Open Access Article
Open Access ArticleA smart NIR fluorescent probe for the highly selective detection of palladium†
Hui-ya Tan‡
a,
Jing-gong Liu‡b,
Lin-fu Zhoua,
Yu-kun Lia,
Jin-wu Yan *a and
Lei Zhang*a
*a and
Lei Zhang*a
aSchool of Bioscience and Bioengineering, South China University of Technology, Guangzhou, P. R. China. E-mail: yjw@scut.edu.cn; lzhangce@scut.edu.cn; Tel: +86 20 39380678
bThe Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou, 510006, P. R. China
First published on 20th January 2017
Abstract
A tailor-made colorimetric and NIR fluorescent probe for Pd0 was developed by introducing allyl chloroformate into the methylene blue (MB) fluorophore. The probe exhibited vivid color change and significant fluorescence enhancement towards Pd0, accompanied with excellent selectivity and sensitivity. No absorption and fluorescence response could be observed towards other metal ions. The fluorescence intensity of this probe showed a linear response to Pd0 in the concentration range of 0–2 μM with a detection limit of 5.7 nM. Moreover, living cell imaging results indicated that this probe holds promising application prospects for detecting intracellular Pd0 species based on its low cytotoxicity and specific turn-on NIR emission.
Introduction
Along with the rapid development of modern industry, heavy and transition metals have been widely applied in many fields such as chemistry, biology and environmental science. Palladium, as an important transition metal, plays an important role in various fields such as glass, fine chemical, electronics, and petroleum industries, as well as in automobiles.1–3 However, the high level of residual palladium due to the wide exploitation of palladium species has raised palladium contamination, which could cause severe adverse health effects.4,5 Therefore, it is urgent to develop highly selective and sensitive methods for palladium detection in environmental and biological samples. Atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectroscopy (ICP-MS), and plasma emission spectroscopy are the conventional approaches for palladium detection, which can achieve accurate and sensitive detection, but expensive facilities, well-controlled experimental conditions and complicated sample-pretreatment procedures are required.6–8 In recent years, optical probes, including colorimetric or fluorescent probes, emerged as powerful alternatives to monitor and sense palladium species because of their convenience, on-site detection, ease of manipulation and biological applications.9–13 In particular, fluorescent probes with near-infrared (NIR) emission are extremely favourable for bioimaging because of deep penetration and minimum auto-fluorescence.14,15 However, most of the reported probes for palladium display absorbance and emissions in the visible region, which greatly restricts their applications in biological imaging. To this end, it is highly desired and urgent to develop NIR fluorescent probes for selective and sensitive detection of palladium.Methylene blue (MB), an oxidised phenothiazin compound already FDA-approved for several indications, was widely used as a medication and stain in both clinical and basic research field.16–18 Recently, MB was also applied as NIR imaging agent in the image-guided surgery.19–22 However, the development of new fluorescent probes based on this NIR fluorophore has rarely been reported.23 It is well-known that MB could be converted to a colorless and non-emissive form called leucomethylene blue under reducing or acidic condition. To obtain the NIR fluorescent probe for Pd0, the allyl chloroformate moiety was introduced into the leucomethylene blue through a carbamate bond, leading to a stable colorless and non-emissive probe MB-APC. We speculated that MB-APC would be uncaged through the Tsuji–Trost reaction in the presence of Pd0,24 leading to the releasing of free MB, accompanied with color change and turn-on NIR emission (Scheme 1).
 | ||
| Scheme 1 Chemical structure and proposed sensing process of MB-APC for Pd0 based on Tsuji–Trost reaction. | ||
Results and discussion
The time-dependent absorption and fluorescence intensity changes of probe MB-APC after adding 3 equivalent of Pd(PPh3)4 were evaluated to investigate its spectral properties and response to Pd0. As shown in Fig. 1A, MB-APC exhibited almost no absorbance in the visible region in the EtOH-PBS (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution. An absorption peak appeared at around 657 nm after adding 3 equivalent of Pd(PPh3)4 solution, which increased gradually with incubation time and reached the maximum values after incubation for 30 min at room temperature. In the fluorescence spectra, no emission could be observed for MB-APC solution (Fig. 1B), which was consistent with leucomethylene blue. After adding 3 equivalent of Pd(PPh3)4 solution, an emission peak at approximately 676 nm appeared and was significantly enhanced with incubation time, accompanied with slight red-shift. The emission peak was shifted to 681 nm and the fluorescence intensity reached a plateau in 30 min at room temperature (Fig. 1B). The turn-on fluorescence may be attributed to the releasing of free MB moiety through the broken of the carbamate bond, which was confirmed by almost the same absorbance and emissive peak with MB from commercial source (Fig. S4†), and the HRMS result (Fig. S5†). It is also noteworthy that both the excitation and emission of MB-APC reach the NIR range, which is very attractive for intracellular sensing and imaging.
1, v/v) solution. An absorption peak appeared at around 657 nm after adding 3 equivalent of Pd(PPh3)4 solution, which increased gradually with incubation time and reached the maximum values after incubation for 30 min at room temperature. In the fluorescence spectra, no emission could be observed for MB-APC solution (Fig. 1B), which was consistent with leucomethylene blue. After adding 3 equivalent of Pd(PPh3)4 solution, an emission peak at approximately 676 nm appeared and was significantly enhanced with incubation time, accompanied with slight red-shift. The emission peak was shifted to 681 nm and the fluorescence intensity reached a plateau in 30 min at room temperature (Fig. 1B). The turn-on fluorescence may be attributed to the releasing of free MB moiety through the broken of the carbamate bond, which was confirmed by almost the same absorbance and emissive peak with MB from commercial source (Fig. S4†), and the HRMS result (Fig. S5†). It is also noteworthy that both the excitation and emission of MB-APC reach the NIR range, which is very attractive for intracellular sensing and imaging.
 | ||
Fig. 1 UV-vis (A) and fluorescence spectral changes (B) of MB-APC (10 μM) against time in the presence of Pd(PPh3)4 (3 equiv.) in EtOH-PBS (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature. 1, v/v) at room temperature. | ||
To further explore the sensing ability of MB-APC to Pd0, UV-vis and fluorescence titration experiments were carried out. As shown in Fig. 2A, the absorption band at 657 nm enhanced with the increasing Pd0 concentrations concomitantly. The significant absorbance enhancement (about 393 fold increase) was accompanied by a marked and vivid color change from achromatic to blue in ambient light (inset of Fig. 2A), demonstrating that MB-APC could be utilized as an on-site and naked-eye indicator for Pd0. Meanwhile, the linear equation was obtained. As shown in Fig. S6A,† the plots of absorption fitted linearly with Pd0 concentration range of 0–6 μM with a correlation coefficient of 0.994. The detection limit of absorption for Pd0 was as low as 12.48 nM. Moreover, the enhancement (about 55 fold increase) of fluorescence intensity can be easily found upon addition of increasing amounts of Pd0, which could also be observed by the naked eye under the UV irradiation (Fig. 2B). As shown in Fig. S6B,† the plots of fluorescence fit linearly with Pd0 concentration range of 0–2 μM with a correlation coefficient of 0.994. The detection limit of fluorescence for Pd0 was deduced to be as low as 5.7 nM. The result indicated that MB-APC had low detection limit in both absorption and fluorescence, which illustrated that the probe can be utilized as an efficient tool for sensing traces of palladium in both colorimetry and fluorescence signal with high sensitivity. Compared with other reported fluorescent probes for Pd0 (Table S1†), NIR excitation and emission, and high sensitivity of MB-APC makes it a robust tool for sensing in both environment and biology samples.
To clarify the specificity of MB-APC towards Pd0 over other metal ions, the absorption and the fluorescent spectra response were further investigated upon addition of various metal ions to EtOH-PBS solution of MB-APC, respectively. 3 equivalent of Pd0 and 10 equivalent of other metal ions were added to the detecting system. As expected, only Pd0 caused such remarkable signal changes in the both absorption and fluorescence spectra of MB-APC (Fig. 3). In contrast, other noble metal with catalysis such as Ru0, and other metal ions such as Ag+, Fe3+, Hg2+, K+, Na+, Pb2+, Ca2+, Mg2+, Cu2+, Ni2+, Co2+, Ba2+, Zn2+, Mn2+, Pt2+, Cd2+, Al3+, Cr3+, showed almost negligible disturbance to the spectra and color (Fig. 3), even up to 10 equivalent concentrations, indicating that MB-APC showed a notable selectivity to Pd0. MB-APC could efficiently discriminate Pd0 from other metal ions by the visual readout (Fig. S7†). Moreover, metal chelators such as EDTA have little influence on this detecting system (Fig. S8†). All these experimental results suggested that this probe MB-APC had an excellent selectivity for Pd0 over other metal ions.
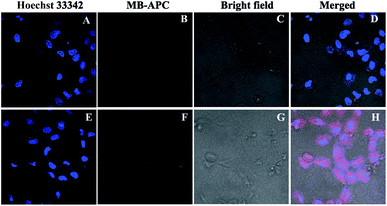
Encouraged by the above findings, the potential intracellular applications of MB-APC were investigated using confocal laser scanning microscopy. We first studied the cytotoxicity of this probe toward using a standard MTT assay. The results revealed that exhibited very low cytotoxicity to living cells for 48 h even up to 100 μM (Fig. S9†). Living cell imaging assay was conducted using HeLa cells. As shown in Fig. 4A–D, co-staining living cells using Hoechst 33342 revealed that free MB-APC exhibited almost no fluorescence signal, which was consistent with fluorescent spectra studies. By contrast, after incubation with Pd0 for 30 min, a distinct strong emission response could be observed (Fig. 4E–H). The results demonstrated that MB-APC is both cell-permeable and capable of sensing palladium in living cells, which makes it a versatile tool for the detection of palladium species in environmental samples and in living cells.
Conclusions
In conclusion, a colorimetric and NIR fluorescent probe MB-APC for Pd0 was rationally developed. The distinct color change and turn-on NIR emission make it an on-site and visual indicator for Pd0. MB-APC also exhibited high specificity and sensitivity, with the detection limit of 5.7 nM. In addition, MB-APC has been successfully applied in detecting and imaging of Pd0 in living cells, which revealed that MB-APC has the potential to track intracellular palladium species taking advantage of its specific turn-on NIR emission. All these results featured its promising application prospects for palladium sensing in both environment and biology field.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21502056), the Natural Science Foundation of Guangdong Province, China (2016A030310463), the Pandeng project for undergraduate in Guangdong Province (j2tw-k315001-16 to L. F. Z.) and National Undergraduate Innovative and Entrepreneurial Training Program (201610561163 to L. F. Z.).Notes and references
- R. R. Barefoot, Trends Anal. Chem., 1999, 18, 702–707 CrossRef CAS.
- K. Pyrznska, J. Environ. Monit., 2000, 2, 99N–103N RSC.
- K. Ravindra, L. Bencs and R. Van Grieken, Sci. Total Environ., 2004, 318, 1–43 CrossRef CAS PubMed.
- R. Merget and G. Rosner, Sci. Total Environ., 2001, 270, 165–173 CrossRef CAS PubMed.
- J. Kielhorn, C. Melber, D. Keller and I. Mangelsdorf, Int. J. Hyg. Environ. Health, 2002, 205, 417–432 CrossRef CAS PubMed.
- B. Dimitrova, K. Benkhedda, E. Ivanova and F. Adams, J. Anal. At. Spectrom., 2004, 19, 1394–1396 RSC.
- C. Locatelli, D. Melucci and G. Torsi, Anal. Bioanal. Chem., 2005, 382, 1567–1573 CrossRef CAS PubMed.
- K. Van Meel, A. Smekens, M. Behets, P. Kazandjian and R. Van Grieken, Anal. Chem., 2007, 79, 6383–6389 CrossRef CAS PubMed.
- H. Li, J. Fan and X. Peng, Chem. Soc. Rev., 2013, 42, 7943–7962 RSC.
- M. P. Tracey, D. Pham and K. Koide, Chem. Soc. Rev., 2015, 44, 4769–4791 RSC.
- J.-w. Yan, X.-l. Wang, Q.-f. Tan, P.-f. Yao, J.-h. Tan and L. Zhang, Analyst, 2016, 141, 2376–2379 RSC.
- J. Zhang, L. Zhang, Y. Zhou, T. Ma and J. Niu, Microchim. Acta, 2013, 180, 211–217 CrossRef CAS.
- Q. Huang, Y. Zhou, Q. Zhang, E. Wang, Y. Min, H. Qiao, J. Zhang and T. Ma, Sens. Actuators, B, 2015, 208, 22–29 CrossRef CAS.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- N. Ormeci, B. Savas, S. Coban, M. Palabıyıkoğlu, A. Ensari, I. Kuzu and N. Kursun, Surg. Endosc., 2008, 22, 693–700 CrossRef CAS PubMed.
- J. P. Tardivo, A. Del Giglio, C. S. de Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, R. de Fátima Turchiello and M. S. Baptista, Photodiagn. Photodyn. Ther., 2005, 2, 175–191 CrossRef CAS PubMed.
- M. Wagner, E. R. Suarez, T. R. Theodoro, C. D. A. S. Machado Filho, M. F. M. Gama, J. P. Tardivo, F. M. Paschoal and M. A. S. Pinhal, Clin. Exp. Dermatol., 2012, 37, 527–533 CrossRef CAS PubMed.
- Y. Ashitate, B. T. Lee, R. G. Laurence, E. Lunsford, M. Hutteman, R. Oketokoun, H. S. Choi and J. V. Frangioni, Ann. Plast. Surg., 2013, 70, 360–365 CrossRef CAS PubMed.
- A. Matsui, E. Tanaka, H. S. Choi, V. Kianzad, S. Gioux, S. J. Lomnes and J. V. Frangioni, Surgery, 2010, 148, 78–86 CrossRef PubMed.
- F. P. R. Verbeek, J. R. van der Vorst, B. E. Schaafsma, R.-J. Swijnenburg, K. N. Gaarenstroom, H. W. Elzevier, C. J. H. van de Velde, J. V. Frangioni and A. L. Vahrmeijer, J. Urology, 2013, 190, 574–579 CrossRef CAS PubMed.
- E. Tanaka, F. Y. Chen, R. Flaumenhaft, G. J. Graham, R. G. Laurence and J. V. Frangioni, J. Thorac. Cardiovasc. Surg., 2009, 138, 133–140 CrossRef CAS PubMed.
- J. Bae, L. E. McNamara, M. A. Nael, F. Mahdi, R. J. Doerksen, G. L. Bidwell, N. I. Hammer and S. Jo, Chem. Commun., 2015, 51, 12787–12790 RSC.
- F. Song, A. L. Garner and K. Koide, J. Am. Chem. Soc., 2007, 129, 12354–12355 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of MB-APC, experimental procedures, and supplemental spectra and graphs. See DOI: 10.1039/c6ra27502h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |