 mL deionized water with/without 10 wt% catalysts (based on dry weight of microalgae). And reactions were carried out at the temperatures of 280 °C with 10 °C min−1 heating rate from room temperature and a constant retention time (30 min), this condition was based on the previously optimized results of process parameters from Euglena sp. HTL. The mixture product of oil–water–solid were obtained after HTL, and then decanted into Buchner funnel. The solid residue was washed by using dichloromethane (DCM) solvent three times (30 mL each time) in the process of the suction filter until the eluent presented colorless, subsequently, the solid residue was transferred into drying oven at 105 °C for 24 h, and weighted to figure out the conversion ratio. Then, the resulting mixture, except the solid residue, was extracted three times using DCM (30 mL each time) and separated in a separating funnel, followed by DCM solvent evaporation by the rotary evaporator to obtain bio-oil and calculate the yield. During the experiment, the gases were not collected and vented in a fume hood when the reactor was opened after the experiment. The HTL experiment without catalyst was used as comparison, which was named as blank in this paper. The compendious process schematic was depicted in Fig. 1.The conversion ratio and the yield of the bio-oil were calculated on the basis of the formulas below, respectively, in which, W was defined as the mass (g).
| CEuglena sp. (wt%) = [1 − (Wsolid residue/Wfeedstock)] × 100% | (1) |
| Ybio-oil (db) (wt%) = (Wbio-oil/Wfeedstock) × 100% | (2) |
2.3 Analytical test instruments
N2 adsorption–desorption isotherms were measured on a Micromeritics ASAP2460 instrument at 77 K. The surface area information of catalysts was calculated using the Brunauer–Emmett–Teller (BET) method. Temperature-programmed desorption of ammonia (NH3-TPD) measurements were carried on a TP-5076 multifunctional automatic adsorption instrument (Xianquan Industry and Trade Development Co., Tianjin, China). Typically, 0.1 g of catalyst was used, the catalyst was pretreated at 450 °C for 1 h under a flow of He (30 mL min−1), then adsorbed NH3 (15 mL min−1) at 100 °C for 30 min, and purged with He until the physically adsorbed NH3 was removed. Later, the catalyst was heated in a flow of He (30 mL min−1) from 100 to 900 °C with a ramp of 5 °C min−1. The signal values of NH3-TPD were recorded by an online thermal conducted detector (TCD). The TGA experiments were carried out in a TA Instruments SDT Q600 thermogravimetric analyzer. 10 mg of sample was heated in 50 mL min−1 of nitrogen atmosphere to 900 °C at 10 °C min−1.The gas chromatography-mass spectrometry (GC-MS) was analyzed on a Trace DSQ GC-MS system with an AB-5MS capillary column (30 m × 0.25 mm id, 0.25 μm film thickness), the high-purity helium was used as carrier gas with a flow rate of 1 mL min−1. The column temperature was programmed from 333 to 573 K at a rate of 10 K min−1 after an initial 2 min isothermal period, and then it was kept at the final temperature for 10 min. The inlet temperature was set to 573 K, and the split ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The mass spectrometer was set to an ionizing voltage of 70 eV with a mass range from 35 to 650 amu. Compounds were identified according to the NIST library.
50. The mass spectrometer was set to an ionizing voltage of 70 eV with a mass range from 35 to 650 amu. Compounds were identified according to the NIST library.
The elemental composition was analyzed using VARIO EL III elemental analyzer to determine the C, H, N content of the samples (including Euglena sp. feedstock and bio-oil) and O determined by difference. For each sample, elemental analysis was carried out in duplicate. Only average values were reported. HHV was calculated via Dulong formula16 which is valuable for comparison across similar bio-oils.
