Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Precise control over reduction potential of fulleropyrrolidines for organic photovoltaic materials†
M. Karakawa*ab,
T. Nagaic,
K. Adachic,
Y. Ieb and
Y. Aso*b
aInstitute for Frontier Science Initiative, Kanazawa University, Kakuma-machi, Kanazawa, Ishikawa 920-1192, Japan. E-mail: karakawa@staff.kanazawa-u.ac.jp; Fax: +81-76-234-4780
bDepartment of Soft Nanomaterials, Nanoscience and Nanotechnology Center, The Institute of Scientific and Industrial Research (ISIR), Osaka University, 8-1 Mihogaoka, Osaka 567-0047, Ibaraki, Japan. E-mail: aso@sanken.osaka-u.ac.jp; Fax: +81-6-6879-8479
cFundamental Technology Group, Chemical R&D Center, Daikin Industries, Ltd, 1-1 Nishi Hitotsuya, Osaka 566-8585, Settsu, Japan. Fax: +81-6-6349-4751
First published on 20th January 2017
Abstract
Organic photovoltaic cells based on two types of organic materials (acceptor and donor) have attracted considerable attention for their low-cost fabrication, and potential for realization of flexible and light weight devices. For many years organic photovoltaic cells have relied on [6,6]-phenyl C61 butyric acid methyl ester, a fullerene derivative that is used as an electron acceptor material. A few reports on bisadduct and C70 derivatives have shown some improvements in device performance; however, further enhancements based on improvements to the fullerene acceptor component have proven challenging. Here we described the device performance of improved acceptor fullerene materials that allow the open circuit voltage to be fine-tuned in organic photovoltaic cells to provide high power conversion efficiency. Our new approach to designing fullerene materials will accelerate development of n-type semiconductor materials and allow for new low cost organic photovoltaic cells.
Introduction
Environmentally friendly energy generation is an important global issue for society. Solar energy harvesting has been recognized as an effective way of addressing our increasing energy needs. Organic photovoltaic (OPV) cells have attracted great attention in recent years owing to the simplicity of their fabrication processes, such as low-cost printing techniques.1–5Current state-of-the art OPV cells are fabricated as a thin film comprising a mixture of p- and n-type organic semiconducting materials.1,2 This organic semiconducting film features a nanoscale phase-segregated structure known as a bulk-hetero junction (BHJ), which provides an effective pathway for a charge generation and separation in the film.
The p-type semiconductors used in such devices are π-conjugated aromatic compounds based on polymers and small molecules, which absorb light leading to generation of excitons in the film.1,2 The absorption of the p-type materials is related to their chemical structures and directly affects their performance in devices. Over the past decade many types of p-type materials have been developed and OPV cells with power conversion efficiencies greater than 10 or 11% have been reported.6–10
In contrast to the remarkable development of p-type donor materials, for n-type acceptor materials there have been few advances over [6,6]-phenyl C61 butyric acid methyl ester (PC61BM), which has dominated reports in this field over the last decade.11 In the term, indene bis-adduct C60 derivative (IC60BA), which is one of the important fullerene derivative, presented an effect to changing a LUMO energy level contributing to obtain a high voltage in OPV cells.12 While a few other n-type materials based on fullerenes have been reported,13–23 the development of non-fullerene acceptors and n-type semiconductors for use in OPV cells has become an active area of research.24–31 Although interest has shifted to non-fullerene derivatives, it remains important to develop new fullerenes as low-cost acceptor materials that may allow for commercially viable OPV modules.
We have previously reported the synthesis and application of new fulleropyrrolidine derivatives having various substituents.32 Our previous study indicated that N-phenyl fulleropyrrolidine and its OPV cells showed a higher reduction potential and better device performance, respectively, than those based on PC61BM.
In this paper, we show that the reduction potential of fulleropyrrolidine derivatives can be controlled based on the structure of the substituent groups. These modifications can in turn control and enhance the open circuit voltage (Voc) of OPV cells. Our newly synthesized fulleropyrrolidine derivatives were designed based on theoretical calculations. The synthesized materials were characterized by UV-vis absorption, cyclic voltammetry and OPV cell measurements. Relationships between the substitution pattern and Voc value were analysed in detail. An OPV cell based our C60-fulleropyrrolidine derivative showed high power conversion efficiency (PCE) of 7.30% with a Voc of 0.80 V. This fulleropyrrolidine represents a next generation fullerene acceptor, which may advance OPV performance beyond that which is possible with PC61BM.
Results and discussion
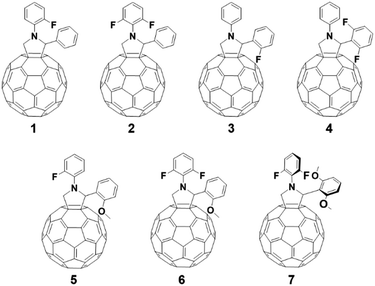
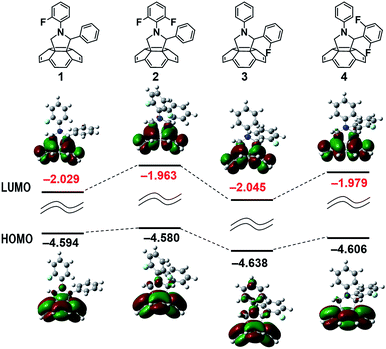
Our new C60-fulleropyrrolidine derivatives are illustrated in Fig. 1. Density functional theory (DFT) calculations of the fulleropyrrolidines were conducted, with Gaussian 09 at the B3LYP/6-31G level, to guide the design of novel fulleropyrrolidine derivatives before their synthesis as shown in Fig. 2 and 3. To simplify the calculations and ensure no-conjugation between the substituent group and the fullerene π-system, the calculations were performed about the substituent group and its surrounding structure.The results of the calculated highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) were related to the structures of the derivatives. When a fluorine (F) atom was introduced at the ortho position of the N-phenyl group (compounds 1 and 2), the HOMO energy level was unchanged; however, compound 2 has a higher LUMO level than that of 1. When an F atom was introduced at the ortho position of the C2 phenyl group (compounds 3 and 4), the energy level changed in a similar way to that for compounds 1 and 2; however, the effects were more pronounced in compounds 3 and 4, which had lower LUMO energy levels. These calculations indicate that the energy levels were mainly affected by the number and position of the F atoms that were introduced into the N- and C2-phenyl groups.
