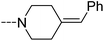
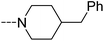
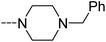
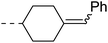
Open Access Article
Open Access ArticleDesign, synthesis, and biological evaluation of oxazolidone derivatives as highly potent N-acylethanolamine acid amidase (NAAA) inhibitors†
Jie Rena,
Yuhang Li*ab,
Hongwei Kec,
Yanting Lia,
Longhe Yangd,
Helin Yua,
Rui Huanga,
Canzhong Lub and
Yan Qiu*a
aMedical College, Xiamen University, Xiamen, Fujian 361102, P. R. China. E-mail: yuhangli@fjirsm.ac.cn; yanqiu@xmu.edu.cn
bXiamen Institute of Rare-earth Materials, Haixi Institutes, Chinese Academy of Sciences, Fujian 361005, P. R. China
cCollege of Ocean and Earth Science, Xiamen University, Xiamen, Fujian 361005, P. R. China
dEngineering Research Center of Marine Biological Resource Comprehensive Utilization, Third Institute of Oceanography, State Oceanic Administration, Xiamen 361005, P. R. China
First published on 21st February 2017
Abstract
N-Acylethanolamine-hydrolyzing acid amidase (NAAA) is a lysosomal enzyme that catalyzes the hydrolysis of endogenous fatty acid ethanolamides (FAEs), such as N-palmitoylethanolamide (PEA). PEA exhibits anti-inflammatory and analgesic activities by engaging peroxisome proliferator-activated receptor α (PPAR-α). Preventing PEA degradation by inhibition of NAAA has been proposed as a novel strategy for the treatment of inflammation and pain. In the present study, we reported the discovery of the oxazolidone derivative as a novel scaffold for NAAA inhibitors, and studied the structure–activity relationship (SAR) by modification of the side chain and terminal lipophilic substituents. The results showed that the link chain length of C5, straight and saturated linkages were the preferred shape patterns for NAAA inhibition. Several nanomolar NAAA inhibitors were described, including 2f, 3h, 3i and 3j with IC50 values of 270 nM, 150 nM, 100 nM and 190 nM, respectively. Enzymatic degradation studies suggested that 2f inhibited NAAA in a selective, noncompetitive and reversible pattern. Moreover, 2f showed high anti-inflammatory and analgesic activities after systemic and oral administration.
1 Introduction
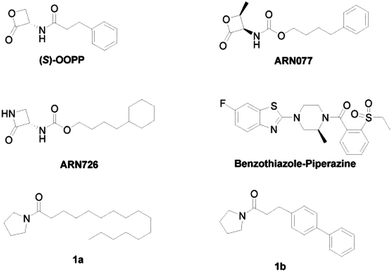
N-Acylethanolamine-hydrolyzing acid amidase (NAAA) is a lysosomal cysteine hydrolase widely expressed in many tissues, especially those associated with immune responses, such as the lungs, spleen, and small intestine. NAAA participates in the control of inflammatory and pain1 via involving in the hydrolysis of endogenous palmitoylethanolamide (PEA). PEA is a multifunctional lipid mediator that has been shown to inhibit peripheral inflammation2–4 and cell degranulation, and exhibits antinociceptive activities in rat and mouse models of acute and chronic pain.5–7 Moreover, PEA also could alleviate skin inflammation8 and neuropathic pain in humans.9 Those effects are mainly attribute to the ability of PEA to engage nuclear receptor peroxisome proliferator-activated receptor-alpha (PPAR-α). In recent years, PEA has attracted increasing attention because of its potential therapeutic effects in inflammation and pain. Preventing PEA degradation by inhibition of NAAA10 may provide a novel strategy for the treatment of inflammatory and pain.4,11 For this reason, NAAA has been pursued as a potential drug target.Despite the increasing interest in the design of NAAA inhibitors as anti-inflammatory and analgesic agents, only few potent NAAA inhibitors have been identified so far.12,13 There are two main categories of NAAA inhibitors reported, irreversible covalent inhibitors, such as β-lactone derivatives14–18 and non-covalent reversible inhibitors, such as benzothiazole-piperazine derivatives.19 Although covalent inhibitors, such as (S)-OOPP,4 ARN077,11 ARN726 (ref. 20) (Fig. 1) demonstrated profound anti-inflammatory effects in several animal models, their application is limited due to the poor chemical and plasma stability. These compounds degraded in phosphate buffered saline (PBS) within 120 min and were rapidly cleaved in plasma.16 Non-covalent reversible inhibitors, such as, benzothiazole-piperazine derivatives (Fig. 1),19 are selective, reversible, and competitive NAAA inhibitors. However, only few potent reversible NAAA inhibitors with drug-like properties have been identified so far. Highly potent, stable and reversible NAAA inhibitors are still highly desired for the treatment of inflammation and pain.
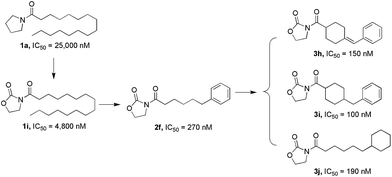
In our previous study, we designed pyrrolidine-based derivatives 1a and 1b (Fig. 1)21 as NAAA inhibitors. Substitution of the saturated alkyl chain of 1a with an aryl-containing lipophilic moiety leaded to a potent NAAA inhibitor 1b (IC50 = 2.1 μM). In vivo pharmacological studies indicated that 1b reduced the mRNA expression levels of inducible nitric oxide synthase and interleukin-6, as well as increased the intracellular PEA levels in mouse macrophages with lipopolysaccharide-induced inflammation.21 Moreover, 1b exhibited excellent chemical stability and plasma stability (t½ > 16 h).
In the present study, we further modified 1a to obtain more potent and biologically stable NAAA inhibitors. A nanomolar NAAA inhibitor 2f was discovered (IC50 = 270 nM), which exerted profound anti-inflammatory and anti-nociceptive effects in rats.22 We then carried out structure–activity relationship (SAR) studies on 2f by modification of the pyrrolidine ring and the lipophilic chain, which led to the identification of several more potent nanomolar NAAA inhibitors, such as 3h, 3i and 3j (Fig. 2). Compound 2f was then selected as a model molecule to study the NAAA inhibition mechanisms and in vivo anti-inflammatory and analgesic activities of oxazolidone derivatives. The results showed that 2f inhibited NAAA in a selective and highly reversible pattern, and efficiently reduced inflammatory and pain after systemic and oral administration. Oral administration of 2f (30 mg kg−1) exhibited the same efficacy with commercial nonsteroidal anti-inflammatory drug ibuprofen at a 6.7-fold higher dose (200 mg kg−1).
2 Results and discussion
2.1 Synthesis of oxazolidone derivatives
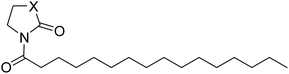
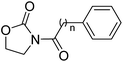
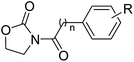
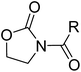
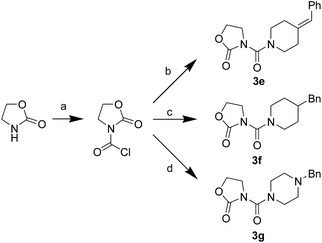
First, we synthesized a series of oxazolidone derivatives with different side chains and terminal lipophilic substituents. Compounds 1a, 1j–1o, and 1q–1t were prepared by the amidation of palmitoyl chloride with the corresponding amine in the presence of triethylamine (Scheme 1).21 1l and 1m were prepared by the methylation of 1q and 1r with iodomethane in the presence of sodium hydride, respectively (Scheme 2).23 1p was prepared by Dess–Martin periodinane oxidation of 1j or 1k (Scheme 3).24 1c and 1d were prepared by the acidic deprotection of pyrrolidine derivatives 1c-1 and 1d-1, which were obtained by the amidation of (R)- or (S)-3-(tert-butoxycarbonylamino)pyrrolidine with palmitoyl chloride, respectively (Scheme 4).25,26 1e and 1f were synthesized by a two-step reaction starting from 1q and 1r, which were converted to iodide derivatives by I2 and PPh3, respectively, followed by hydrogenation with H2 (Scheme 5).27,28 1g, 2a–2h, 3a–3d and 3k–3s were synthesized by the imidate reaction, employing the corresponding acyl chloride with 2-pyrrolidone, 2-imidazolidone, or 2-oxazolidone in the presence of n-BuLi, respectively (Scheme 6).29 3e–3g were synthesized by the amidation of 2-oxooxazolidine-3-carbonyl chloride, generated by reaction of 2-oxazolidone and bis(trichloromethyl) carbonate, with the corresponding substituted piperazine or piperidine (Scheme 7).30 HPLC analysis indicated >99% purity for compound 1g, 1e–1i, 2b, 2d, 2f–2h, 3c, 3e, and 3g, >98% purity for 1c–1d, 1f, 2a, 2e, 3a, 3b and 3g–3i, and >97% purity for 2c and 3d (Table S3†).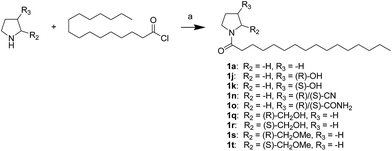 | ||
| Scheme 1 The synthesis of compounds 1a, 1j, 1k, 1n, 1o, and 1q–1t. Reagents and conditions: (a) Et3N, CH2Cl2, room temperature. | ||
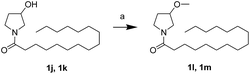 | ||
| Scheme 2 The synthesis of compounds 1l and 1m. Reagents and conditions: (a) NaH, MeI, tetrahydrofuran (THF), 0 °C. | ||
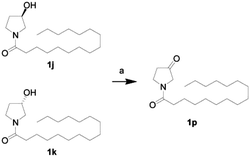 | ||
| Scheme 3 The synthesis of compound 1p. Reagents and conditions: (a) Dess–Martin periodinane, CH2Cl2, room temperature. | ||
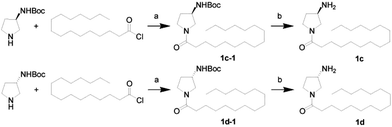 | ||
| Scheme 4 The synthesis of compounds 1c and 1d. Reagents and conditions: (a) Et3N, CH2Cl2, room temperature; (b) HCl, dioxane, 0 °C. | ||
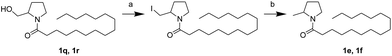 | ||
| Scheme 5 The synthesis of compounds 1e and 1f. Reagents and conditions: (a) PPH3, I2, THF, room temperature; (b) H2, Pd/C, CH3OH, room temperature. | ||
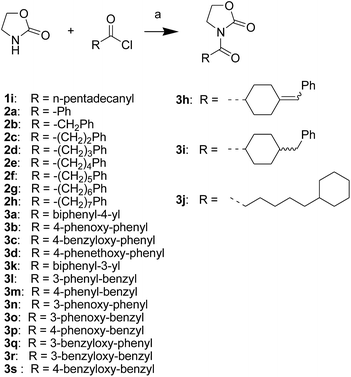 | ||
| Scheme 6 The synthesis of compounds 1i, 2a–2h, 3a–3d and 3h–3s. Reagents and conditions: (a) n-BuLi, THF, −78 °C. | ||
2.2 NAAA inhibition by oxazolidone derivatives
The NAAA inhibition capability of these newly synthesized compounds were tested using heptadecenoylethanolamide as a substrate. Since many NAAA inhibitors also block fatty acid amide hydrolase (FAAH), the FAAH inhibition of these compounds also was examined to obtain NAAA selective inhibitors. (S)-OOPP and well known FAAH inhibitor URB597 were used as standard compounds in the enzymatic assay. The results showed that substitution at 3-position of pyrrolidine with an amino group (1c, 1d) slightly increased NAAA and FAAH inhibition, and substitutions at the 2-position with (S)-methyl (1f) or carbonyl (1g) slightly or moderately enhanced inhibition towards NAAA without changing inhibition against FAAH. However, (R)-methyl (1e), hydroxy (1j, 1k), methoxy (1l, 1m), cyano (1n), formamide (1o), hydroxymethyl (1q, 1r) and methoxymethyl (1s, 1t) substituents diminished the NAAA and FAAH inhibitory effects (Table S1†). 1g exhibited low-micromolar inhibition on NAAA activity (IC50 = 4.6 ± 0.48 μM), and it was over 5-fold more potent towards NAAA than FAAH (Table 1). To further optimize the hydrophilic moieties, a series of 1g isosteres were synthesized and tested (1h, 1i) (Table 2). Enzymatic assay results showed that 1i with oxazolidone ring had similar effects on NAAA inhibition as 1g, but 1i exhibited dramatically enhanced selectivity towards NAAA. Less than 10% FAAH activity was inhibited by 1i even at a concentration of 100 μM. Therefore, 2-oxazolidone ring was selected as the template for further optimization.It has been reported that substitutions of the saturated alkyl chain with aryl-containing lipophilic moieties could enhance the inhibitory potency on NAAA and improved the drug-like properties.14,21 Therefore, a series of oxazolidone derivatives incorporating a phenyl ring at the terminus were synthesized and tested. As shown in Table 3, 2a with the chain length of C0 exhibited low-micromolar inhibitory potency toward NAAA (IC50 = 3.4 ± 0.42 μM), while 2f with the chain length of C5 exhibited excellent potency (IC50 = 0.27 ± 0.04 μM), 3-fold more potent than (S)-OOPP tested under the same experimental conditions. Interestingly, sharply reduced or even abolished activity was observed as the chain length decreased from C5 to C1 or increased from C5 to C7. We are not quite sure why their potency dramatically changed when we varied the link chain length, probably because these oxazolidone imides with different chain length are activated to different extent when contacting the NAAA.
To further optimize the NAAA inhibition activity of 2f, we replaced the rotatable bond of the acyl chain of 2f with an additional phenyl group. As shown in Table 4 and S2,† a series of analogs corresponding to biphenyl (3a, 3k–3m), phenoxyphenyl (3b, 3n–3p), and benzyloxyphenyl (3c, 3q–3s) were obtained and examined. Compared to 2a, 1,4-substituted phenoxyphenyl (3b, IC50 = 0.68 ± 0.11 μM) and benzyloxyphenyl (3c, IC50 = 2.3 ± 0.36 μM) analogs were approximately 10-fold and 2-fold more potent. However, 1,4-substituted bipheny analog 3a (IC50 = 9.4 ± 1.2 μM) was 2-fold less potent (Table 4), and 1,3-substituted isomers (3k, 3l, 3n, 3o, 3q, 3r) were completely inert for NAAA inhibition (Table S2†). The results indicated that straight structures are preferred shape for the hydrophobic channel of NAAA, which is in agreement with previous reports.14,17 The binding pocket of NAAA proximal to the carbonyl moieties seemed to be narrow, as such, only a phenyl moiety is tolerated at this region. Therefore, incorporation of an extra methylene group between the biaryl and carbonyl groups (3m, 3p and 3s) significantly decreased NAAA inhibition.
It's worth noting that the position of the terminal phenyl groups in biaryl series compounds (3a–3d) is corresponding to the location of phenyl moieties in the phenylalkyl series compounds (2e–2h). Increasing the length of the terminal aryl group of 3a by insertion of an oxygen atom or a methyleneoxy unit between the phenyl rings led to 14-fold (3b) or 4-fold (3c) higher potency, whereas the insertion of an additional methylene unit to the aliphatic linker of 3c significantly decreased activity (3d). These results highlighted the importance of the optimal length and connecting pattern of the aryl-containing lipophilic moieties.
It has been reported that NAAA prefer saturated substrates e.g., N-myristoylethanolamine and PEA, rather than unsaturated substrates, e.g., AEA.1 Therefore, we studied whether the replacement of a phenyl ring with a 6-member ring can improve the NAAA inhibition. A series of additional derivatives of 2f were then synthesized and examined (Table 5). The results showed that introducing hexane ring further enhanced the NAAA inhibitory potency. We found two highly potent NAAA inhibitors 3h and 3i with a IC50 value of 150 nM and 100 nM, respectively, around 2-fold more potent than 2f. Moreover, saturated linkages were found to be more beneficial for the activity than unsaturated benzene ring (3h, 3i vs. 3a–3c; 3j vs. 2f). However, nitrogen atoms substitution of the saturated hexane significantly reduced the activity (3e–3g).
2.3 NAAA inhibition pattern
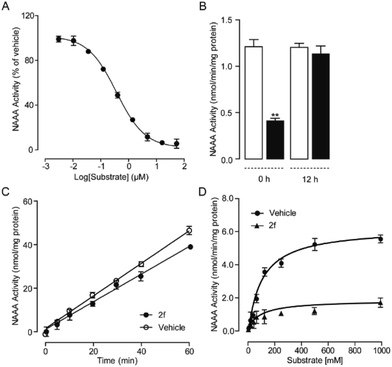
To further understand the NAAA inhibition pattern of these oxazolidone derivatives, compound 2f was selected as a model molecule and its interaction mode with NAAA protein in cells was studied. The results showed that 2f displayed a profound inhibition toward recombinant human NAAA (IC50 = 0.27 ± 0.04 μM, Fig. S1A†) and recombinant rat NAAA in NAAA-transfected HEK293 cells (IC50 = 0.42 ± 0.04 μM, Fig. S1B†), which were similar to the data obtained with the NAAA protein extract. Dialysis (Fig. 3B) and rapid dilution (Fig. 3C) of the 2f–NAAA interaction complex almost completely restored the NAAA activity. Moreover, Michaelis–Menten kinetic analysis revealed that 2f induced changes in the maximal catalytic velocity (Vmax) of NAAA (Vmax in nmol per min per mg protein, vehicle: 6.0 ± 0.51; and 2f, 0.3 μM: 1.9 ± 0.15, Fig. 3D) but did not affect Michaelis–Menten constant Km (Km in μM, vehicle: 174 ± 26; and 2f, 0.3 μM: 212 ± 24). These results indicated that 2f is a reversible and noncompetitive NAAA inhibitor.2.4 In vivo anti-inflammatory and analgesic activities of 2f
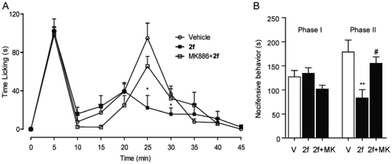
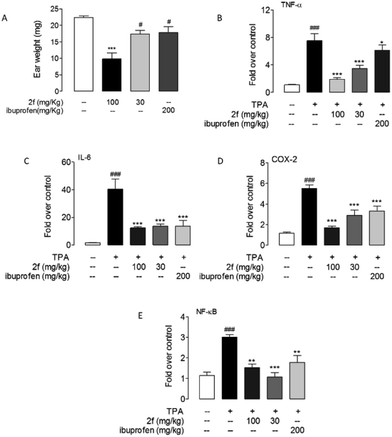
Finally, the in vivo anti-inflammation and analgesic activities of 2f was studied in different pain and inflammation mice models. Experimental mice were treated with 2f via intraperitoneal injection or intragastrical administration. The behavioural response of Mice to formalin stimulation was divided to the first-phase of acute pain (0–10 min) and the second-phase of chronic pain (15–35 min). Single treatment of 2f (10 mg kg−1, i.p.) decreased second-phase but not first-phase (Fig. 4), suggesting a central mechanism of action of 2f. The reduced licking time by 2f at the second-phase was reversed by PPAR-α antagonist MK886 (2 mg kg−1, i.p.), which indicated that 2f relieve pain in a PPAR-α dependent pathway.The anti-inflammatory activity of 2f was tested in 12-o-tetradecanoylphorbol-13-acetate (TPA) induced mice ear edema model. A commercial nonsteroidal anti-inflammatory drug, ibuprofen (200 mg kg−1) was intragastrically administrated as a positive control. The results showed that intragastrical administration of 2f (30, 100 mg kg−1) significantly reduce ears edema. Oral administration of compound 2f (30 mg kg−1) presented the same efficacy with ibuprofen (200 mg kg−1). Moreover, 2f significantly prevent the production of proinflammatory cytokines, including TNF-α, IL-6, COX-2 and NF-κB in mRNA level (Fig. 5).
For many nonsteroidal anti-inflammatory drugs, such as COX-2 inhibitors, adverse gastrointestinal effects and increased cardiovascular risks are two main limitations for their clinical application. We tested the toxicity of 2f by monitoring its influence on food intake, body weight and ether-a-go-go-related-gene (hERG) channel activity. Long-term studies suggested that intragastrical administration of 2f (30 mg kg−1 for 30 days, 100 mg kg−1 for 10 days) had no significant effects on food intake and body weight in mice (Fig. S2†). Mice stomach anatomy indicated that 2f did not cause gastrointestinal hemorrhages, while indomethacin (20 mg kg−1 for 3 days, ig) resulted in obvious hemorrhages (Fig. S3†). The low gastrointestinal side effects of 2f may be because it induces analgesia and anti-inflammatory via enhancement of PEA rather than reducing prostaglandins (PGs) in the gut. Moreover, the activity of hERG channels in CHO cells was only inhibited by 38.3% by 40 μM 2f (Fig. S4†), a concentration far above its threshold for NAAA inhibition. However, cisapride, a COX-2 inhibitor, inhibited 50% hERG at a concentration of 0.1 μM. These results suggested that with lower gastrointestinal and cardiovascular side effects, oxazolidone derivatives are promising anti-inflammatory drug candidates in clinical translation.
3 Conclusion
In conclusion, we report the discovery of oxazolidone derivatives as a novel scaffold of NAAA inhibitors. The NAAA inhibition activity of oxazolidone derivatives was modulated by the side chain and terminal lipophilic substituents. The results showed that the link chain length of C5, straight and saturated linkages were the preferred shape patterns. Several nanomolar NAAA inhibitors (2f, 3b, 3h, 3i and 3j) were developed. Compound 2f exhibited highly potent and selective inhibitory activity towards NAAA via a competitive and reversible pattern. Moreover, in vivo study showed that 2f had significant anti-inflammatory and analgesic effects with low side effects. Our study demonstrated that oxazolidone derivatives is a class of potent NAAA inhibitors holds great promise for clinical translation. We anticipate that these oxazolidone derivatives will help better understand the function of NAAA and facilitate the development of improved therapies for inflammation and pain.4 Materials and methods
4.1 Materials and instruments
All reagents used in the present study were purchased from Sigma-Aldrich (Shanghai, China) unless otherwise indicated. Tetrahydrofuran was distilled prior to use from sodium benzophenone ketyl. Dichloromethane (CH2Cl2) was distilled from phosphorus pentoxide. Dimethylformamide (DMF) was distilled from calcium hydride. Silica gel (300–400 mesh) from Yantai Athy Chemical Technology Co. Ltd. (Zhifu, China) was used for column chromatography, and compounds were eluted with an ethyl acetate/petroleum ether (PE) (60–90 °C) mixture (unless otherwise stated).The 1H-NMR and 13C-NMR spectra were recorded on a Bruker 400 spectrometer (400 MHz for 1H, 100 MHz for 13C), using CDCl3, d6-DMSO, or CD3OD as solvent and Me4Si as internal substance. IR spectra were recorded on a Nicolet Avatar 360 RT-IR spectrophotometer. Mass spectra were recorded on an Applied Bio systems MDS SCIEX 3200Q-TRAP mass spectrometry (MS) system with electro spray ionization (ESI†) and direct injection. The HRFABMS spectra were recorded on a Bruker APEX-FTMS apparatus. Elemental analysis was performed using a Vario RL analyzer. Melting points were determined on a Yanaco MP-500 melting point apparatus and are uncorrected. HPLC analysis were run on Agilent-1200 series HPLC system equipped with a photodiode array detector, using a Hypersil Gold C18 column (dimensions 250 × 4.6 mm, particle size 5 μm). Photodiode array (PDA) detector range was set as 210–600 nm.
4.2 Synthesis of oxazolidone derivatives
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH/CH2Cl2 = 1
MeOH/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) to afford 1c and 1d.25,26
15) to afford 1c and 1d.25,26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc/PE 1
EtOAc/PE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford 1e and 1f.27,28
5) to afford 1e and 1f.27,28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of aqueous 2 M NaOH and MeOH (16 mL), stirred at room temperature for 4 h, then treated with 2 M HCl, and extracted with EtOAc (3 × 5 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel to afford 3q-1 and 3t-1.14
1 mixture of aqueous 2 M NaOH and MeOH (16 mL), stirred at room temperature for 4 h, then treated with 2 M HCl, and extracted with EtOAc (3 × 5 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel to afford 3q-1 and 3t-1.144.3 In vitro biological evaluation of oxazolidone derivatives
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 95
water 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, each containing 0.25% acetic acid and 5 mmol L−1 ammonium acetate, pH = 7.4. The molecular ions were monitored by ESI† negative mode at m/z = 267 for heptadecenoic acid, m/z 303 for arachidonic acid, and m/z 269 for C17:0 FFAs.
5, v/v, each containing 0.25% acetic acid and 5 mmol L−1 ammonium acetate, pH = 7.4. The molecular ions were monitored by ESI† negative mode at m/z = 267 for heptadecenoic acid, m/z 303 for arachidonic acid, and m/z 269 for C17:0 FFAs.4.4 In vivo anti-inflammatory and analgesic activity of compound 2f
All animal experiments were performed in accordance with ‘Guide and Care and Use of Laboratory Animals’ from National Institutes of Health (NIH) and approved by the Animal Care and Use Committees of Xiamen University in China.Acknowledgements
We thank Dr Daniele Piomelli at the University of California, Irvine for the kind gifts of the HEK293-rNAAA cells and the HEK293-rFAAH cells. YL also wants to thank William stung for English revision. This work was supported by funds from the National Natural Science Foundation of China (Grant No. 81373273 to QY and 81602974 to YL), Xiamen Southern Ocean Research Center Project (No. 14GYY018NF18) to QY, the Fundamental Research Funds for the Central Universities (No. 20720150054) to QY and China Postdoctoral Science Foundation Funded Project (No. 2016M592876XB) to YL.References
- K. Tsuboi, Y. X. Sun, Y. Okamoto, N. Araki, T. Tonai and N. Ueda, J. Biol. Chem., 2005, 280, 11082–11092 CrossRef CAS PubMed.
- L. Facci, R. Dal Toso, S. Romanello, A. Buriani, S. D. Skaper and A. Leon, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 3376–3380 CrossRef CAS.
- J. Lo Verme, J. Fu, G. Astarita, G. La Rana, R. Russo, A. Calignano and D. Piomelli, Mol. Pharmacol., 2005, 67, 15–19 CrossRef CAS PubMed.
- C. Solorzano, C. Zhu, N. Battista, G. Astarita, A. Lodola, S. Rivara, M. Mor, R. Russo, M. Maccarrone, F. Antonietti, A. Duranti, A. Tontini, S. Cuzzocrea, G. Tarzia and D. Piomelli, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20966–20971 CrossRef CAS PubMed.
- S. Petrosino, T. Iuvone and V. Di Marzo, Biochimie, 2010, 92, 724–727 CrossRef CAS PubMed.
- A. Calignano, G. La Rana and D. Piomelli, Eur. J. Pharmacol, 2001, 419, 191–198 CrossRef CAS PubMed.
- B. Costa, S. Conti, G. Giagnoni and M. Colleoni, Br. J. Pharmacol., 2002, 137, 413–420 CrossRef CAS PubMed.
- S. Petrosino, L. Cristino, M. Karsak, E. Gaffal, N. Ueda, T. Tuting, T. Bisogno, D. De Filippis, A. D'Amico, C. Saturnino, P. Orlando, A. Zimmer, T. Iuvone and V. Di Marzo, Allergy, 2010, 65, 698–711 CrossRef CAS PubMed.
- J. M. Hesselink and T. A. Hekker, J. Pain Res., 2012, 5, 437–442 CrossRef PubMed.
- B. F. Cravatt, D. K. Giang, S. P. Mayfield, D. L. Boger, R. A. Lerner and N. B. Gilula, Nature, 1996, 384, 83–87 CrossRef CAS PubMed.
- O. Sasso, G. Moreno-Sanz, C. Martucci, N. Realini, M. Dionisi, L. Mengatto, A. Duranti, G. Tarozzo, G. Tarzia, M. Mor, R. Bertorelli, A. Reggiani and D. Piomelli, Pain, 2013, 154, 350–360 CrossRef CAS PubMed.
- J. M. West, N. Zvonok, K. M. Whitten, S. K. Vadivel, A. L. Bowman and A. Makriyannis, PLoS One, 2012, 7, e43877 CAS.
- T. Bandiera, S. Ponzano and D. Piomelli, Pharmacol. Res., 2014, 86, 11–17 CrossRef CAS PubMed.
- C. Solorzano, F. Antonietti, A. Duranti, A. Tontini, S. Rivara, A. Lodola, F. Vacondio, G. Tarzia, D. Piomelli and M. Mor, J. Med. Chem., 2010, 53, 5770–5781 CrossRef CAS PubMed.
- A. Armirotti, E. Romeo, S. Ponzano, L. Mengatto, M. Dionisi, C. Karacsonyi, F. Bertozzi, G. Garau, G. Tarozzo, A. Reggiani, T. Bandiera, G. Tarzia, M. Mor and D. Piomelli, ACS Med. Chem. Lett., 2012, 3, 422–426 CrossRef CAS PubMed.
- A. Duranti, A. Tontini, F. Antonietti, F. Vacondio, A. Fioni, C. Silva, A. Lodola, S. Rivara, C. Solorzano, D. Piomelli, G. Tarzia and M. Mor, J. Med. Chem., 2012, 55, 4824–4836 CrossRef CAS PubMed.
- S. Ponzano, F. Bertozzi, L. Mengatto, M. Dionisi, A. Armirotti, E. Romeo, A. Berteotti, C. Fiorelli, G. Tarozzo, A. Reggiani, A. Duranti, G. Tarzia, M. Mor, A. Cavalli, D. Piomelli and T. Bandiera, J. Med. Chem., 2013, 56, 6917–6934 CrossRef CAS PubMed.
- R. Vitale, G. Ottonello, R. Petracca, S. M. Bertozzi, S. Ponzano, A. Armirotti, A. Berteotti, M. Dionisi, A. Cavalli, D. Piomelli, T. Bandiera and F. Bertozzi, ChemMedChem, 2014, 9, 323–336 CrossRef CAS PubMed.
- M. Migliore, S. Pontis, A. L. Fuentes de Arriba, N. Realini, E. Torrente, A. Armirotti, E. Romeo, S. Di Martino, D. Russo, D. Pizzirani, M. Summa, M. Lanfranco, G. Ottonello, P. Busquet, K. M. Jung, M. Garcia-Guzman, R. Heim, R. Scarpelli and D. Piomelli, Angew. Chem., Int. Ed., 2016, 55, 11193–11197 CrossRef CAS PubMed.
- A. Ribeiro, S. Pontis, L. Mengatto, A. Armirotti, V. Chiurchiu, V. Capurro, A. Fiasella, A. Nuzzi, E. Romeo, G. Moreno-Sanz, M. Maccarrone, A. Reggiani, G. Tarzia, M. Mor, F. Bertozzi, T. Bandiera and D. Piomelli, ACS Chem. Biol., 2015, 2015(10), 1838–1846 CrossRef PubMed.
- Y. Li, L. Yang, L. Chen, C. Zhu, R. Huang, X. Zheng, Y. Qiu and J. Fu, PLoS One, 2012, 7, e43023 CAS.
- L. Yang, L. Li, L. Chen, Y. Li, H. Chen, Y. Li, G. Ji, D. Lin, Z. Liu and Y. Qiu, Sci. Rep., 2015, 5, 13565 CrossRef CAS PubMed.
- E. M. Smith, G. F. Swiss, B. R. Neustadt, E. H. Gold, J. A. Sommer, A. D. Brown, P. J. Chiu, R. Moran, E. J. Sybertz and T. Baum, J. Med. Chem., 1988, 31, 875–885 CrossRef CAS PubMed.
- C. M. Yea, C. E. Allan, D. M. Ashworth, J. Barnett, A. J. Baxter, J. D. Broadbridge, R. J. Franklin, S. L. Hampton, P. Hudson, J. A. Horton, P. D. Jenkins, A. M. Penson, G. R. Pitt, P. Riviere, P. A. Robson, D. P. Rooker, G. Semple, A. Sheppard, R. M. Haigh and M. B. Roe, J. Med. Chem., 2008, 51, 8124–8134 CrossRef CAS PubMed.
- B. J. Backes, K. Longenecker, G. L. Hamilton, K. Stewart, C. Lai, H. Kopecka, T. W. von Geldern, D. J. Madar, Z. Pei, T. H. Lubben, B. A. Zinker, Z. Tian, S. J. Ballaron, M. A. Stashko, A. K. Mika, D. W. Beno, A. J. Kempf-Grote, C. Black-Schaefer, H. L. Sham and J. M. Trevillyan, Bioorg. Med. Chem. Lett., 2007, 17, 2005–2012 CrossRef CAS PubMed.
- S. J. Macdonald, D. J. Belton, D. M. Buckley, J. E. Spooner, M. S. Anson, L. A. Harrison, K. Mills, R. J. Upton, M. D. Dowle, R. A. Smith, C. R. Molloy and C. Risley, J. Med. Chem., 1998, 41, 3919–3922 CrossRef CAS PubMed.
- I. Izquierdo, M. T. Plaza, J. A. Tamayo, F. Franco and F. Sánchez-Cantalejo, Tetrahedron, 2008, 64, 4993–4998 CrossRef CAS.
- S. Mitsumori, H. Zhang, P. Ha-Yeon Cheong, K. N. Houk, F. Tanaka and C. F. Barbas 3rd, J. Am. Chem. Soc, 2006, 128, 1040–1041 CrossRef CAS PubMed.
- L. Huang and W. D. Wulff, J. Am. Chem. Soc, 2011, 133, 8892–8895 CrossRef CAS PubMed.
- J. T. Randolph, C. A. Flentge, P. P. Huang, D. K. Hutchinson, L. L. Klein, H. B. Lim, R. Mondal, T. Reisch, D. A. Montgomery, W. W. Jiang, S. V. Masse, L. E. Hernandez, R. F. Henry, Y. Liu, G. Koev, W. M. Kati, K. D. Stewart, D. W. Beno, A. Molla and D. J. Kempf, J. Med. Chem., 2009, 52, 3174–3183 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, characterization, and 1H-NMR and 13C-NMR spectra of the intermediate and final compounds. See DOI: 10.1039/c6ra28734d |
| This journal is © The Royal Society of Chemistry 2017 |

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O