| HHV (MJ kg−1) = 0.3383 × C + 1.442 × (H − O/8) | (3) |
The energy recovery ratio (ERR) proposed by Minowa et al.17 and Yokoyama et al.18 was aimed to study the energy of the resultant products relative to the energy input of the material. The energy recovery of feedstock to oil was calculated based on the following equation
| ERR (%) = (Ep/Efeedstock) × 100% = (Ybio-oil × HHVbio-oil)/HHVfeedstock × 100% | (4) |
3. Results and discussion
3.1 Catalytic HTL of Euglena sp.
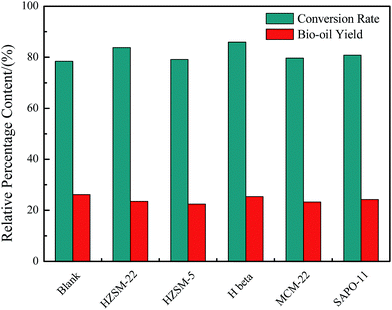
The proximate analysis, biochemical composition analysis, and elemental compositions of Euglena sp. feedstock are presented in Table 1. The main biochemical compositions were carbohydrates (49.5%), protein (30.4%), and lipids (11.8%). Meanwhile, the ash content was substantially high and reached 29.42%. These results illustrate that the Euglena sp. feedstock belonged to microalgae with low lipid and high ash contents. EA revealed that the contents of C, H, N, and O of the Euglena sp. feedstock were 32.76%, 5.14%, 3.81%, and 28.87%, respectively. The HHV was 13.29 MJ kg−1 as calculated by the Dulong formula. Microalgae, such as Euglena sp., are greatly suitable to the HTL process because the whole feedstock could be entirely converted, and different biochemical components could exhibit different transformation processes under HTL.The conversion ratio of the Euglena sp. feedstock and the bio-oil yield with/without catalysts from HTL at 280 °C for 30 min are displayed in Fig. 2. All the conversion ratios rose when the catalysts of HZSM-22, HZSM-5, H beta, MCM-22, and SAPO-11 were introduced to the HTL process. The ratios were 83.82%, 79.1%, 85.89%, 79.62%, and 80.81%, respectively, relative to the blank, which attained a conversion ratio of 78.39%. This finding indicates that the catalysts introduced were slightly beneficial in enhancing the conversion ratio of Euglena sp. Meanwhile, the conversion ratios were in the order H beta > HZSM-22 > SAPO-11 > MCM-22 > HZSM-5, which generally conformed to the order of decreasing amount of acidity sites listed in Table 2. Hence, the acid sites in the catalysts helped convert the biomass. By contrast, the bio-oil yields presented an opposite tendency when catalysts were added, that is, 23.54%, 22.45%, 25.41%, 23.23%, and 24.3% for HZSM-22, HZSM-5, H beta, MCM-22, and SAPO-11, respectively, and 26.13% for the blank. This result might be explained by the different acidic properties of the catalysts. The different acidic properties may have promoted the occurrence of cracking reaction during conversion and slightly decreased the bio-oil yield. Although the bio-oil yield in the presence of catalysts was reduced in different degrees, the obtained constituent for each bio-oil showed obvious beneficial changes. The following points discuss the detailed analysis achieved by EA and GC-MS. As already reported, the reduced bio-oil yield could be compensated by the higher bio-oil quality (e.g., lower nitrogen and oxygen contents; higher HHV).19 The total acidity and acid strength distribution of the catalysts have been listed, and all the catalysts possessed both weak and strong acid sites (Table 2). Usually, the weak acid sites were assigned as silanol groups at the external surface or at lattice defects, and OH groups bonded to the extra-framework aluminum species. Meanwhile, the strong acid sites generally resulted from the framework tetrahedral aluminum species.15,20 The H beta catalyst exhibited the highest total acidity sites and the strongest acid sites, which were 0.19 and 0.35, respectively. Meanwhile, during conversion, the H beta catalyst also showed the highest conversion ratio relative to those of the other catalyst and a lower bio-oil yield with respect to that of the blank. In addition, the large surface area (582 m2 g−1) of the H beta catalyst facilitated the reaction of additional organic molecules.
| Catalyst | Surface area/m2 g−1 | Weak acid sites/unit | Strong acid sites/unit | Total acid/unit |
|---|---|---|---|---|
| HZSM-22 | 150 | 0.12 | 0.16 | 0.29 |
| HZSM-5 | 354 | 0.13 | 0.12 | 0.25 |
| H Beta | 582 | 0.15 | 0.19 | 0.35 |
| MCM-22 | 347 | 0.07 | 0.14 | 0.22 |
| SAPO-11 | 262 | 0.09 | 0.17 | 0.27 |
Table 3 displays the elemental composition, HHV, and the corresponding energy recovery ratio (ERR) of the obtained bio-oils. These parameters are of great importance to study of bio-oil quality. The contents of C (73.59%) and H (7.90%) of the blank bio-oil were much higher than those of Euglena sp. Meanwhile, the content of O (12.77%) significantly declined with respect to that of Euglena sp. (28.87%). This result illustrates that a higher energy density of bio-oil could be obtained from Euglena sp. feedstock by HTL treatment. By contrast, the N content of bio-oil (5.74%) showed an increasing trend compared with that of Euglena sp. (3.81%). This result was attained because more N entered the oil phase than those that entered the aqueous and gas phases through the interactions between amino acid (protein hydrolysate) and other hydrolysis products from lipid and carbohydrate. Concurrently, the HHV (33.99 MJ kg−1) and ERR (66.83%) rose relative to those of Euglena sp. The finding suggests that the majority of the energy content of Euglena sp. entered the bio-oil. With catalyst introduction, the contents of C and H increased distinctly, whereas the contents of O and N declined. The HHV further rose, and the order of HHV of the obtained bio-oils was consistent with that of the amount of acidity sites of the corresponding catalysts and the conversion ratios. This consistency implies that the catalytic conversion helped improve the bio-oil quality. Despite the nonprominence of O content variation with catalyst addition (except H beta, O content lowered ca. 20%) compared with that of the blank, the types of oxygen-containing compounds changed obviously. Desirable components with good flammability and suitability for fuels, such as hydrocarbons and esters, increased significantly. Meanwhile, undesirable components, such as carboxylic acid and N-containing compounds, decreased remarkably. Among these catalysts, H beta showed the optimum catalytic activity as the bio-oil contained the most contents of C (76.09%) and H (9.14%) and the lowest content of O (10.21%) and N (4.56%) relative to others. Besides, the bio-oil obtained when H beta was used as catalyst also attained a maximum HHV of 37.08 MJ kg−1. This value approached that of petroleum (43 MJ kg−1).21 The above-mentioned results imply that the H beta catalyst played a favorable role in improving the bio-oil quality in deoxygenation and denitrification.
| Elemental analysis (wt% daf) | Bio-oil | |||||
|---|---|---|---|---|---|---|
| Blank | HZSM-22 | HZSM-5 | H beta | MCM-22 | SAPO-11 | |
| a daf = dry ash free.b Determined by difference. | ||||||
| C | 73.59 | 74.72 | 73.64 | 76.09 | 73.7 | 74.31 |
| H | 7.90 | 8.34 | 8.09 | 9.14 | 7.97 | 8.26 |
| Ob | 12.77 | 12.00 | 12.74 | 10.21 | 12.67 | 12.18 |
| N | 5.74 | 4.94 | 5.53 | 4.56 | 5.66 | 5.25 |
| H/C (molar ratio) | 1.29 | 1.34 | 1.32 | 1.44 | 1.30 | 1.33 |
| O/C (molar ratio) | 0.13 | 0.12 | 0.13 | 0.10 | 0.13 | 0.12 |
| N/C (molar ratio) | 0.07 | 0.06 | 0.06 | 0.05 | 0.07 | 0.06 |
| Empirical formula | CH1.29O0.13N0.07 | CH1.34O0.12N0.06 | CH1.32O0.13N0.06 | CH1.44O0.10N0.05 | CH1.30O0.13N0.07 | CH1.33O0.12N0.06 |
| HHV (MJ kg−1) | 33.99 | 35.13 | 34.28 | 37.08 | 34.14 | 34.86 |
| ERR | 66.83 | 62.23 | 57.91 | 70.89 | 59.67 | 63.74 |
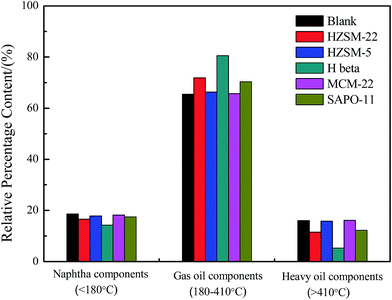
To determine the boiling-point distribution of the obtained bio-oil from Euglena sp., a simulated method of distillation was carried out by TGA.5,11,22 The boiling points of the major components of the obtained bio-oils lay within the temperature range of 180 °C to 410 °C (Fig. 3), similar to the boiling point range of the gas oil components. Meanwhile, the other components that correspond to naphtha oil and heavy oil in the boiling range were relatively less in amount. Moreover, the presence of HZSM-22, H beta, and SAPO-11 catalysts distinctly altered the boiling-point distribution of the bio-oils. In particular, the contents of the gas oil components were further enhanced, whereas the other contents decreased. Especially, when the H beta was used as catalyst, the content of gas oil components reached 80.53%, simultaneously, and the heavy oil content decreased to 5.27%. This aspect indicated that H beta with high acid amount, as well as large surface area, could promote the cracking of additional macromolecules in the heavy oil components.