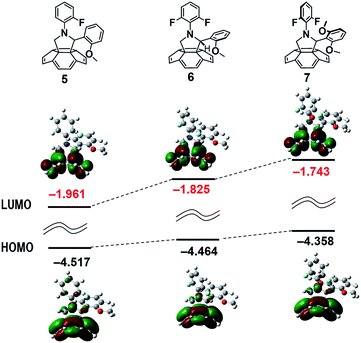
When a methoxy group was introduced at the C2-phenyl group instead of an F atom, the energy levels of 5, 6 and 7 shifted higher, as observed for the introduction of an F atom into the N-phenyl group (Fig. 3). In compound 7, the combined effects of both the F atoms and the methoxy groups gave the highest calculated LUMO energy level in this series. Based on the LUMO level of our unsubstituted compound (PNP),32 we could estimate a Voc value of 0.82 V for 7 and 0.80 V for the mono-methoxy compound 6. Notably these small changes in substitution might have a considerable influence on the LUMO level of the fulleropyrrolidine derivative but did not result in any break down of the fullerene π-system. Maintaining the fullerene π-system is important for forming an ideal BHJ structured thin film and for obtaining material that is stable under electron injection conditions.
We synthesized compounds shown in Fig. 1, 1–7 from corresponding amino acids and aldehyde derivatives under one step Prato reaction conditions.33,34 The full synthetic procedures are described in ESI (Fig. S1†).
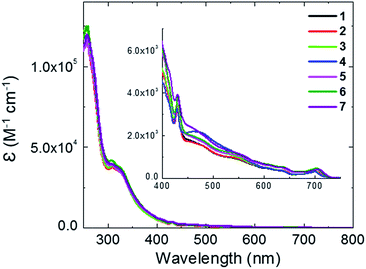
UV-vis absorption spectra for fullerene derivatives, 1–7, were measured in chloroform solution (Fig. 4). An absorption band between 250 and 400 nm with a maximum located at 257 nm was observed in all compounds. A sharp peak at 420 nm, which is also observed for PC61BM, was assigned to the 58π-system of the fullerene derivative. A weak absorption at 710 nm was attributed to π–π* transitions of the fullerenes. Small absorption differences were observed in the region between 430 and 600 nm, indicating that the substituent groups on the fullerenes had little effect on the electronic structure of the fullerene π-system.
Cyclic voltammetry (CV) measurements were performed in a three-electrode cell to estimate the LUMO energy level of the derivatives from their reduction potential. The results obtained are summarized in Table 1. For comparison, the CV value of PNP32 is also listed. Reversible redox processes were observed in all the fulleropyrrolidine derivatives, as shown in Fig. S2†. The following equation was used to calculate the LUMO levels: ELUMO = −(E11/2 + 4.8 eV), where E11/2 is the first half-wave reduction potential relative to ferrocene (Fc).35
| Compound | λ (nm) | ε (×105) | E11/2 (V vs. Fc/Fc+) | ELUMO (eV) |
|---|---|---|---|---|
| 1 | 255 | 1.18 | −1.141 | −3.659 |
| 2 | 256 | 1.15 | −1.162 | −3.638 |
| 3 | 256 | 1.23 | −1.129 | −3.671 |
| 4 | 256 | 1.19 | −1.133 | −3.667 |
| 5 | 257 | 1.20 | −1.151 | −3.651 |
| 6 | 257 | 1.25 | −1.158 | −3.642 |
| 7 | 257 | 1.20 | −1.175 | −3.625 |
| PNP | 257 | 1.12 | −1.128 | −3.672 |
The reduction potentials of the fulleropyrrolidines varied depending on their substitution pattern as shown in Table 1. The LUMO energy level was raised by around 0.02 eV per F atom from 1 to 2. The F atoms on the C2-phenyl group had little effect on the LUMO level and raised the energy by less than 0.01 eV per F atom. The trends of the CV results were consistent with the trends of our DFT calculations, as shown in Fig. 2. The DFT calculations indicated that the methoxy-substituted compound 7 would have the highest LUMO energy level, and indeed, 7 featured the most negative reduction potential in the series, as determined by CV. Notably there were no π-orbital interactions between the substituent group and the fullerene π-system. The differences in the reduction potentials were likely caused by through space π–π orbital interactions between the fullerene and the C2-phenyl ring. In a recent report, Swager and co-workers described a relationship between the cyclobutadiene structure and the fullerene π-system, which resulted in a higher LUMO level.36
Our CV results, suggest that the reduction potential can be controlled by introducing substituents that have through space interactions, but do not disrupt the fullerene π-system.
OPV cells were fabricated from the newly synthesized fulleropyrrolidine derivatives as acceptor materials and PTB7 as a donor material to evaluate the potential of the materials. A device structure of ITO/PFN/PTB7:fullerene derivative/MoOx/Al under simulated AM 1.5G solar irradiation at 100 mW cm−2 was used. The active layer was spin-cast from chlorobenzene (CB) solutions for compounds 1, 2, 5 and 6, and o-dichlorobenzene (DCB) solutions for 3, 4 and 7 owing to the compounds different solubilities. The current density–voltage (J–V) characteristics and the external quantum efficiency (EQE) spectra are shown in Fig. 5. The OPV parameters are summarized in Table 2. As reported by Janssen and co-workers PC71BM-based OPV devices generally show higher performance than those based on PC61BM, which has been attributed to the broader absorption of PC71BM than that of PC61BM.37 However, C70 fullerene derivatives are expensive, which makes them less well suited to commercial production of OPV modules. We believe that development of C60 fullerene-based acceptors is important to realizing economically viable OPV cells. The data reported in Table 2 are comparable to reports on the performance of OPV cells with PC71BM as an acceptor.38,39
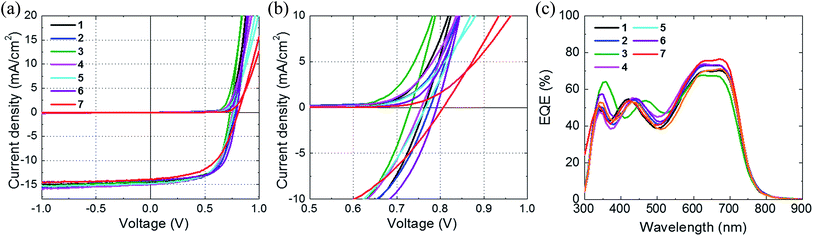 | ||
| Fig. 5 J–V curves for the ranges (a) −1.0 to 1.0 V and (b) 0.5–1.0 V, and (c) external quantum efficiency (EQE) spectra of OPV cells with fulleropyrrolidine derivatives. | ||
| PTB7:1 | PTB7:2 | PTB7:3 | PTB7:4 | PTB7:5 | PTB7:6 | PTB7:7 | |
|---|---|---|---|---|---|---|---|
| a Mean values and standard deviations of 10 devices shown in parentheses. | |||||||
| Jsc [mA cm−2] | 14.468 (14.312 ± 0.305) | 14.866 (14.507 ± 0.579) | 14.327 (14.143 ± 0.376) | 14.848 (13.997 ± 0.489) | 14.758 (14.493 ± 0.325) | 14.104 (14.105 ± 0.173) | 13.875 (13.552 ± 0.235) |
| Voc [V] | 0.764 (0.761 ± 0.003) | 0.776 (0.784 ± 0.005) | 0.731 (0.734 ± 0.006) | 0.756 (0.757 ± 0.005) | 0.766 (0.768 ± 0.003) | 0.792 (0.795 ± 0.003) | 0.807 (0.809 ± 0.008) |
| FF | 0.644 (0.631 ± 0.021) | 0.614 (0.606 ± 0.019) | 0.653 (0.631 ± 0.014) | 0.613 (0.625 ± 0.007) | 0.598 (0.593 ± 0.017) | 0.654 (0.629 ± 0.036) | 0.545 (0.538 ± 0.005) |
| PCE [%] | 7.12 (6.87 ± 0.17) | 7.09 (6.88 ± 0.17) | 6.84 (6.54 ± 0.22) | 6.88 (6.62 ± 0.19) | 6.76 (6.59 ± 0.13) | 7.30 (7.05 ± 0.33) | 6.10 (5.90 ± 0.12) |
The J–V curves show that the devices generated high short circuit current density (Jsc) values. High Jsc values were achieved in OPV cells based on compounds 2 and 5. The device featuring compound 7 had a slightly lower Jsc, which was attributed to a thinner active layer (∼90 nm) in this device and reduced photo absorption. The OPV cell with 7 also had a slightly higher series resistance than the others and a relatively low fill factor.
We focused on the Voc of the OPV cells based on our new acceptors. Our CV measurements and calculations revealed a trend in the reduction potential of the compounds. The data in Table 2 and Fig. 5b clearly show that the Voc values reflected the different reduction potentials of the derivatives. Relationships between the chemical structure and Voc are illustrated in Fig. 6.
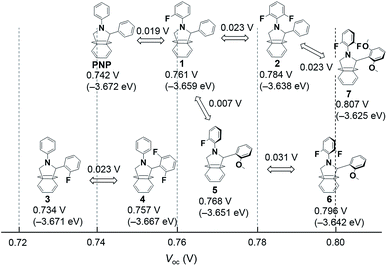 | ||
| Fig. 6 Relationships between chemical structure and obtained Voc. The values in brackets are the LUMO levels calculated from CV measurements. | ||
In changing the basic PNP structure (0.742 V, −3.672 eV), by introducing fluorine atoms in compounds 1 and 2, we observed that the Voc increased by 0.02 eV per F atom and enhanced the Voc to 0.78 V. For the case of C2-phenyl substitution the measured Voc values were slightly lower than those of 1 and 2, even in compound 4, which had two F atoms. The C2-phenyl fluorination had little positive effect on the Voc value. A tetrafluoro compound (4F in ESI†) was also synthesized. The 4F showed above trade off relations of introducing F atoms in phenyl rings, resulting in not so high Voc of 0.780 V.
For the methoxy-substituted series, Voc increased to ca. 0.77, 0.80 and 0.81 for the OPV cells based on 5, 6 and 7, respectively. This trend also agreed with the DFT calculations and CV results. Thus, the Voc in cells with PTB7 (typically 0.74 V) was increased to more than 0.80 V without any disruption to the pristine fullerene π-system. We showed that modifying the substitution pattern is a promising method to fine tune the LUMO level of fullerene derivatives and achieve the best alignment of energy levels in a cell.
Conclusions
In conclusion, we designed new fulleropyrrolidine acceptor molecules guided by theoretical calculations. On the basis of these calculations we synthesized new fulleropyrrolidine derivatives with controlled reduction potentials. The trend in the LUMO levels of the compounds was determined by DFT calculations and was consistent with the results of CV measurements. We fabricated OPV devices and evaluated the performance of the compounds. Introduction of an F atom into the N-phenyl group contributed to enhanced Voc of OPV cells. Conversely, an F atom on the C2 phenyl group made little contribution to the Voc of OPV cells. The position and number of the F atoms introduced into the phenyl group affected the shifting of the LUMO level. Furthermore, compounds having methoxy-substituted C2 phenyl groups were used as acceptors in the active layer of OPV cells. The energy level of these derivatives also affected the Voc value of the OPV cells. A Voc as high as 0.81 V was achieved with PTB7 as the donor material. In this fulleropyrrolidine series, we were able to tune the Voc value by 0.01–0.07 V without breaking the C60 fullerene π-system. We achieved a high PCE of 7.30% using our C60 fulleropyrrolidine derivatives. To date, development of new p-type materials with improved light absorption, have mainly contributed to improved energy conversion efficiency in OPV. In this report, we successfully improved the PCE of OPV cells with fulleropyrrolidine acceptor materials, by enhancing the Voc of the cell. This is an important step that may contribute to new gains in already highly optimized OPV cells.Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor (1
acceptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5)/MoOx/Al, the PFN interlayer material was dissolved in methanol (2 mg mL−1) and spin-coated on top of the clean ITO substrate based on previous reports.9 The organic active layer, with a thickness of 90–100 nm, was prepared by spin-coating a blended solution (25 mg mL−1) in chlorobenzene (for 1, 2, 5 and 6) with 1,8-diiodoctane (3%, v/v) or dichlorobenzene (for 3, 4 and 7) with 1,8-diiodoctane (3%, v/v) at 1000 rpm for 120 s in a nitrogen atmosphere. A 10 nm MoOx layer and an 80 nm Al layer were then evaporated through a shadow mask to define the active area (0.09 cm2) of the devices and form the top electrode using EO-5 metal evaporation chamber (Eiko engineering co. Ltd.). Current density–voltage characteristics of the photovoltaic cells were measured in the dark and under simulated solar light, with a Keithley 2400 source meter and a XES-301S solar simulator (San-Ei Electric Co., Ltd.), calibrated to produce 100 mW cm−2 AM 1.5G illumination. All device measurements were performed in N2 filled measurement-apparatus at room temperature.
1.5)/MoOx/Al, the PFN interlayer material was dissolved in methanol (2 mg mL−1) and spin-coated on top of the clean ITO substrate based on previous reports.9 The organic active layer, with a thickness of 90–100 nm, was prepared by spin-coating a blended solution (25 mg mL−1) in chlorobenzene (for 1, 2, 5 and 6) with 1,8-diiodoctane (3%, v/v) or dichlorobenzene (for 3, 4 and 7) with 1,8-diiodoctane (3%, v/v) at 1000 rpm for 120 s in a nitrogen atmosphere. A 10 nm MoOx layer and an 80 nm Al layer were then evaporated through a shadow mask to define the active area (0.09 cm2) of the devices and form the top electrode using EO-5 metal evaporation chamber (Eiko engineering co. Ltd.). Current density–voltage characteristics of the photovoltaic cells were measured in the dark and under simulated solar light, with a Keithley 2400 source meter and a XES-301S solar simulator (San-Ei Electric Co., Ltd.), calibrated to produce 100 mW cm−2 AM 1.5G illumination. All device measurements were performed in N2 filled measurement-apparatus at room temperature.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 20
toluene, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and by GPC to give the compound 1 (177 mg, 38%); 1H NMR (700 MHz, CDCl3, δ): 4.74 (1H, d, J = 9.6 Hz), 5.66 (1H, d, J = 9.6 Hz), 6.10 (1H, s), 7.10–7.38 (7H, m), 7.77 (2H, d, J = 7.3 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −119.50 to −119.75 (m); 13C NMR (175 MHz, CDCl3, δ): 66.68, 66.71, 68.66, 75.91, 116.42 (d, 2JFC = 20.2 Hz), 123.77, 124.36 (d, 3JFC = 7.7 Hz), 128.37, 128.71, 128.82, 134.59 (d, 2JFC = 10.6 Hz), 135.86, 136.06, 136.59, 136.65, 136.88, 139.34, 139.83, 140.22, 140.26, 141.49, 141.68, 141.88, 141.93, 142.02, 142.05, 142.08, 142.14, 142.20, 142.23, 142.41, 142.59, 142.61, 142.72, 143.00, 143.18, 144.38, 144.43, 144.60, 144.78, 145.19, 145.28, 145.29, 146.34, 145.44, 145.46, 145.55, 145.63, 145.79, 145.97, 146.04, 146.15, 146.18, 146.25, 146.28, 146.33, 146.36, 146.46, 146.87, 147.36, 147.40, 152.99, 153.49, 156.02, 157.81 (d, 1JFC = 248.5 Hz); UV-vis (CHCl3): λmax (ε) = 255 nm (118
1) and by GPC to give the compound 1 (177 mg, 38%); 1H NMR (700 MHz, CDCl3, δ): 4.74 (1H, d, J = 9.6 Hz), 5.66 (1H, d, J = 9.6 Hz), 6.10 (1H, s), 7.10–7.38 (7H, m), 7.77 (2H, d, J = 7.3 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −119.50 to −119.75 (m); 13C NMR (175 MHz, CDCl3, δ): 66.68, 66.71, 68.66, 75.91, 116.42 (d, 2JFC = 20.2 Hz), 123.77, 124.36 (d, 3JFC = 7.7 Hz), 128.37, 128.71, 128.82, 134.59 (d, 2JFC = 10.6 Hz), 135.86, 136.06, 136.59, 136.65, 136.88, 139.34, 139.83, 140.22, 140.26, 141.49, 141.68, 141.88, 141.93, 142.02, 142.05, 142.08, 142.14, 142.20, 142.23, 142.41, 142.59, 142.61, 142.72, 143.00, 143.18, 144.38, 144.43, 144.60, 144.78, 145.19, 145.28, 145.29, 146.34, 145.44, 145.46, 145.55, 145.63, 145.79, 145.97, 146.04, 146.15, 146.18, 146.25, 146.28, 146.33, 146.36, 146.46, 146.87, 147.36, 147.40, 152.99, 153.49, 156.02, 157.81 (d, 1JFC = 248.5 Hz); UV-vis (CHCl3): λmax (ε) = 255 nm (118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 934 (M + 1); HRMS calcd for C74H13FN 934.1032; found 934.1052; calcd C 95.17, H 1.30, N 1.50; found C 95.25, H 1.78, N 1.55.
000); MS (FAB) m/z 934 (M + 1); HRMS calcd for C74H13FN 934.1032; found 934.1052; calcd C 95.17, H 1.30, N 1.50; found C 95.25, H 1.78, N 1.55.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 20
toluene, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and by GPC to give the compound 2 (108 mg, 23%); 1H NMR (700 MHz, CDCl3, δ): 5.12 (1H, d, J = 9.1 Hz), 5.26 (1H, d, J = 9.1 Hz), 6.46 (1H, s), 6.96 (2H, t, J = 8.7 Hz), 7.12–7.35 (4H, m), 7.77 (2H, d, J = 7.5 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −117.06 to −117.15 (m); 13C NMR (175 MHz, CDCl3, δ): 65.73, 68.93, 76.51, 77.84, 112.42 (d, 2JFC = 20.4 Hz), 122.15 (t, 2JFC = 13.9 Hz), 126.89 (t, 3JFC = 10.2 Hz), 128.40, 128.48, 129.12, 136.10, 136.15, 136.41, 136.59, 137.04, 139.44, 139.89, 140.14, 140.22, 141.49, 141.67, 141.88, 142.01, 142.05, 142.09, 142.15, 142.20, 142.26, 142.47, 142.58, 142.60, 142.70, 143.00, 143.18, 144.39, 144.42, 144.63, 144.78, 145.18, 145.28, 145.30, 146.34, 145.42, 145.58, 145.63, 145.78, 145.97, 146.08, 146.14, 146.18, 146.23, 146.30, 146.36, 146.56, 146.81, 147.36, 153.08, 153.14, 153.36, 156.29, 161.10 (dd, 1JFC = 249.7 Hz, 3JFC = 6.3 Hz); UV-vis (CHCl3): λmax (ε) = 256 nm (115
1) and by GPC to give the compound 2 (108 mg, 23%); 1H NMR (700 MHz, CDCl3, δ): 5.12 (1H, d, J = 9.1 Hz), 5.26 (1H, d, J = 9.1 Hz), 6.46 (1H, s), 6.96 (2H, t, J = 8.7 Hz), 7.12–7.35 (4H, m), 7.77 (2H, d, J = 7.5 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −117.06 to −117.15 (m); 13C NMR (175 MHz, CDCl3, δ): 65.73, 68.93, 76.51, 77.84, 112.42 (d, 2JFC = 20.4 Hz), 122.15 (t, 2JFC = 13.9 Hz), 126.89 (t, 3JFC = 10.2 Hz), 128.40, 128.48, 129.12, 136.10, 136.15, 136.41, 136.59, 137.04, 139.44, 139.89, 140.14, 140.22, 141.49, 141.67, 141.88, 142.01, 142.05, 142.09, 142.15, 142.20, 142.26, 142.47, 142.58, 142.60, 142.70, 143.00, 143.18, 144.39, 144.42, 144.63, 144.78, 145.18, 145.28, 145.30, 146.34, 145.42, 145.58, 145.63, 145.78, 145.97, 146.08, 146.14, 146.18, 146.23, 146.30, 146.36, 146.56, 146.81, 147.36, 153.08, 153.14, 153.36, 156.29, 161.10 (dd, 1JFC = 249.7 Hz, 3JFC = 6.3 Hz); UV-vis (CHCl3): λmax (ε) = 256 nm (115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 951 (M+); HRMS calcd for C74H11F2N 951.0860; found 951.0861; calcd C 93.37, H 1.16, N 1.47; found C 93.47, H 1.67, N 1.45.
000); MS (FAB) m/z 951 (M+); HRMS calcd for C74H11F2N 951.0860; found 951.0861; calcd C 93.37, H 1.16, N 1.47; found C 93.47, H 1.67, N 1.45.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 20
toluene, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and by GPC to give the compound 3 (72.1 mg, 15.4%); 1H NMR (700 MHz, CDCl3, δ): 5.09 (1H, d, J = 9.9 Hz), 5.65 (1H, d, J = 9.9 Hz), 6.61 (1H, s), 7.02–7.18 (3H, m), 7.20–7.28 (1H, m), 7.28–7.42 (4H, m), 7.84 (1H, dd, J = 6.3, 6.3 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −114.0 to −115.5 (m); 13C NMR (175 MHz, CDCl3, δ): 67.90 (bs), 68.43, 75.60, 115.93 (d, 2JFC = 22.4 Hz), 120.64 (bs), 122.28 (bs), 124.91, 125.47 (d, 3JFC = 11.7 Hz), 129.38, 129.80 (d, 3JFC = 7.7 Hz), 130.12, 135.44, 135.82, 136.43, 136.57, 139.45, 139.95, 140.26, 140.31, 141.78, 142.04, 142.08, 142.11, 142.15, 142.21, 142.24, 142.32, 142.63, 142.65, 142.67, 142.70, 143.07, 143.13, 144.47, 144.51, 144.56, 144.64, 145.18, 145.27, 145.32, 146.36, 145.52, 145.55, 145.59, 145.60, 145.64, 145.97, 146.04, 146.12, 146.16, 146.19, 146.27, 146.32, 146.36, 146.73, 147.40, 147.44, 152.87, 153.65, 153.91, 156.67, 160.99 (d, 1JFC = 249.9 Hz); UV-vis (CHCl3): λmax (ε) = 256 nm (123
1) and by GPC to give the compound 3 (72.1 mg, 15.4%); 1H NMR (700 MHz, CDCl3, δ): 5.09 (1H, d, J = 9.9 Hz), 5.65 (1H, d, J = 9.9 Hz), 6.61 (1H, s), 7.02–7.18 (3H, m), 7.20–7.28 (1H, m), 7.28–7.42 (4H, m), 7.84 (1H, dd, J = 6.3, 6.3 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −114.0 to −115.5 (m); 13C NMR (175 MHz, CDCl3, δ): 67.90 (bs), 68.43, 75.60, 115.93 (d, 2JFC = 22.4 Hz), 120.64 (bs), 122.28 (bs), 124.91, 125.47 (d, 3JFC = 11.7 Hz), 129.38, 129.80 (d, 3JFC = 7.7 Hz), 130.12, 135.44, 135.82, 136.43, 136.57, 139.45, 139.95, 140.26, 140.31, 141.78, 142.04, 142.08, 142.11, 142.15, 142.21, 142.24, 142.32, 142.63, 142.65, 142.67, 142.70, 143.07, 143.13, 144.47, 144.51, 144.56, 144.64, 145.18, 145.27, 145.32, 146.36, 145.52, 145.55, 145.59, 145.60, 145.64, 145.97, 146.04, 146.12, 146.16, 146.19, 146.27, 146.32, 146.36, 146.73, 147.40, 147.44, 152.87, 153.65, 153.91, 156.67, 160.99 (d, 1JFC = 249.9 Hz); UV-vis (CHCl3): λmax (ε) = 256 nm (123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 934 (M + 1); HRMS calcd for C74H13FN 934.1032; found 934.1023; calcd C 95.17, H 1.30, N 1.50; found C 94.42, H 1.69, N 1.43.
000); MS (FAB) m/z 934 (M + 1); HRMS calcd for C74H13FN 934.1032; found 934.1023; calcd C 95.17, H 1.30, N 1.50; found C 94.42, H 1.69, N 1.43.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 20
toluene, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and by GPC to give the compound 4 (103 mg, 43%); 1H NMR (700 MHz, CDCl3, δ): 5.40 (1H, dd, J = 9.9, 5.9 Hz), 5.66 (1H, dd, J = 9.9, 2.4 Hz), 6.88–7.02 (2H, m), 7.14 (1H, d, J = 7.5 Hz), 7.20–7.30 (3H, m), 7.37 (1H, t, J = 7.5 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −105.30 to −105.39 (1F, m), −114.35 to −114.45 (1F, m); 13C NMR (175 MHz, CDCl3, δ): 62.81 (dd, 3JFC = 9.2 Hz), 65.26, 68.44, 74.21, 111.67 (d, 2JFC = 24.5 Hz), 112.94 (d, 2JFC = 22.9 Hz), 115.50, 115.64 (t, 2JFC = 34.0 Hz), 119.38, 129.56, 130.59 (t, 3JFC = 10.6 Hz), 134.56, 135.06, 136.26, 137.04, 139.72, 140.16, 140.26, 140.33, 141.71, 141.82, 141.86, 142.10, 142.13, 142.15, 142.22, 142.42, 142.62, 142.65, 142.68, 143.10, 143.18, 144.37, 144.61, 144.66, 145.10, 145.25, 145.29, 146.32, 145.47, 145.57, 145.73, 145.77, 145.97, 146.06, 146.09, 146.11, 146.31, 147.46, 147.55, 151.84, 154.44, 154.78, 155.27, 160.26 (dd, 1JFC = 257.3 Hz, 3JFC = 6.3 Hz), 161.99 (dd, 1JFC = 247.3 Hz, 3JFC = 8.9 Hz); UV-vis (CHCl3): λmax (ε) = 256 nm (119
1) and by GPC to give the compound 4 (103 mg, 43%); 1H NMR (700 MHz, CDCl3, δ): 5.40 (1H, dd, J = 9.9, 5.9 Hz), 5.66 (1H, dd, J = 9.9, 2.4 Hz), 6.88–7.02 (2H, m), 7.14 (1H, d, J = 7.5 Hz), 7.20–7.30 (3H, m), 7.37 (1H, t, J = 7.5 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −105.30 to −105.39 (1F, m), −114.35 to −114.45 (1F, m); 13C NMR (175 MHz, CDCl3, δ): 62.81 (dd, 3JFC = 9.2 Hz), 65.26, 68.44, 74.21, 111.67 (d, 2JFC = 24.5 Hz), 112.94 (d, 2JFC = 22.9 Hz), 115.50, 115.64 (t, 2JFC = 34.0 Hz), 119.38, 129.56, 130.59 (t, 3JFC = 10.6 Hz), 134.56, 135.06, 136.26, 137.04, 139.72, 140.16, 140.26, 140.33, 141.71, 141.82, 141.86, 142.10, 142.13, 142.15, 142.22, 142.42, 142.62, 142.65, 142.68, 143.10, 143.18, 144.37, 144.61, 144.66, 145.10, 145.25, 145.29, 146.32, 145.47, 145.57, 145.73, 145.77, 145.97, 146.06, 146.09, 146.11, 146.31, 147.46, 147.55, 151.84, 154.44, 154.78, 155.27, 160.26 (dd, 1JFC = 257.3 Hz, 3JFC = 6.3 Hz), 161.99 (dd, 1JFC = 247.3 Hz, 3JFC = 8.9 Hz); UV-vis (CHCl3): λmax (ε) = 256 nm (119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 951 (M+); HRMS calcd for C74H11F2N 951.0860; found 951.0867; calcd C 93.37, H 1.16, N 1.47; found C 93.22, H 1.34, N 1.56.
000); MS (FAB) m/z 951 (M+); HRMS calcd for C74H11F2N 951.0860; found 951.0867; calcd C 93.37, H 1.16, N 1.47; found C 93.22, H 1.34, N 1.56.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 20
toluene, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and by GPC to give the compound 5 (82.5 mg, 34%); 1H NMR (700 MHz, CDCl3, δ): 3.75 (3H, s), 4.71 (1H, d, J = 10.3 Hz), 5.65 (1H, d, J = 10.3 Hz), 6.68 (1H, s), 6.82–6.93 (2H, m), 7.02–7.29 (5H, m), 7.77 (1H, dd, J = 7.9, 1.6 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −121.23 to −121.34 (m); 13C NMR (175 MHz, CDCl3, δ): 55.29, 66.39, 68.95, 69.16, 74.99, 110.78, 116.28 (d, 2JFC = 20.2 Hz), 121.21, 123.07, 124.42 (d, 2JFC = 11.6 Hz), 125.09, 129.05 (d, 3JFC = 7.6 Hz), 129.72, 134.65, 135.02 (d, 3JFC = 10.2 Hz), 136.63, 136.51, 136.63, 139.33, 140.18, 140.23, 141.50, 141.68, 141.74, 141.82, 142.00, 142.09, 142.17, 142.24, 142.35, 142.55, 142.57, 142.62, 142.65, 142.96, 143.05, 144.36, 144.38, 144.54, 144.61, 145.11, 145.20, 145.27, 145.36, 145.51, 145.55, 145.59, 145.72, 145.90, 145.94, 146.07, 146.15, 146.19, 146.25, 146.54, 146.73, 147.33, 147.38, 153.41, 153.77, 154.71, 156.52, 157.32, 157.58 (d, 3JFC = 245.3 Hz); UV-vis (CHCl3): λmax (ε) = 257 nm (120
1) and by GPC to give the compound 5 (82.5 mg, 34%); 1H NMR (700 MHz, CDCl3, δ): 3.75 (3H, s), 4.71 (1H, d, J = 10.3 Hz), 5.65 (1H, d, J = 10.3 Hz), 6.68 (1H, s), 6.82–6.93 (2H, m), 7.02–7.29 (5H, m), 7.77 (1H, dd, J = 7.9, 1.6 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −121.23 to −121.34 (m); 13C NMR (175 MHz, CDCl3, δ): 55.29, 66.39, 68.95, 69.16, 74.99, 110.78, 116.28 (d, 2JFC = 20.2 Hz), 121.21, 123.07, 124.42 (d, 2JFC = 11.6 Hz), 125.09, 129.05 (d, 3JFC = 7.6 Hz), 129.72, 134.65, 135.02 (d, 3JFC = 10.2 Hz), 136.63, 136.51, 136.63, 139.33, 140.18, 140.23, 141.50, 141.68, 141.74, 141.82, 142.00, 142.09, 142.17, 142.24, 142.35, 142.55, 142.57, 142.62, 142.65, 142.96, 143.05, 144.36, 144.38, 144.54, 144.61, 145.11, 145.20, 145.27, 145.36, 145.51, 145.55, 145.59, 145.72, 145.90, 145.94, 146.07, 146.15, 146.19, 146.25, 146.54, 146.73, 147.33, 147.38, 153.41, 153.77, 154.71, 156.52, 157.32, 157.58 (d, 3JFC = 245.3 Hz); UV-vis (CHCl3): λmax (ε) = 257 nm (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 964 (M + 1); HRMS calcd for C75H15FNO 963.1059; found 963.1036; calcd C 93.45, H 1.46, N 1.45; found C 93.38, H 1.61, N 1.52.
000); MS (FAB) m/z 964 (M + 1); HRMS calcd for C75H15FNO 963.1059; found 963.1036; calcd C 93.45, H 1.46, N 1.45; found C 93.38, H 1.61, N 1.52.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 10
toluene, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the compound 6 (219 mg, 45%); 1H NMR (700 MHz, CDCl3, δ): 3.86 (3H, s), 5.20 (1H, d, J = 9.1 Hz), 5.34 (1H, d, J = 9.1 Hz), 6.88–6.95 (2H, m), 6.99–7.07 (2H, m), 7.16 (1H, s), 7.20–7.29 (2H, m), 7.81 (1H, d, J = 7.9 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −114.80 to −114.98 (m); 13C NMR (175 MHz, CDCl3, δ): 55.41, 65.48, 69.13, 69.95, 111.02, 112.38 (d, 2JFC = 23.9 Hz), 120.82, 122.54, 125.31, 126.26 (t, 2JFC = 21.2 Hz), 128.22, 128.96, 129.32, 134.81, 136.57, 136.66, 139.39, 140.10, 140.17, 141.50, 141.66, 141.75, 141.89, 141.97, 142.07, 142.10, 142.18, 142.28, 142.42, 142.54, 142.63, 142.96, 143.04, 144.32, 144.45, 144.58, 144.63, 145.07, 145.11, 145.12, 145.20, 145.26, 145.28, 145.33, 145.59, 145.60, 145.71, 145.93, 145.96, 146.06, 146.14, 146.20, 146.25, 146.57, 146.85, 147.23, 147.31, 153.21, 153.81, 154.66, 156.78, 157.91, 159.67, 160.81 (dd, 1JFC = 252.4 Hz, 3JFC = 7.7 Hz); UV-vis (CHCl3): λmax (ε) = 257 nm (125
1) to give the compound 6 (219 mg, 45%); 1H NMR (700 MHz, CDCl3, δ): 3.86 (3H, s), 5.20 (1H, d, J = 9.1 Hz), 5.34 (1H, d, J = 9.1 Hz), 6.88–6.95 (2H, m), 6.99–7.07 (2H, m), 7.16 (1H, s), 7.20–7.29 (2H, m), 7.81 (1H, d, J = 7.9 Hz); 19F-NMR (372.5 MHz, CDCl3, δ): −114.80 to −114.98 (m); 13C NMR (175 MHz, CDCl3, δ): 55.41, 65.48, 69.13, 69.95, 111.02, 112.38 (d, 2JFC = 23.9 Hz), 120.82, 122.54, 125.31, 126.26 (t, 2JFC = 21.2 Hz), 128.22, 128.96, 129.32, 134.81, 136.57, 136.66, 139.39, 140.10, 140.17, 141.50, 141.66, 141.75, 141.89, 141.97, 142.07, 142.10, 142.18, 142.28, 142.42, 142.54, 142.63, 142.96, 143.04, 144.32, 144.45, 144.58, 144.63, 145.07, 145.11, 145.12, 145.20, 145.26, 145.28, 145.33, 145.59, 145.60, 145.71, 145.93, 145.96, 146.06, 146.14, 146.20, 146.25, 146.57, 146.85, 147.23, 147.31, 153.21, 153.81, 154.66, 156.78, 157.91, 159.67, 160.81 (dd, 1JFC = 252.4 Hz, 3JFC = 7.7 Hz); UV-vis (CHCl3): λmax (ε) = 257 nm (125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 981 (M+); HRMS calcd for C75H13F2NO 981.0965; found 981.0972; calcd C 91.74, H 1.33, N 1.43; found C 91.65, H 1.47, N 1.54.
000); MS (FAB) m/z 981 (M+); HRMS calcd for C75H13F2NO 981.0965; found 981.0972; calcd C 91.74, H 1.33, N 1.43; found C 91.65, H 1.47, N 1.54.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 10
toluene, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the compound 7 (205 mg, 41%); 1H NMR (700 MHz, CDCl3, δ): 3.66 (3H, s), 3.78 (3H, s), 5.04 (1H, d, J = 9.1 Hz), 5.52 (1H, d, J = 9.1 Hz), 6.38 (1H, d, J = 7.7 Hz), 6.55 (1H, d, J = 7.7 Hz), 6.86–6.95 (1H, m), 6.97–7.06 (1H, m), 7.11–7.20 (2H, m), 7.38 (1H, s); 19F-NMR (372.5 MHz, CDCl3, δ): −119.23 to −118.31 (2F, m); 13C NMR (175 MHz, CDCl3, δ): 54.55, 56.13, 64.96, 69.15, 69.91, 75.73, 104.03, 104.20, 111.81, 111.94 (d, 4JFC = 4.6 Hz), 113.10, 123.34 (t, 2JFC = 20.2 Hz), 124.06 (d–d, 2JFC = 20.2 Hz, 4JFC = 10.2 Hz), 129.71, 134.43, 136.24, 136.88, 137.09, 139.13, 139.70, 139.91, 139.99, 141.36, 141.63, 141.75, 142.06, 142.09, 142.12, 142.39, 142.46, 142.54, 142.56, 143.02, 144.38, 144.59, 144.67, 144.70, 145.05, 145.08, 145.10, 145.13, 145.20, 145.23, 145.24, 145.56, 145.70, 145.73, 145.81, 145.86, 145.90, 145.94, 145.98, 146.12, 146.14, 146.19, 146.24, 146.25, 146.72, 146.96, 147.24, 147.29, 154.34, 154.37, 155.79, 156.85, 159.02, 159.76, 160.17 (dd, 1JFC = 249.9 Hz, 3JFC = 8.8 Hz); UV-vis (CHCl3): λmax (ε) = 257 nm (120
1) to give the compound 7 (205 mg, 41%); 1H NMR (700 MHz, CDCl3, δ): 3.66 (3H, s), 3.78 (3H, s), 5.04 (1H, d, J = 9.1 Hz), 5.52 (1H, d, J = 9.1 Hz), 6.38 (1H, d, J = 7.7 Hz), 6.55 (1H, d, J = 7.7 Hz), 6.86–6.95 (1H, m), 6.97–7.06 (1H, m), 7.11–7.20 (2H, m), 7.38 (1H, s); 19F-NMR (372.5 MHz, CDCl3, δ): −119.23 to −118.31 (2F, m); 13C NMR (175 MHz, CDCl3, δ): 54.55, 56.13, 64.96, 69.15, 69.91, 75.73, 104.03, 104.20, 111.81, 111.94 (d, 4JFC = 4.6 Hz), 113.10, 123.34 (t, 2JFC = 20.2 Hz), 124.06 (d–d, 2JFC = 20.2 Hz, 4JFC = 10.2 Hz), 129.71, 134.43, 136.24, 136.88, 137.09, 139.13, 139.70, 139.91, 139.99, 141.36, 141.63, 141.75, 142.06, 142.09, 142.12, 142.39, 142.46, 142.54, 142.56, 143.02, 144.38, 144.59, 144.67, 144.70, 145.05, 145.08, 145.10, 145.13, 145.20, 145.23, 145.24, 145.56, 145.70, 145.73, 145.81, 145.86, 145.90, 145.94, 145.98, 146.12, 146.14, 146.19, 146.24, 146.25, 146.72, 146.96, 147.24, 147.29, 154.34, 154.37, 155.79, 156.85, 159.02, 159.76, 160.17 (dd, 1JFC = 249.9 Hz, 3JFC = 8.8 Hz); UV-vis (CHCl3): λmax (ε) = 257 nm (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); MS (FAB) m/z 1012 (M + 1); HRMS calcd for C76H16F2NO2 1012.1149; found 1012.1116; calcd C 90.20, H 1.49, N 1.38; found C 90.31, H 1.65, N 1.36.
000); MS (FAB) m/z 1012 (M + 1); HRMS calcd for C76H16F2NO2 1012.1149; found 1012.1116; calcd C 90.20, H 1.49, N 1.38; found C 90.31, H 1.65, N 1.36.Acknowledgements
This work was supported by a cooperative research with Daikin Co., Ltd. Thanks are extended to the Comprehensive Analysis Center (CAC), The Institute of Scientific and Industrial Research (ISIR), Osaka University for their assistance in the elemental analyses and NMR measurements.Notes and references
- L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642 CrossRef CAS PubMed.
- C.-C. Chueh, C.-Z. Li and A. K.-Y. Jen, Energy Environ. Sci., 2015, 8, 1160 CAS.
- F. C. Krebs, N. Espinosa, M. Hösel, R. R. Søndergaard and M. Jørgensen, Adv. Mater., 2014, 26, 29 CrossRef CAS PubMed.
- R. Po, A. Bernardi, A. Calabrese, C. Carbonera, G. Corso and A. Pellegrino, Energy Environ. Sci., 2014, 7, 925 CAS.
- T. P. Osedach, T. L. Andrewb and V. Bulović, Energy Environ. Sci., 2013, 6, 711 CAS.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- V. Vohra, K. Kawashima, T. Kakara, T. Koganezawa, I. Osaka, K. Takimiya and H. Murata, Nat. Photonics, 2015, 9, 403 CrossRef CAS.
- L. Huo, T. Liu, X. Sun, Y. Cai, A. J. Heeger and Y. Sun, Adv. Mater., 2015, 27, 2938 CrossRef CAS PubMed.
- J.-D. Chen, C. Cui, Y.-Q. Li, L. Zhou, Q.-D. Ou, C. Li, Y. Li and J.-X. Tang, Adv. Mater., 2015, 27, 1035 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- G. J. Zhao, Y. He and Y. Li, Adv. Mater., 2010, 22, 4355 CrossRef CAS PubMed.
- K. Zhao, Q. Wang, B. Xu, W. Zhao, X. Liu, B. Yang, M. Sun and J. Hou, J. Mater. Chem. A, 2016, 4, 9511 CAS.
- K. Zhao, L. Ye, W. Zhao, S. Zhang, H. Yao, B. Xu, M. Sun and J. Hou, J. Mater. Chem. C, 2015, 3, 9565 RSC.
- Y. He and Y. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970 RSC.
- Y. Yamane, K. Sugawara, N. Nakamura, S. Hayase, T. Nokami and T. Itoh, J. Org. Chem., 2015, 80, 4638 CrossRef CAS PubMed.
- Y. Morinaka, M. Nobori, M. Murata, A. Wakamiya, T. Sagawa, S. Yoshikawa and Y. Murata, Chem. Commun., 2013, 49, 3670 RSC.
- K. Yoshimura, K. Matsumoto, Y. Uetani, S. Sakumichi, S. Hayase, M. Kawatsura and T. Itoh, Tetrahedron, 2012, 68, 3605 CrossRef CAS.
- P. A. Troshin, E. A. Khakina, M. Egginger, A. E. Goryachev, S. I. Troyanov, A. Fuchsbauer, A. S. Peregudov, R. N. Lyubovskaya, V. F. Razumov and N. S. Sariciftci, ChemSusChem, 2010, 3, 356 CrossRef CAS PubMed.
- K. Matsumoto, K. Hashimoto, M. Kamo, Y. Uetani, S. Hayase, M. Kawatsura and T. Itoh, J. Mater. Chem., 2010, 20, 9226 RSC.
- C. Yang, J. Y. Kim, S. Cho, J. K. Lee, A. J. Heeger and F. Wudl, J. Am. Chem. Soc., 2008, 130, 6444 CrossRef CAS PubMed.
- M. Lenes, G.-J. A. H. Wetzelaer, F. B. Kooistra, S. C. Veenstra, J. C. Hummelen and P. W. M. Blom, Adv. Mater., 2008, 20, 2116 CrossRef CAS.
- G. Zhao, Y. He, Z. Xu, J. Hou, M. Zhang, J. Min, H.-Y. Chen, M. Ye, Z. Hong, Y. Yang and Y. Li, Adv. Funct. Mater., 2010, 20, 1480 CrossRef CAS.
- Y. Lin and X. Zhan, Mater. Horiz., 2014, 1, 470 RSC.
- K. Cnops, B. P. Rand, D. Cheyns, B. Verreet, M. A. Empl and P. Heremans, Nat. Commun., 2014, 5, 3406 Search PubMed.
- Y. Liu, T. T. Larsen-Olsen, X. Zhao, B. Andreasen, R. R. Søndergaard, M. Helgesen, K. Norrman, M. Jørgensen, F. C. Krebs and X. Zhan, Sol. Energy Mater. Sol. Cells, 2013, 112, 157 CrossRef CAS.
- D. Mori, H. Benten, H. Ohkita, S. Ito and K. Miyake, ACS Appl. Mater. Interfaces, 2012, 4, 3325 CAS.
- E. Zhou, J. Cong, M. Zhao, L. Zhang, K. Hashimoto and K. Tajima, Chem. Commun., 2012, 48, 5283 RSC.
- Y. Vaynzof, T. J. K. Brenner, D. Kabra, H. Sirringhaus and R. H. Friend, Adv. Funct. Mater., 2012, 22, 2418 CrossRef CAS.
- M. Schubert, D. Dolfen, J. Frisch, S. Roland, R. Steyrleuthner, B. Stiller, Z. Chen, U. Scherf, N. Koch, A. Facchetti and D. Neher, Adv. Energy Mater., 2012, 2, 369 CrossRef CAS.
- A. Facchetti, Chem. Mater., 2011, 23, 733 CrossRef CAS.
- M. Karakawa, T. Nagai, K. Adachi, Y. Ie and Y. Aso, J. Mater. Chem. A, 2014, 2, 20889 CAS.
- M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798 CrossRef CAS.
- P. Wang, B. Chen, R. M. Metzger, T. Da Ros and M. Prato, J. Mater. Chem., 1997, 7, 2397 RSC.
- J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bässler, M. Porsch and J. Daub, Adv. Mater., 1995, 7, 551 CrossRef CAS.
- G. D. Han, W. R. Collins, T. L. Andrew, V. Bulović and T. M. Swager, Adv. Funct. Mater., 2013, 23, 3061 CrossRef CAS.
- M. M. Wienk, J. M. Kroon, W. J. H. Verhees, J. Knol, J. C. Hummelen, P. A. van Hal and R. A. J. Janssen, Angew. Chem., Int. Ed, 2003, 42, 3371 CrossRef CAS PubMed.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135 CrossRef CAS PubMed.
- H. J. Son, W. Wang, T. Xu, Y. Liang, Y. Wu, G. Li and L. Yu, J. Am. Chem. Soc., 2011, 133, 1885 CrossRef CAS PubMed.
- F. Huang, H. Wu, D. Wang, W. Yang and Y. Cao, Chem. Mater., 2004, 16, 708 CrossRef CAS.
- M. A. Khadlm and L. D. Colebrook, J. Chem. Eng. Data, 1985, 30, 239 CrossRef.
- I. Waterhouse, A. Naylor, C. J. Wallis and F. Ellis, EP Pat., 0340030, 1989.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental information for synthesis of compounds, CV curves, atomic force microscopy images. See DOI: 10.1039/c6ra27661j |
| This journal is © The Royal Society of Chemistry 2017 |