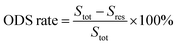Open Access Article
Open Access ArticleTrifluoromethanesulfonic acid-based DESs as extractants and catalysts for removal of DBT from model oil
Chunfeng Mao ,
Rongxiang Zhao*,
Xiuping Li and
Xiaohan Gao
,
Rongxiang Zhao*,
Xiuping Li and
Xiaohan Gao
College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001, China. E-mail: maochunfeng555@163.com; zylhzrx@126.com
First published on 23rd February 2017
Abstract
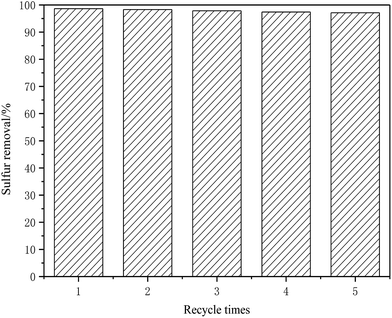
A series of deep eutectic solvents (DESs) of ChCl/XCF3SO3H (X from 1.0 to 2.0) were synthesized by stirring a mixture of choline chloride (ChCl) and trifluoromethanesulfonic acid (CF3SO3H) at room temperature. The DESs were characterized by Fourier transform infrared (FT-IR) and 1H nuclear magnetic resonance (1H NMR). The oxidative desulfurization of model oil was investigated using ChCl/1.5CF3SO3H as a catalyst and extraction agent, and H2O2 as the oxidant. Some reaction parameters such as type of DES, molar ratio of CF3SO3H and ChCl in DESs, H2O2 dose, reaction temperature, DES dose and type of sulfur compound were investigated. Under the optimum conditions, the removal rate of dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) can reach up to 98.65% and 96.8%, respectively. After five recycling runs, the removal rate of DBT can still reach 97.16%.
1 Introduction
SOx emission, which is mainly produced by vehicles, not only pollutes the environment, such as acid rain,1 but also harms the health of humans, such as cancer. In order to decrease the pollution caused by SOx, many countries have laid down a series of rules for restricting the sulfur content of fuel oil. It is an aim to achieve little sulfur in oil (sulfur content < 10 μg g−1).2 The traditional hydrodesulfurization (HDS) process, removing aliphatic and acyclic sulfur compounds, has been considered as a mature technology. However, HDS finds it difficult to remove aromatic sulfur compounds3,4 such as thiophene (TH), benzothiophene (BT) dibenzothiophene (DBT) and their derivatives because of their large steric hindrance. Therefore, some non-hydrodesulfurization processes, such as oxidative desulfurization (ODS),5 biodesulfurization (BDS),6 extractive desulfurization (EDS)7 and adsorption desulfurization (ADS),8 etc., have attracted wide attention.Among these non-hydrodesulfurization processes, oxidative desulfurization (ODS) was considered as one of the most promising technologies due to its advantages,9 such as the high desulfurization rate and the mild reaction conditions. Various oxidants such as H2O2, NO2, O2, O3, K2FeO4, and organic peroxides have been investigated in the oxidative desulfurization system. Among these oxidants, H2O2 is widely employed due to its high activity and low price.10
In recent years, a Brønsted acid based ionic liquid was used in the oxidative desulfurization process. Fang et al.11 reported the synthesis of 1-butyl-3-methyl-imidazolium trifluoroacetic acid ([C4mim]TFA) ionic liquids and their application in oxidative desulfurization and found that the anion of the ionic liquid has an important influence on the desulfurization rate. Lu et al.12 synthesized 1-methylimidazolium tetrafluoroborate ([HMIm]BF4) ionic liquids and the sulfur removal of DBT in model oil can reach 93% under optimum conditions. Gao et al.13 synthesized an acidic ionic liquid N-butyl-N-methylimidazolium hydrogen sulfate ([BMIm]HSO4), its oxidative desulfurization rate can reach 100% for DBT in model oil. The [BMIm]HSO4 ionic liquid can be recycled 5 times with only a slight reduction in activity. Zhao et al.14 synthesized the Brønsted acid ionic liquid N-methyl-pyrrolidonium tetrafluoroborate ([Hnmp]BF4) and used as the extractant and catalyst for desulfurization (ODS) of model oil. The experimental results indicated that 99.4% sulfur compounds in diesel fuel can be removed. Although these ionic liquids have better desulfurization activity in the oxidative desulfurization system, the raw materials of ionic liquids are expensive and the preparation process is complex.
As a new type of solvent, deep eutectic solvents have attracted great attention because of its excellent physical and chemical properties, which is friendly for environment. DESs are composed of two or more components that interact via intermolecular hydrogen bonds.15 Compared with the traditional ionic liquids, DESs have many advantages such as cheap and easily accessible raw material, a simple preparation process, and wide range of applications.16 In developmental history of DESs, ChCl-based DESs are first reported by Abbott and his co-workers, its physical properties have been researched.17 Thereafter, DESs are used in various fields such as electrochemistry, organic synthesis and separation processes.18–21 DESs also are used in the desulfurization process of fuels. For example, Zhu et al.22 reported the synthesis of ChCl·2CH3COOH and it was used as the extractant of oxidation desulfurization process. The study found that the removal rate of DBT in model oil can up to 98.6% under UV light irradiation. Gano Z. S. et al.23 reported that the FeCl3-based deep eutectic solvents for the extractive desulfurization of liquid fuels. Experimental results show the desulfurization efficiencies of 64% and 44% can be achieved for DBT and thiophene, respectively. Yin et al.24 reported that the synthesis of ChCl/p-TsOH and the desulfurization efficiencies of ChCl/p-TsOH can reach up to 97.25%, which are much higher than those obtained with traditional and functional ionic liquids. Our research group has synthesized phenylpropanoic acid-based DESs and was applied to the oxidation desulfurization process.25 In the synthesis, the acid-based DESs was synthesized by heating method. As is known to all, simpler preparation process and higher acidity of DESs are favorable for the desulfurization.26 Therefore, it is necessary to develop a new DES using a simple preparation method and apply it to the oxidative desulfurization process.
Trifluoromethanesulfonic acid (CF3SO3H) is one of the strongest organic acids, it is widely used in the synthesis reaction. In this work, the novel CF3SO3H-based DESs were synthesized by stirring a mixture of ChCl and CF3SO3H at the room temperature. The removal rate of DBT from model oil was investigated using CF3SO3H-based DESs as an extraction agent and catalyst, H2O2 as the oxidant. The influence of different type DESs, the molar ratio of ChCl to CF3SO3H, the oxygen to sulfur (O/S) molar ratio, the reaction temperature and the amount of DESs on the desulfurization rate was investigated. Under the optimum conditions, the removal rate of dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) can reach up to 98.65% and 96.8%, respectively. After five recycling runs, the removal rate of DBT can still reach 97.16%.
2 Experimental
2.1 Chemical regent and instrument
Dibenzothiophene (DBT, 98%), benzothiophene (BT, 97%), thiophene (TH, 99.8%) and 4,6-dimethyldibenzothiophene (4,6-DMDBT, 97%) were purchased from Aladdin Chemistry Co. Ltd. The choline chloride (ChCl, AR), tetrabutylammonium chloride (TBAC, AR), tetraethylammonium chloride (TEAC, AR), n-octane, carbon tetrachloride (CCl4, 99.5%), trifluoromethanesulfonic acid (CF3SO3H, 98%), and hydrogen peroxide (H2O2, 30 wt%) were purchased from Sinopharm Chemical Reagent Co. Ltd. Gas chromatography was carried out on an Agilent 7890A GC with an FID detector using a 30 m packed HP5 column. Infrared spectra of DBT, oxidation of DBT, DESs and raw materials were determined on Fourier-transform infrared spectrometer (WQF-520; Beijing Beifen Ruili Instrument Company, China). 1H NMR of DESs were determined by using the Mercury Plus 400 MHz spectrometer (Varian Co., Ltd. American).2.2 Synthesis of ChCl/XCF3SO3H
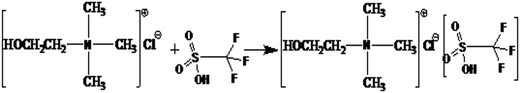
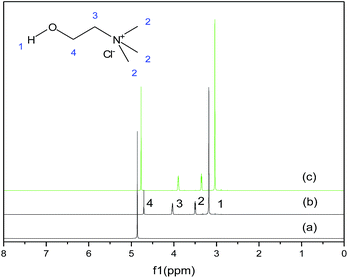
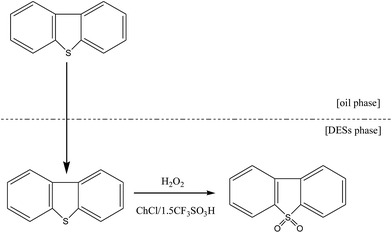
ChCl/XCF3SO3H was synthesized by stirring a mixing of ChCl and CF3SO3H at room temperature. The specific process is as follows: ChCl was added to a 100 mL round-bottomed flask. Then, the CF3SO3H was carefully added according to a certain molar ratio under stirring conditions. The two raw materials were stirred vigorously for 1 h in order to fully release the reaction heat. After the reaction, the ChCl/XCF3SO3H (X from 1.0 to 2.0) DESs homogeneous liquid was obtained. The reaction was shown in Fig. 1. TBAC/CF3SO3H and TEAC/CF3SO3H were synthesized according to the same method.1H NMR spectra of CF3SO3H, ChCl and ChCl/CF3SO3H were obtained on hydrogen spectrum detector. Coupling constants (J1) in Hz and chemical shifts (d) are given in ppm. The multiplicities of signals in 1H NMR are given with chemical shifts (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), and the data are listed as follows:
CF3SO3H for 1H NMR (500 MHz, D2O) δ 4.86 (s, 1H).
ChCl for 1H NMR (500 MHz, D2O) δ 4.71 (d, 26H), 4.08 (m, 59H), 3.56–3.46 (m, 39H), 3.25 (s, J = 68.7 Hz, 30H), 3.18 (d, 176H), 3.03 (s, 2H).
ChCl/CF3SO3H for 1H NMR (500 MHz, D2O) δ 4.77 (s, 2H), 3.90 (ddd, J = 7.7, 5.3, 2.8 Hz, 1H), 3.35 (dd, J = 5.8, 4.2 Hz, 1H), 3.04 (s, 5H).
2.3 Desulfurization experiment
The 500 μg g−1 model oil was prepared by dissolving 1.437 g DBT in 500 mL n-octane. The model oil, DESs, and 30 wt% H2O2 were added into a three-necked flask. The mixture was stirred in a water bath at a certain temperature. A small amount of the upper oil phase was removed every 20 min and determined by gas chromatography. The desulfurization rate was calculated using the following formula:where Stot (500 μg g−1) is the initial concentration of the sulfur compound in the model oil and Sres is the residual concentration of the sulfur compound after the ODS process.
3 Results and discussion
3.1 Characterization
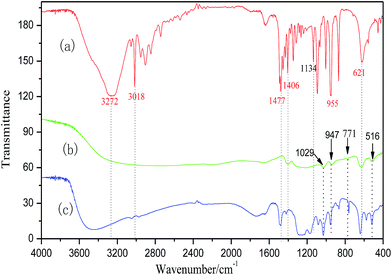
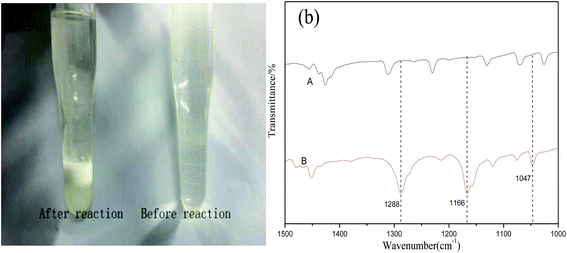
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1406 cm−1 in ChCl, and stretching vibration peak of F–C at 947 cm−1 and bending vibration peak of F–C at 621 cm−1 in CF3SO3H shift to 3466, 3048, 1416, 960 and 644 cm−1 in ChCl/1.5CF3SO3H were clearly seen (Fig. 2). The peaks happening to shift are attribute to the formation of hydrogen bond in the DES.27
O at 1406 cm−1 in ChCl, and stretching vibration peak of F–C at 947 cm−1 and bending vibration peak of F–C at 621 cm−1 in CF3SO3H shift to 3466, 3048, 1416, 960 and 644 cm−1 in ChCl/1.5CF3SO3H were clearly seen (Fig. 2). The peaks happening to shift are attribute to the formation of hydrogen bond in the DES.27
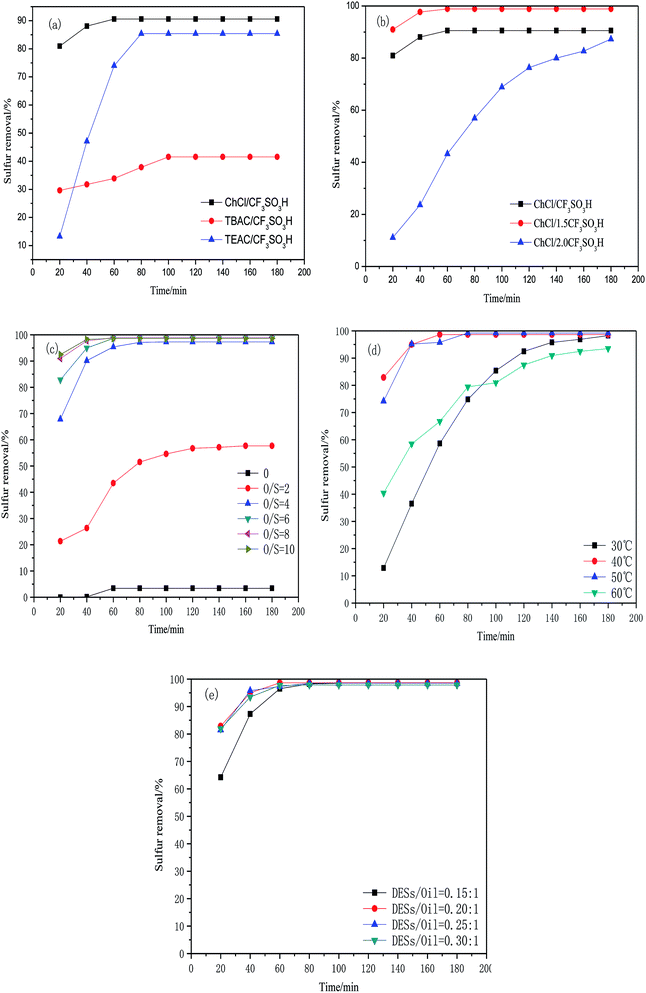
3.2 Oxidative desulfurization reaction conditions
| DESs | Conductivity (δ)/(μs cm−1) | Viscosity (η)/(mPa s) |
|---|---|---|
| ChCl/1.0CF3SO3H | 5200 | 100 |
| ChCl/1.5CF3SO3H | 4200 | 85 |
| ChCl/2.0CF3SO3H | 4000 | 70 |
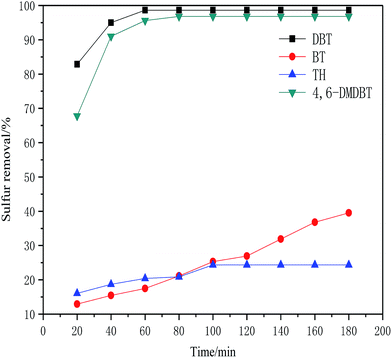 | ||
| Fig. 5 Influence of different sulfur compounds on desulfurization rate (5 mL model oil, O/S molar ratio of 6, 1 mL DESs, 40 °C). | ||
According to the experimental results, ChCl/1.5CF3SO3H show excellent oxidative desulfurization activity. Table 2 shows a comparison of the desulfurization activity of ChCl/1.5CF3SO3H and other acidic ionic liquids. It can be seen that ChCl/1.5CF3SO3H has a high desulfurization rate under mild experimental conditions. Furthermore, ChCl/1.5CF3SO3H can be prepared by a simple method and the raw material are easily obtained.
| Catalysts | S content (μg g−1) | Reaction conditions | Removal of DBT | Ref. |
|---|---|---|---|---|
| [C4mim]TFA | 202 | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 1 oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, H2O2 = 1 mL, 70 °C, 30 min 35, H2O2 = 1 mL, 70 °C, 30 min |
100% | 20 |
| [HMIm]BF4 | 1000 | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 5 oil = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2, O/S = 10, 90 °C, 360 min 3.2, O/S = 10, 90 °C, 360 min |
93% | 21 |
| [BMIM][HSO4] | 1000 | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 1 oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, O/S = 5, 25 °C, 90 min 2, O/S = 5, 25 °C, 90 min |
99.6% | 22 |
| [Hnmp]BF4 | 1550 | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 1 oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, O/S = 3, 60 °C, 60 min 1, O/S = 3, 60 °C, 60 min |
100% | 23 |
| ChCl/1.5CF3SO3H | 500 | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 1 oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, O/S = 6, 40 °C, 60 min 5, O/S = 6, 40 °C, 60 min |
98.65% | This work |
3.3 Catalytic oxidative desulfurization of diesel oil
The S-removal activity of ChCl/1.5CF3SO3H for the real diesel oil with a total S-content of 375 mg L−1 was also investigated. Under the optimal condition, the desulfurization rate of 68.5% was obtained. Sulfur removal efficiency was found to be lower in diesel oil than that obtained in model oil (98.65%), which may be attributed to the more complex chemical composition of diesel.384 Conclusion
A series of ChCl/XCF3SO3H (X from 1.0 to 2.0) were synthesized by stirring a mixture of ChCl and CF3SO3H. The DESs were used as extractant and catalyst in the oxidative desulfurization process. Compared to other acidic ionic liquids, the synthesis method of ChCl/XCF3SO3H is simple. The ChCl/1.5CF3SO3H exhibits a high desulfurization activity for DBT in model oil and can be recycled 5 times without a significant decrease in activity. The results demonstrate the DESs exhibit the high catalytic activity and stability for the desulfurization system.Acknowledgements
The authors also acknowledge the financial support of the Natural Science Foundation of China (Project no. 21003069); the authors also acknowledge the financial support of the Doctoral Fund of Liaoning Province (201501105).References
- X. M. Yan, Z. Mei and P. Mei, et al., J. Porous Mater., 2014, 21(5), 729–737 CrossRef CAS.
- H. Li, W. Zhu and J. Lu, et al., React. Kinet., Mech. Catal., 2009, 96(1), 165–173 CrossRef CAS.
- J. Zhang, W. Zhu and H. Li, et al., Green Chem., 2009, 11(11), 1801–1807 RSC.
- X. Tang, Y. Zhang and J. Li, et al., Ind. Eng. Chem. Res., 2015, 54(16), 4625–4632 CrossRef CAS.
- R. Wang, G. Zhang and H. Zhao, Catal. Today, 2010, 149(1), 117–121 CrossRef CAS.
- J. M. Campos-Martin, M. C. Capel-Sanchez and P. Perez-Presas, et al., J. Chem. Technol. Biotechnol., 2010, 85(7), 879–890 CrossRef CAS.
- Z. S. Gano, F. S. Mjalli and T. Al-Wahaibi, et al., Chem. Eng. Process., 2015, 93, 10–20 CrossRef CAS.
- H. X. Zhang, H. L. Huang and C. X. Li, et al., Ind. Eng. Chem. Res., 2012, 51(38), 12449–12455 CAS.
- S. Ribeiro, C. M. Granadeiro and P. Silva, et al., Catal. Sci. Technol., 2013, 3(9), 2404–2414 CAS.
- H. Yang, B. Jiang and Y. Sun, et al., Chem. Eng. J., 2016, 306, 131–138 CrossRef CAS.
- D. Fang, Q. Wang and Y. Liu, et al., Energy Fuels, 2014, 28(10), 6677–6682 CrossRef CAS.
- L. Lu, S. Cheng and J. Gao, et al., Energy Fuels, 2007, 21(1), 383–384 CrossRef CAS.
- H. Gao, C. Guo and J. Xing, et al., Green Chem., 2010, 12(7), 1220–1224 RSC.
- D. Zhao, J. Wang and E. Zhou, Green Chem., 2007, 9(11), 1219–1222 RSC.
- A. Paiva, R. Craveiro and I. Aroso, et al., ACS Sustainable Chem. Eng., 2014, 2(5), 1063–1071 CrossRef CAS.
- C. Li, D. Li and S. Zou, et al., Green Chem., 2013, 15(10), 2793–2799 RSC.
- A. P. Abbott, D. Boothby and G. Capper, et al., J. Am. Chem. Soc., 2004, 126(29), 9142–9147 CrossRef CAS PubMed.
- Q. Zhang, K. D. O. Vigier and S. Royer, et al., Chem. Soc. Rev., 2012, 41(21), 7108–7146 RSC.
- A. P. Abbott, G. Capper and K. J. McKenzie, et al., J. Electroanal. Chem., 2007, 599(2), 288–294 CrossRef CAS.
- B. P. Wu, Q. Wen and H. Xu, et al., J. Mol. Catal. B: Enzym., 2014, 101, 101–107 CrossRef CAS.
- K. Pang, Y. Hou and W. Wu, et al., Green Chem., 2012, 14(9), 2398–2401 RSC.
- W. Zhu, C. Wang and H. Li, et al., Green Chem., 2015, 17(4), 2464–2472 RSC.
- Z. S. Gano, F. S. Mjalli and T. Al-Wahaibi, et al., Chem. Eng. Prog., 2015, 93, 10–20 CrossRef CAS.
- J. Yin, J. Wang and Z. Li, et al., Green Chem., 2015, 17(9), 4552–4559 RSC.
- C. Mao, R. Zhao and X. Li, Fuel, 2017, 189, 400–407 CrossRef CAS.
- P. S. Kulkarni and C. A. M. Afonso, Green Chem., 2010, 12(7), 1139–1149 RSC.
- P. Liu, J. W. Hao and S. J. Liang, et al., Monatsh. Chem., 2016, 147(4), 801–808 CrossRef CAS.
- W. Jiang, W. Zhu and Y. Chang, et al., Chem. Eng. J., 2014, 250, 48–54 CrossRef CAS.
- Y. Dong, Y. Nie and Q. Zhou, Chem. Eng. Technol., 2013, 36(3), 435–442 CrossRef CAS.
- F. Li, B. Wu and R. Liu, et al., Chem. Eng. J., 2015, 274, 192–199 CrossRef CAS.
- X. Chen, H. Guo and A. A. Abdeltawab, et al., Energy Fuels, 2015, 29(5), 2998–3003 CrossRef CAS.
- W. Jiang, W. Zhu and H. Li, et al., RSC Adv., 2013, 3(7), 2355–2361 RSC.
- W. S. Zhu, H. Li and Q. Q. Gu, et al., J. Mol. Catal. A: Chem., 2011, 336(1), 16–22 CrossRef CAS.
- D. Wang, E. W. Qian and H. Amano, et al., Appl. Catal., A, 2003, 253(1), 91–99 CrossRef CAS.
- W. Zhu, H. Li and X. Jiang, et al., Green Chem., 2008, 10(6), 641–646 RSC.
- C. Mao, R. Zhao and X. Li, Fuel, 2017, 189, 400–407 CrossRef CAS.
- M. Zhang, W. Zhu and S. Xun, et al., Chem. Eng. J., 2013, 220, 328–336 CrossRef CAS.
- W. Zhu, G. Zhu and H. Li, et al., J. Mol. Catal. A: Chem., 2011, 347, 8–14 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |