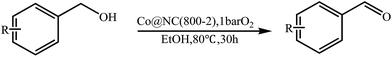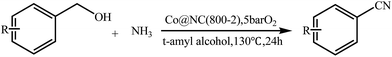Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterogeneous cobalt catalysts for selective oxygenation of alcohols to aldehydes, esters and nitriles†
Fei Mao,
Zhengliang Qi,
Haipeng Fan,
Dejun Sui,
Rizhi Chen and
Jun Huang *
*
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing 210009, P. R. China. E-mail: junhuang@njtech.edu.cn; Fax: +86-25-83172261
First published on 5th January 2017
Abstract
Efficient and green oxygenation of alcohols to the corresponding aldehydes, esters and nitriles was developed with high selectivity. Functional alcohols, including some heterocyclic and allylic alcohols can be oxygenated to the corresponding aldehydes, esters and nitriles respectively. Moreover, the catalyst can be recycled and reused without significant deactivation. Noteworthy, the Co@NC (800-2h) catalyzed oxygenation of alcohols can be regulated easily by changing the reaction conditions, and then the corresponding aldehydes, esters and nitriles can be obtained in high yields respectively.
Introduction
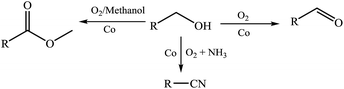
Selective oxygenation of alcohols is not only of wide concern in basic chemistry research, but it is also a great challenge for the production of bulk chemical.1 Selective oxidation of alcohols is a powerful tool in the synthesis of complex organic molecules such as natural products, pharmaceuticals and polymers.2 Generally, esters are prepared by a two-step synthetic procedure, involving the synthesis of the carboxylic acids or activated acid derivatives, and a subsequent reaction with alcohols.3 As a result, the multistep process is often accompanied by the formation of toxic and harmful byproducts.4 At the same time, nitriles are important building blocks of dyes, natural products, herbicides, agrochemicals, and pharmaceuticals, and the preparation of nitriles is often through multistep process using toxic HCN or metal cyanides.5 The ammoxidation has been developed for the production of some nitriles under drastic conditions in industrial application.6 The development of selective oxidation reactions is an important topic and is crucial for the advancement of green and sustainable industrial processes.7 In recent years, much effort has been devoted to the oxidation of alcohols to esters by a single step, which could represent a big step forward toward green, economic, and sustainable processes because of the availability and low cost of alcohols in comparison to their oxidation products such as aldehydes and carboxylic acids.8 But the relevant reports are limited to noble metal catalysts, such as palladium, gold, and ruthenium. Moreover, some homogeneous systems are used for the oxidation process, which are economically unfavorable.9 So, it is desirable to develop cost-effective, heterogeneous catalysts with good selectivity.10 A possible solution to this problem can be the increased utilization of cheap metal catalysts, such as iron, cobalt and copper catalysts.11Recently, some cobalt and iron catalysts were reported for the oxidation of alcohols to the corresponding esters and nitriles, and good yields were obtained.12 A heterogeneous Co catalyst system was reported by us for the reductive amination of aldehydes and ketones, and the Co catalyst system exhibited good activity and excellent selectivity.13 Herein, the heterogeneous Co catalysts were used for the selective oxidation of alcohols. Through controlling the reaction conditions, the Co catalyzed oxygenation of alcohols can give esters, aldehydes and nitriles selectively (Scheme 1).
Results and discussion
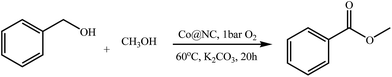
As developed previously, the supported cobalt catalysts (Co@NC) were prepared by pyrolysis of Co(OAc)2 with 1-methyl-3-cyanomethyl-1H-imidazolium chloride ([MCNIm]Cl) in activated carbon (detailed experimental see ESI†). The prepared Co@NC catalysts were tested for the oxidative cross esterification of benzyl alcohol with methanol to methyl benzoate with O2 as the oxidant with addition of K2CO3 as a base. The optimization of the catalyst preparation conditions was performed and the results are shown in Table 1.| Entry | Catalyst | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co (mol) Co (mol) |
Convb. (%) | Selb. (%) |
|---|---|---|---|---|
| a Reaction conditions: 1.0 mmol of benzyl alcohol, 3.0 mol% Co with Co@NC, 4.0 mL of CH3OH, 0.2 mmol of K2CO3, under 1 bar O2, at 60 °C, 20 h.b Determined by GC analysis.c Without base.d Without O2.e Without base and O2. In case of lower yields, benzaldehyde was detected as a minor product. IL = [MCNIm]Cl, C = active carbon. | ||||
| 1 | Co@NC (500-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 30 |
| 2 | Co@NC (600-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 64 |
| 3 | Co@NC (700-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 80 |
| 4 | Co@NC (700-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 88 |
| 5 | Co@NC (800-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 91 |
| 6 | Co@NC (800-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 95 |
| 7 | Co@NC (800-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
100 | 98 |
| 8 | C/Co (800-2h) | — | 20 | 12 |
| 9 | C/IL (800-2) | — | 16 | 2 |
| 10 | C (800-2h) | — | 3 | <1 |
| 11c | Co@NC (800-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
100 | 30 |
| 12d | Co@NC (800-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
21 | 6 |
| 13e | Co@NC (800-2h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
14 | 8 |
| 14 | — | — | 2 | <1 |
The Co@NC (800-2h) (carbonation at 800 °C under N2 for 2 hours) was the most active catalyst for the oxidative cross esterification of benzyl alcohol with methanol to methyl benzoate in 98% yield at 60 °C within 20 h (Table 1, entry 7). The effect of calcination temperature for catalyst preparation was studied, and the Co@NC catalysts calcined at low temperature showed lower selectivity for the methyl benzoate (Table 1, entries 1–4). The ratio of the IL/Co played a role, and the ratio of 3/1 was best for the catalyst preparation (Table 1, entries 5–7). The catalyst Co/C (800-2h) (pyrolyzed a mixture of cobalt(II) acetate in activated carbon) showed low activity for the oxidative esterification of benzyl alcohol with methanol to methyl benzoate in only 12% yield (Table 1, entry 8). Catalysts without adding Co metal, both C/IL (800-2h) and C (800-2h) have no catalytic activity (Table 1, entries 9 and 10). In addition, reaction conditions were optimized for the oxidative esterification of benzyl alcohol with methanol. The oxidation gave methyl benzoate in only 30% yield without base (Table 1, entry 11). If the reaction was performed without O2 (under N2) or without both base and O2, the yield was quite low (Table 1, entries 12 and 13). The oxidation cannot give methyl benzoate without catalyst, base and O2 (Table 1, entry 14).
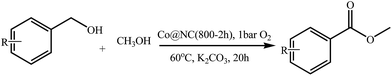
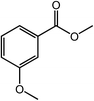
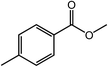
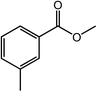
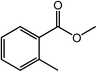
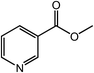
With the optimized reaction conditions, the applying scope of the Co@NC (800-2h) for the oxidative cross esterification of benzyl alcohols with methanol was investigated, and the results are presented in Table 2. We are delighted that a wide range of benzyl alcohols can be oxidized to the corresponding methyl esters in high yields. Benzyl alcohols substituted with p-OMe, m-OMe, p-Me, m-Me and o-Me groups can be converted to the corresponding methyl esters in high yields (88–97%) (Table 2, entries 2–6). Moreover, the oxidation of nitrobenzyl alcohol in methanol gave methyl nitrobenzoate in 94% yield (Table 2, entry 7). Notably, the oxidation of halides (including F, Cl and Br) substituted benzyl alcohols afforded the corresponding methyl benzoates in good yields (Table 2, entries 8–13). Naphthalenemethanol and (methylenedioxy) phenylmethanol were oxidized into the corresponding methyl esters in good yields (Table 2, entries 14, 15). The oxidation of cinnamic alcohol in methanol gave methyl cinnamate in 91% yield. Besides, heterocycle alcohols, furfuralcohol, and pyridine-2-methanol and pyridine-3-methanol were transferred into the corresponding heterocyclic carboxylic acid esters in good yields (Table 2, entries 17–19).
| Entry | Alcohol | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1.0 mmol of benzyl alcohol, 3.0 mol% Co with Co@NC (800-2h), 4.0 mL of CH3OH, 0.2 mmol of K2CO3, under 1 bar O2, at 60 °C, 20 h.b Isolated yields. | |||
| 1 |  |
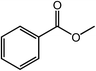 |
98 |
| 2 | 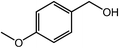 |
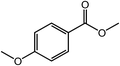 |
96 |
| 3 | 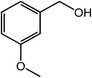 |
 |
93 |
| 4 | 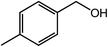 |
 |
97 |
| 5 | 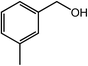 |
 |
93 |
| 6 | 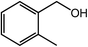 |
 |
88 |
| 7 | 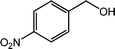 |
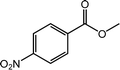 |
94 |
| 8 | 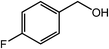 |
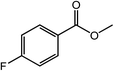 |
95 |
| 9 | 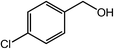 |
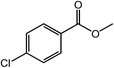 |
98 |
| 10 | 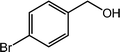 |
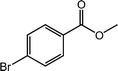 |
99 |
| 11 | 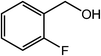 |
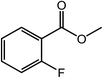 |
85 |
| 12 | 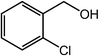 |
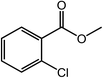 |
85 |
| 13 | 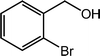 |
 |
86 |
| 14 |  |
 |
87 |
| 15 |  |
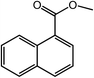 |
98 |
| 16 | 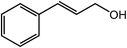 |
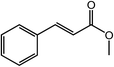 |
91 |
| 17 | 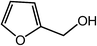 |
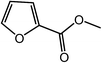 |
88 |
| 18 | 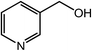 |
 |
95 |
| 19 | 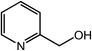 |
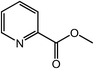 |
90 |
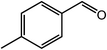
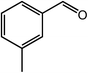
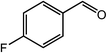
As benzaldehyde was obtained as a byproduct in the cross oxidative esterification of benzyl alcohol with methanol, the selective oxidation of benzyl alcohol to benzaldehyde was tested. Without base added, Co@NC (800-2h) catalyzed selective oxidation of benzyl alcohol gave benzaldehyde in excellent yield (Table 3, entry 1). Encouraged by the success, we examined various benzyl alcohols for the oxidation, and the results are listed in Table 3. Benzyl alcohols with functional groups, such as CH3, NO2, F, Cl, Br and MeO, can be oxidized efficiently to the corresponding benzaldehydes in good to excellent yields (Table 3, entries 2–9). The Co@NC (800-2h) catalyzed oxidation of diphenylmethanol afforded the benzophenone in good yield (Table 3, entry 10). Besides, cinnamyl alcohol can also be applied in this system, and cinnamaldehyde was obtained in 90% yield (Table 3, entry 11). Additionally, heterocyclic substrates such as 2-pyridinemethanol and furfuralcohol, were converted to the pyridylaldehyde and furaldehyde in 90% and 89% yields respectively (Table 3, entries 12 and 13). Base can speed up the oxidation of alcohols but reduce the selectivity. Without base, aldehydes can be obtained selectively under mild conditions.
| Entry | Alcohol | Product | Yieldb,c (%) |
|---|---|---|---|
| a Reaction conditions: 1.0 mmol of alcohol, 2.0 mL of EtOH, 5.0 mol% Co with Co@NC (800-2h), under 1 bar O2, at 80 °C, 30 h.b GC yields.c Isolated yields (isolated yields are slightly lower than related GC yields for volatile products). | |||
| 1 | 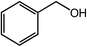 |
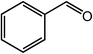 |
99, 96 |
| 2 | 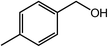 |
 |
99, 95 |
| 3 | 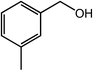 |
 |
95, 93 |
| 4 | 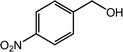 |
 |
95, 94 |
| 5 | 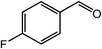 |
 |
92, 88 |
| 6 | 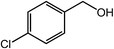 |
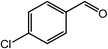 |
96, 93 |
| 7 | 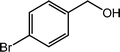 |
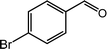 |
97, 94 |
| 8 | 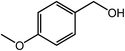 |
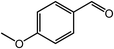 |
94, 90 |
| 9 | 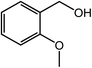 |
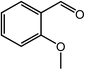 |
90, 87 |
| 10 | 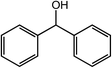 |
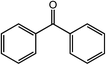 |
94, 94 |
| 11 | 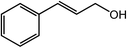 |
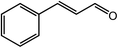 |
90, 87 |
| 12 | 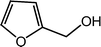 |
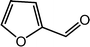 |
89, 93 |
| 13 | 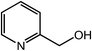 |
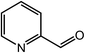 |
90, 85 |
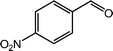
Next, we tried the ammoxidation of alcohols to nitriles, as nitriles are widely applicable in pharmaceuticals and biology active molecules. Delightedly, the Co@NC (800-2h) catalyst also allows the straightforward synthesis of nitriles from alcohols using aqueous ammonia and molecular oxygen. It was observed that the oxidation of substituted benzyl alcohols gave the corresponding nitriles in good to excellent yields (Table 4, entries 1–14). But slightly low yields of nitriles were obtained when ortho-substituted benzyl alcohols were used for the ammoxidation reaction (Table 4, entries 10–12). Moreover, cinnamonitrile can be obtained in high yield from the ammoxidation of cinnamyl alcohol (Table 4, entry 15).
| Entry | Alcohol | Product | Yieldb,c (%) |
|---|---|---|---|
| a Reaction conditions: 1.0 mmol of alcohol, 2.0 mL of t-amyl alcohol, 5.0 mol% Co with Co@NC (800-2h), 200 μl of aq. NH3, under 5 bar O2, at 130 °C, 24 h.b GC yields.c Isolated yields (isolated yields are slightly lower than related GC yields for volatile products). | |||
| 1 | 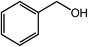 |
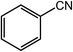 |
99, 94 |
| 2 | 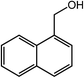 |
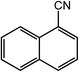 |
97, 93 |
| 3 | 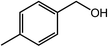 |
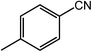 |
98, 94 |
| 4 | 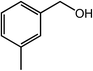 |
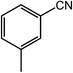 |
95, 92 |
| 5 | 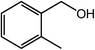 |
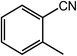 |
92, 89 |
| 6 | 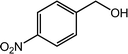 |
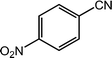 |
98, 95 |
| 7 | 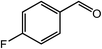 |
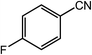 |
96, 93 |
| 8 | 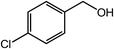 |
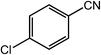 |
97, 93 |
| 9 | 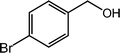 |
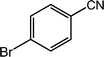 |
97, 94 |
| 10 | 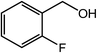 |
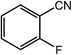 |
91, 87 |
| 11 | 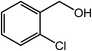 |
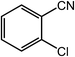 |
93, 90 |
| 12 | 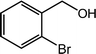 |
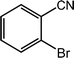 |
92, 90 |
| 13 | 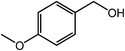 |
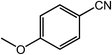 |
95, 93 |
| 14 | 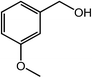 |
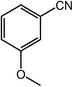 |
94, 92 |
| 15 | 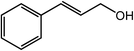 |
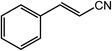 |
97, 93 |
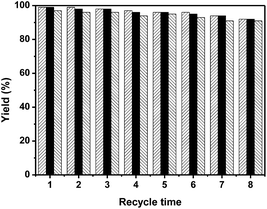
The reusability of Co@NC (800-2h) was investigated for the oxidation of benzyl alcohol to methyl benzoate, benzaldehyde and benzonitrile respectively. The recovered catalyst was reused after filtered, washed and dried under vacuum at room temperature. Under the same reaction conditions, methyl benzoate, benzaldehyde and benzonitrile can be obtained in excellent yields respectively with the reused Co@NC (800-2h) catalyst (Fig. 1). No evident deactivation was found after 8 times of reusability of the Co@NC (800-2h) catalyst in all the 3 oxidation reactions (Fig. 1). Moreover, no Co was detected (ICP-AES) in the solution after removal of the Co@NC (800-2h) by filtration.
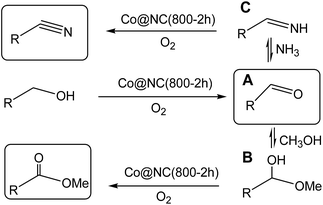
To investigate the relationship of the catalyst structure with the performance, the catalysts were characterized by XRD, XPS, TEM, SEM and N2 adsorption analysis. Since the catalysts were applied for the reductive amination of aldehydes with amines, and the catalyst structure was studied extensively.13 As we can see only metallic β-Co peaks but no CoO or Co3O4 peaks from XRD spactra, we can image that the Co(0) particles were coated with CoO in the Co@NC (800-2h) since Co2+ was detected by XPS spectra (Fig. S1 and S2†). It is interesting that the recycled Co@NC (800-2h) was nearly the same XRD spectra, we can see only metallic β-Co peaks from the XRD spectra (Fig. S2B†), which means Co in the recycled Co@NC (800-2h) was also Co(0) particles coated with CoO. With variable chemical valences, cobalt is able to transfer electrons and act as the active site for oxygen transfer.14 Thus, the obtained Co@NC (800-2h) is responsible for the catalytic activity of the oxidations. Based on above oxidation reaction results, we proposed the pathway of the oxidation of alcohols to aldehydes, esters and nitriles (Scheme 2). The alcohols are oxidized to the corresponding aldehydes (A) firstly, and then reacted with methanol or NH3 to the intermediates B or C (Scheme 2). Finally, the B and the C can be oxidized to esters and nitriles respectively.
Conclusions
In summary, effective Co catalysts were developed and applied for the selective oxygenation of alcohols to aldehydes, esters and nitriles. The catalysts Co@NC can be prepared simply and applied widely for the selective oxidations. Noteworthy, the Co@NC (800-2h) catalyzed oxygenation of alcohols can be regulated easily by changing the reaction conditions, and then the corresponding aldehydes, esters and nitriles can be obtained respectively. In addition, functional alcohols, including heterocyclic and allylic alcohols can also be oxygenated to the corresponding aldehydes, esters and nitriles respectively. Moreover, the catalyst can be recycled and reused without significant deactivation. Thus, green, economical and sustainable processes to aldehydes, esters and nitriles were developed using oxygen as final oxidant with high selectivity.Acknowledgements
This work was supported by the National Natural Science of Foundation of China (grant no. 21676140), the fund from the State Key Laboratory of Materials-Oriented Chemical Engineering (grant ZK201402) and the Project of Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- (a) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780–1824 CrossRef CAS PubMed; (b) S. Gaspa, A. Porcheddu and L. D. Luca, Adv. Synth. Catal., 2016, 358, 154–158 CrossRef CAS; (c) B. Karimi, M. Rafiee, S. Alizadeha and H. Vali, Green Chem., 2015, 17, 991–1000 RSC; (d) J. B. Xie, J. J. Bao, H. X. Li, D. W. Tan, H. Y. Li and J. P. Lang, RSC Adv., 2014, 4, 54007–54017 RSC.
- (a) L. L. Chang, J. H. Yang and J. Y. Ying, ChemSusChem, 2015, 8, 1916–1925 CrossRef PubMed; (b) R. V. Jagadeesh, H. Junge, M. Pohl, J. Radnik, A. Bruckner and M. Beller, J. Am. Chem. Soc., 2013, 135, 10776–10782 CrossRef CAS PubMed; (c) L. Y. Wang, S. S. Shang, G. S. Li, L. H. Ren, Y. Lv and S. Gao, J. Org. Chem., 2016, 81, 2189–2193 CrossRef CAS PubMed; (d) R. V. Jagadeesh, H. Junge and M. Beller, Nat. Commun., 2014, 5, 4123 CAS; (e) R. A. Molla, K. Ghosh, K. Tuhina and S. M. Islam, New J. Chem., 2015, 39, 921–930 RSC.
- J. Otera, Esterification: Methods, Reactions, and Applications, Wiley-VCH, Weinheim, 2003 Search PubMed.
- (a) K. D. Chau, F. Duus and T. N. Le, Green Chem. Lett. Rev., 2013, 6, 89–93 CrossRef CAS; (b) J. D. Nguyen, E. M. D'Amato, J. M. Narayanam and C. R. Stephenson, Nat. Chem., 2012, 4, 854–859 CrossRef CAS PubMed.
- (a) P. Anbarasan, T. Schareina and M. Beller, Chem. Soc. Rev., 2011, 40, 5049–5067 RSC; (b) M. Sundermeier, A. Zapf, S. Mutyala and W. Baumann, Chem.–Eur. J., 2003, 9, 1828–1836 CrossRef CAS PubMed.
- (a) S. J. Guo, G. Wan, S. Sun, Y. Jiang, J. T. Yu and J. Cheng, Chem. Commun., 2015, 51, 5085–5088 RSC; (b) C. P. Kumar, K. R. Reddy, V. V. Rao and K. V. R. Chary, Green Chem., 2002, 4, 513–516 RSC; (c) L. Wang, B. Guo, H. X. Li, Q. Li, H. Y. Li and J. P. Lang, Dalton Trans., 2013, 42, 15570–15580 RSC.
- (a) Y. X. Zhou, Y. Z. Chen, L. N. Cao, J. L. Lu and H. L. Jiang, Chem. Commun., 2015, 51, 8292–8295 RSC; (b) J. Miura, T. Akaogi and H. Ishida, ACS Catal., 2013, 3, 1845–1849 CrossRef.
- (a) C. Liu, J. Wang, L. Meng, Y. Deng, Y. Li and A. Lei, Angew. Chem., Int. Ed., 2011, 50, 5144–5148 CrossRef CAS PubMed; (b) H. Wang, L. Li, X. F. Bai, J. Y. Shang, K. F. Yang and L. W. Xu, Adv. Synth. Catal., 2013, 355, 341–347 CAS; (c) C. H. Bai, A. Q. Li, X. F. Yao, H. L. Liu and Y. W. Li, Green Chem., 2016, 18, 1061–1069 RSC; (d) A. M. Whittaker and V. M. Dong, Angew. Chem., Int. Ed., 2015, 54, 1312–1315 CrossRef CAS PubMed; (e) X. L. Lang, M. Q. Shi, Y. K. Jiang, H. Chen and C. N. Ma, RSC Adv., 2016, 6, 13873–13880 RSC.
- (a) A. Corma, H. Garcia and F. X. Llabrés, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed; (b) L. M. Dornan and M. J. Muldoon, Catal. Sci. Technol., 2015, 5, 1428–1432 RSC; (c) C. Liu, S. Tang and A. Lei, Chem. Commun., 2013, 49, 1324 RSC; (d) J. H. Xia, A. L. Shao, S. Tang, X. L. Gao, M. Gao and A. W. Lei, Org. Biomol. Chem., 2015, 13, 6154–6157 RSC; (e) S. Wang, S. T. Yin, G. W. Chen, L. Li and H. Zhang, Catal. Sci. Technol., 2016, 6, 4090–4104 RSC; (f) H. Miyamura, T. Yasukawa and V. Kobayashi, Green Chem., 2010, 12, 776–778 RSC; (g) K. Kaizuka, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2010, 132, 15096–15098 CrossRef CAS PubMed; (h) C. Gunanathan, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2009, 131, 3146–3147 CrossRef CAS PubMed; (i) C. M. Nicklaus, P. H. Phua, T. Buntara, S. Noel, H. J. Heeres and J. G. Vries, Adv. Synth. Catal., 2013, 355, 2839–2844 CrossRef CAS; (j) T. L. Gianetti, S. P. Annen, G. S. Quinones, M. Reiher, M. Driess and H. Grîtzmacher, Angew. Chem., Int. Ed., 2016, 55, 1854–1858 CrossRef CAS PubMed.
- (a) W. Zhong, H. L. Liu, C. H. Bai, S. J. Liao and Y. W. Li, ACS Catal., 2015, 5, 1850–1856 CrossRef CAS; (b) C. Parmeggiani and F. Cardona, Green Chem., 2012, 14, 547–564 RSC; (c) K. Ghosh, M. A. Iqubal, R. A. Molla, A. Mishra, Kamaluddin and S. Manirul Islam, Catal. Sci. Technol., 2015, 5, 1606–1622 CAS; (d) J. Gao, Z. G. Ren and J. P. Lang, J. Organomet. Chem., 2015, 792, 88–92 CrossRef CAS.
- (a) A. Boddien, D. Mellmann, F. Gartner, R. Jackstell, H. Junge, P. J. Dyson, G. Laurenczy, R. Ludwig and M. Beller, Science, 2011, 333, 1733–1736 CrossRef CAS PubMed; (b) H. Song, B. Kang and S. H. Hong, ACS Catal., 2014, 4, 2889–2895 CrossRef CAS; (c) V. Lyaskovskyy, A. I. O. Suarez, H. Lu, H. Jiang, X. P. Zhang and B. Bruin, J. Am. Chem. Soc., 2011, 133, 12264–12273 CrossRef CAS PubMed; (d) K.-I. Shimizu, K. Kon, M. Seto, K. Shimura, H. Yamazaki and J. N. Kondo, Green Chem., 2013, 15, 418 RSC; (e) J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302–1315 RSC; (f) C. Chen, B. Liu and W. Chen, Synthesis, 2013, 45, 3387–3391 CrossRef CAS; (g) M. Liu, T. Q. Chen and S. F. Yin, Catal. Sci. Technol., 2016, 6, 690–693 RSC; (h) D. W. Tan, J. B. Xie, Q. Li, H. X. Li, J. C. Li, H. Y. Li and J. P. Lang, Dalton Trans., 2014, 43, 14061–14071 RSC.
- (a) R. V. Jagadeesh, T. Stemmler, A.-E. Surkus, M. Bauer, M.-M. Pohl, J. Radnik, K. Junge, H. Junge, A. Brückner and M. Beller, Nat. Protoc., 2015, 10, 916–926 CrossRef PubMed; (b) T. Y. Cheng, H. Yu, F. Peng, H. J. Wang, B. S. Zhang and D. S. Su, Catal. Sci. Technol., 2016, 6, 1007–1015 RSC; (c) L. L. Geng, M. Zhang, W. X. Zhang, M. J. Jia, W. F. Yan and G. Liu, Catal. Sci. Technol., 2015, 5, 3097–3102 RSC.
- F. Mao, D. J. Sui, Z. L. Qi, H. P. Fan, R. Z. Chen and J. Huang, RSC Adv., 2016, 6, 94068–94073 RSC.
- (a) H. Y. Jin, J. Wang, D. F. Su, Z. Z. Wei, Z. F. Pang and Y. Wang, J. Am. Chem. Soc., 2015, 137, 2688–2694 CrossRef CAS PubMed; (b) A. M. Ullman and D. G. Nocera, J. Am. Chem. Soc., 2013, 135, 15053–15061 CrossRef CAS PubMed; (c) P. X. Li, R. G. Ma, Y. Zhou, Y. F. Chen, Q. Liu, G. H. Peng and J. C. Wang, RSC Adv., 2016, 6, 70763–70769 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra27073e |
| This journal is © The Royal Society of Chemistry 2017 |