The GC-MS was used in this paper to identify the main components of the obtained bio-oil. Notably, the present percentage area values in this paper only illustrate the relative content of each organics in the bio-oil vaporized and passed through the GC column. The constituents of the bio-oils from Euglena sp. HTL with/without catalyst were analyzed, and the major compositions are listed in Table S1 (ESI†). To study the differences of each bio-oil, the components were categorized into N-containing compounds, phenols, ketones, hydrocarbons, alcohols, esters, and acids on the basis of the functional groups (Fig. 4). The bio-oil constituents obtained from direct HTL were N-containing compounds (30.87%), phenols (3.55%), ketones (15.28%), hydrocarbons (26.25%), alcohols (7.61%), and acids (16.44%), respectively. We conclude that the introduction of these five catalysts changed the components of the bio-oil to some extent (ESI Table S1†). The N-containing compounds, which reduced obviously compared with that of the bio-oil obtained in the blank, indicate that the catalysts with different acidities positively affected the denitrification of the HTL process. N-Containing compounds of pyrroles and pyridine were partly eliminated, and indole and their derivatives were removed completely. Meanwhile, L-proline-derived compound was also fully converted over the added catalysts. The catalysts added likely inhibited the Maillard reaction between the amino acids and reduced sugars or the self-condensation reaction of the amino acids to some degree.
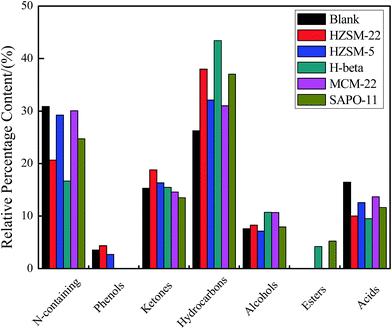 | ||
| Fig. 4 The major component content from the bio-oil obtained from Euglena HTL with/without catalysts. | ||
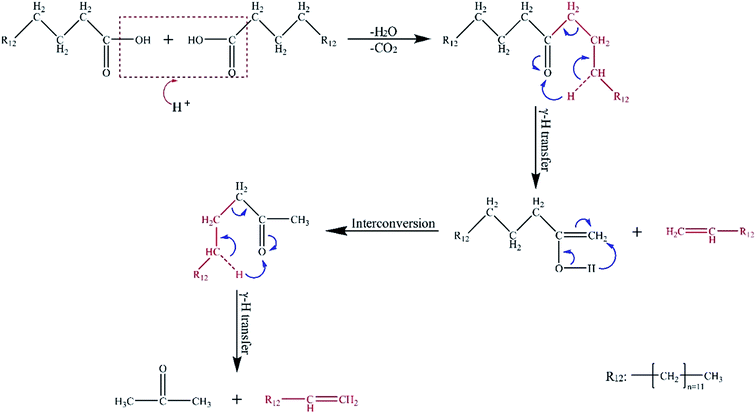
After the catalysts were added, the contents of acids, including tridecanoic acid, tetradecanoic acid, and hexadecanoic acid, showed a downward trend. However, the content of hydrocarbons, especially 1-tetradecene, presented a remarkable rising trend. These results were attributed to the inter-reaction of the two molecules of hexadecanoic acid. The possible reaction paths are described in Fig. 5. First, condensation reaction occurs between the two molecules of hexadecanoic acid derived from the hydrolysis of lipids. A molecule each of H2O and CO2 is removed in the acidic condition. Then, symmetrical ketone intermediates are formed. Finally, the intermediates become the terminal olefins and enol through the γ-H transfer reaction. Given its instability, the enol molecule is easily converted into methyl ketones. Then, a γ-H transfer reaction transpires to generate other terminal olefins and acetone.23,24
During direct HTL, the existence of 1-tetradecene resulted from the increased ion product that led to the increased natural levels of hydronium ions in hot-compressed water at 280 °C. These conditions could result in acid-catalyzed reactions.5 The introduction of catalysts with different acidities would further accelerate the rates of the acid-catalyzed reactions and cause the 1-tetradecene content to rise again. Additionally, the alcohol content of the bio-oils with different catalysts increased slightly relative to that of the blank. The ketone content also slightly rose except in those with MCM-22 and SAPO-11. Phenols presented in the bio-oils of the blank sample and with HZSM-22 and HZSM-5 catalysts only. A certain amount of esters emerged under the effects of H beta and SAPO-11. By contrast, among the five catalysts, H beta exhibited excellent catalytic properties. The obtained bio-oil consisted of 43.43% hydrocarbons, 10.71% alcohols, 15.49% ketones, 4.20% esters, 16.68% N-containing compounds, and 9.50% acids. Furthermore, the hexadecanoic acid was absolutely converted in the presence of H beta. These findings show that the H beta catalyst not only exerts a strong denitrification effect but also holds an outstanding conversion ability for acids. Meanwhile, the existing 1-tetradecanol may be attributed to the hydration reaction of a portion of the 1-tetradecene with water over H beta in the HTL process.25,26 Our purpose is to confirm which zeolite would be effective specifically for Euglena sp. HTL. Hence, we did not consider the problems of catalyst recyclability. Future work should focus on the recycle and reuse of catalysts after application in industrialized microalgal HTL.
3.2 Reaction mechanism of the main hydrocarbon component
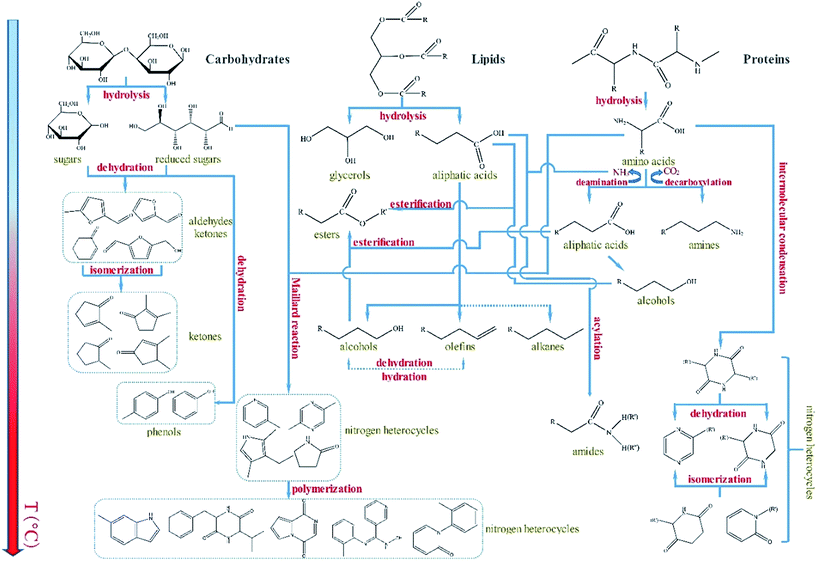
Currently, many studies investigate the reaction mechanism of HTL using different microalgae as feedstock.27–29 The reaction mechanism for specific microalgae may help in the control of the properties of the obtained bio-oils. This information may guide the modulation of the process conditions for HTL and the use different catalyst types. However, clarifying each reaction step is highly difficult because of the complexity of the bio-oil composition and the limited vaporization temperature of GC-MS. The components of bio-oils could not be completely detected, especially the high-boiling-point compounds.4 Hence, only a portion of the compounds confirmed by GC-MS may be representative to reasonably speculate the overall possible reaction paths.The general reaction network for the HTL of Euglena sp. was plausibly speculated mainly on the basis of GC-MS experimental results as well as previous published studies (Fig. 6). In the initial stage, the bio-macromolecules, such as carbohydrates, lipids, and proteins, were hydrolyzed with water and generated their corresponding monomer products and small molecular compounds, including monosaccharides, aliphatic acids, and amino acids.19,30,31 With increasing temperature, part of the monosaccharides produced furfural, furfural derivatives, or ketones through dehydration and isomerization. Such furfurals and furfural derivatives were determined by GC-MS in the bio-oils obtained by HTL at low temperatures (<200 °C). Meanwhile, the aliphatic acids generated from the lipid hydrolysis could produce hydrocarbons and alcohols through a series of reactions, including condensation, decomposition, and deoxygenation. For the amino acids, deamination and decarboxylation were concurrent, and the corresponding products were NH3, aliphatic acids and CO2, and amines. A part of NH3 further reacted with the aliphatic acids derived from lipids to form amides. The rest of NH3, as well as CO2, entered into the gas phase. Meanwhile, the resultant amino acids also underwent intermolecular condensation, followed by dehydration and isomerization, to generate N-heterocycles. In the reaction system, the esters were mainly formed by two reaction pathways. In the first pathway, aliphatic acids were derived from the hydrolysis of lipids esterified with the alcohols obtained from aliphatic acid hydrolysis. In the second pathway, the alcohols were produced from the conversion of aliphatic acids obtained from the hydrolysis of lipids esterified with the aliphatic acids derived from deamination during protein hydrolysis. Another important and complicated reaction, named as Maillard reaction,32,33 occurred between reduced sugars and amino acids. The reaction chiefly generated N-heterocycles, such as pyridine, pyrazine, pyrrole, indole, pyridinone, pyrrolidinone, and their derivatives. As mentioned above, the catalysts added not only modified the progress of the reactions and the distribution of the bio-oil products but also eventually improved the quality of the obtained bio-oil in the entire HTL process of Euglena sp.
4. Conclusions
Euglena sp. microalgae were first converted to bio-oil with a relatively high HHV (33.99 MJ kg−1) via HTL without catalysts at 280 °C for 30 min. The results illustrated that Euglena sp. is a suitable feedstock for bio-oil production. Five zeolite catalysts with different acidities were also introduced to improve the bio-oil quality. Among these catalysts, H beta exhibited the best catalytic performance. Specifically, the contents of N-containing compounds and carboxylic acids were reduced significantly to 16.68% and 9.50%, respectively, and the content of hydrocarbons remarkably increased to 43.43%. The formation mechanism of the main hydrocarbon component 1-tetradecene was proposed, and a general reaction network for Euglena sp. HTL was plausibly speculated.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21576155, 21376140 and 21376136), the Research Project of Guangdong Provincial Department of Science and Technology Department (No. 2015B020215004), the Program for Changjiang Scholars and the Innovative Research Team in University (IRT13026) and Program for New Century Excellent Talents in University (No. NCET-12-0308).References
- IPCC, Climate Change 2013: The Physical Science Basis, Cambridge University Press, Cambridge, 2013 Search PubMed.
- P. Biller, C. Friedman and A. B. Ross, Bioresour. Technol., 2013, 136, 188–195 CrossRef CAS PubMed.
- Y. Chen, Y. L. Wu, D. R. Hua, C. Li, M. P. Harold, J. L. Wang and M. D. Yang, RSC Adv., 2015, 5, 18673–18701 RSC.
- D. L. Barreiro, B. R. Gomez, F. Ronsse, U. Hornung, A. Kruse and W. Prins, Fuel Process. Technol., 2016, 148, 117–127 CrossRef.
- T. M. Yeh, J. G. Dickinson, A. Franck, S. Linic, L. T. Thompson and P. E. Savage, J. Chem. Technol. Biotechnol., 2013, 88, 13–24 CrossRef CAS.
- A. B. Ross, P. Biller, M. L. Kubacki, H. Li, A. Lea-Langton and J. M. Jones, Fuel, 2010, 89, 2234–2243 CrossRef CAS.
- D. Zhou, L. A. Zhang, S. C. Zhang, H. B. Fu and J. M. Chen, Energy Fuels, 2010, 24, 4054–4061 CrossRef CAS.
- P. G. Duan and P. E. Savage, Ind. Eng. Chem. Res., 2011, 50, 52–61 CrossRef CAS.
- U. Jena, K. C. Das and J. R. Kastner, Appl. Energy, 2012, 98, 368–375 CrossRef CAS.
- P. Biller, R. Riley and A. B. Ross, Bioresour. Technol., 2011, 102, 4841–4848 CrossRef CAS PubMed.
- Y. Chen, Y. L. Wu, R. R. Ding, P. Zhang, J. Liu, M. D. Yang and P. Zhang, AIChE J., 2015, 61, 1118–1128 CrossRef CAS.
- Y. F. Xu, X. J. Zheng, H. Q. Yu and X. G. Hu, Bioresour. Technol., 2014, 156, 1–5 CrossRef CAS PubMed.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- W. B. Widayatno, G. Q. Guan, J. Rizkiana, X. Du, X. G. Hao, Z. L. Zhang and A. Abudula, Bioresour. Technol., 2015, 179, 518–523 CrossRef CAS PubMed.
- P. Duan, Y. Xu, F. Wang, B. Wang and W. Yan, Biochem. Eng. J., 2015, 116, 105–112 CrossRef.
- H. J. Huang, X. Z. Yuan, G. M. Zeng, J. Y. Wang, H. Li, C. F. Zhou, X. K. Pei, Q. A. You and L. A. Chen, Fuel Process. Technol., 2011, 92, 147–153 CrossRef CAS.
- T. Minowa, T. Kondo and S. T. Sudirjo, Biomass Bioenergy, 1998, 14, 517–524 CrossRef CAS.
- S. Yokoyama, A. Suzuki, M. Murakami, T. Ogi, K. Koguchi and E. Nakamura, Fuel, 1987, 66, 1150–1155 CrossRef CAS.
- J. X. Zhang, W. T. Chen, P. Zhang, Z. Y. Luo and Y. H. Zhang, Bioresour. Technol., 2013, 133, 389–397 CrossRef CAS PubMed.
- Q. Wang, Z. M. Cui, C. Y. Cao and W. G. Song, J. Phys. Chem. C, 2011, 115, 24987–24992 CAS.
- T. M. Brown, P. G. Duan and P. E. Savage, Energy Fuels, 2010, 24, 3639–3646 CrossRef CAS.
- C. Jazrawi, P. Biller, Y. Y. He, A. Montoya, A. B. Ross, T. Maschmeyer and B. S. Haynes, Algal Res., 2015, 8, 15–22 CrossRef.
- A. Leung, D. G. B. Boocock and S. K. Konar, Energy Fuels, 1995, 9, 913–920 CrossRef CAS.
- E. Vonghia, D. G. B. Boocock, S. K. Konar and A. Leung, Energy Fuels, 1995, 9, 1090–1096 CrossRef CAS.
- K. Eguchi, T. Tokiai and H. Arai, Appl. Catal., 1987, 34, 275–287 CrossRef CAS.
- H. Wang, T. Huang, J. Du and Z. M. Liu, Chin. J. Catal., 2005, 26, 451–452 CAS.
- C. Gai, Y. H. Zhang, W. T. Chen, P. Zhang and Y. P. Dong, Energy Convers. Manage., 2015, 96, 330–339 CrossRef CAS.
- D. L. Barreiro, M. Beck, U. Hornung, F. Ronsse, A. Kruse and W. Prins, Algal Res., 2015, 11, 234–241 CrossRef.
- W. Costanzo, R. Hilten, U. Jena, K. C. Das and J. R. Kastner, Algal Res., 2016, 13, 53–68 CrossRef.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef CAS.
- S. D. Yin, R. Dolan, M. Harris and Z. C. Tan, Bioresour. Technol., 2010, 101, 3657–3664 CrossRef CAS PubMed.
- C. Torri, L. G. Alba, C. Samori, D. Fabbri and D. W. F. Brilman, Energy Fuels, 2012, 26, 658–671 CrossRef CAS.
- L. G. Alba, C. Torri, C. Samori, J. van der Spek, D. Fabbri, S. R. A. Kersten and D. W. F. Brilman, Energy Fuels, 2012, 26, 642–657 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28747f |
| This journal is © The Royal Society of Chemistry 2017 |