Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
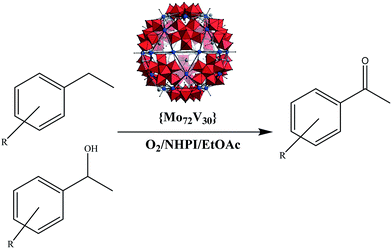
Selective aerobic benzylic C–H oxidation co-catalyzed by N-hydroxyphthalimide and Keplerate {Mo72V30} nanocluster†
Abdolreza Rezaeifard *,
Ashkan Khoshyan,
Maasoumeh Jafarpour* and
Mehrdad Pourtahmasb
*,
Ashkan Khoshyan,
Maasoumeh Jafarpour* and
Mehrdad Pourtahmasb
Catalysis Research Laboratory, Department of Chemistry, Faculty of Science, University of Birjand, Birjand, 97179-414 Iran. E-mail: rrezaeifard@birjand.ac.ir; mjafarpour@birjand.ac.ir
First published on 10th March 2017
Abstract
The catalytic efficiency of a Keplerate {Mo72V30} nanocapsule in the aerobic benzylic C–H oxidation of alcohols and hydrocarbons was exploited. The aerobic reactions were conducted using catalytic amounts of {Mo72V30} and under the co-catalytic effects of NHPI (N-hydroxyphthalimide) to furnish desired aldehydes and ketones in high yields and excellent selectivity. Different benzylic alcohols and hydrocarbons were shown to be amenable to oxidation under standard conditions presented here. Spectral data and leaching experiments revealed that the cluster had a long-term stability and could be used repeatedly in consecutive runs.
1. Introduction
The selective aerobic oxidation of alcohols is an important strategy for the synthesis of desired carbonyl compounds as valuable chemical intermediates with widespread applications in the perfumery, dye and agro chemical industries.1–3 The undesirable features of traditional reagents and methods such as chromium or manganese oxides and hypervalent iodine oxidants used for alcohol oxidation have forced chemists to use transition metal catalysts to reduce the economic and environmental costs of chemical production.4,5 Nevertheless, metal organic catalysts such as porphyrin and Schiff base complexes are disadvantaged by their oxidative instability and the consequent drawbacks in separating the products and contamination by residual catalysts.6,7 Further, the use of inexpensive and green oxidants such as O2 or H2O2 with high active oxygen content producing water as the sole by-product is another main issue in catalytic oxidation of alcohols and other substrates. Hence, development of clean, selective and atom-efficient methods is a fundamental challenge in modern oxidation methods and a hot topic within both academic and industrial arenas.4,5Polyoxometalates (POMs), with controllable redox and acidic properties at atomic or molecular levels have been well considered as alternative oxidation catalysts in the past few decades. POM-catalyzed oxidation of alcohols to aldehydes and ketones have been well documented mostly by employing Keggin type POMs and to a lesser extent by Wells–Dawson scaffolds.8 Among different transition metal containing POMs used for alcohol oxidation, V-substituted ones exhibited to be more competent to promote this transformation.9 The use of polyoxovanadates (POVs) in the oxidation of alcohols are disadvantaged by their high catalyst loadings and high reaction temperatures up to 135 °C, leading to the catalyst overheating, termed cooking, which results in concomitant catalyst deactivation.9
Giant nanosized porous Keplerate-type POMs, as a significant class of inorganic nanocapsules with variety of fascinating and unusual properties, have attracted more and more attention of scientists in the areas of chemistry, physics, biology, and materials.10,11 Nevertheless, unlike the commonly explored Keggin and Dawson POMs, they are largely unexplored for catalytic reactions.8
Following our discovery on catalytic activity of Keplerate {Mo132-OAc} nanoball in aerobic olefin epoxidation,12 as well as oxidation of sulfides and olefins with H2O2,13 a few works on catalytic activity of Keplerates has been done.14–18
In continuation of our recent research on development of selective aerobic C–H oxidation of benzylic alcohols and hydrocarbons,19–21 herein the catalytic efficiency of nanosized {Mo72V30} Keplerate POM,22 as a heterogeneous catalyst is exploited for the first time using O2/NHPI (N-hydroxyphthalimide) oxidation system, in ethyl acetate as a safe solvent (Scheme 1). The solid catalyst exhibited desired efficiency and selectivity in the oxidation of benzylic alcohols as well as benzylic hydrocarbons and proved to maintain its integrity under oxidation conditions and could be recyclable for many times.
2. Experimental
2.1. General remarks
All chemicals were purchased from Merck and Fluka Chemical Companies. Powder X-ray diffraction (XRD) was performed on a Bruker D8-advance X-ray diffractometer with Cu Ka (λ = 1.5406 Å) radiation. The FT-IR spectra were recorded on a Shimadzu 800 FT-IR system using a KBr pellet. UV-Vis spectra were recorded on a SPECORD® 210 PLUS. The TGA measurements were obtained by a TGA-50 (Shimadzu) at the heating rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 under 20 mL min−1 flowing air. Progresses of the reactions were monitored by TLC using silica-gel SIL G/UV 254 plates and also by GC on a Shimadzu GC-16A instrument using a 25 m CBP1-M25 (0.32 mm ID, 0.5 mm coating) capillary column.
°C min−1 under 20 mL min−1 flowing air. Progresses of the reactions were monitored by TLC using silica-gel SIL G/UV 254 plates and also by GC on a Shimadzu GC-16A instrument using a 25 m CBP1-M25 (0.32 mm ID, 0.5 mm coating) capillary column.
2.2. Synthesis of {Mo72V30}
{Mo72V30} was synthesized according to literature.22 A solution of NaVO3 (2.6 g, 21.3 mmol) dissolved in 55 mL H2O at 70 °C and then cooled to room temperature was added to a solution of Na2MoO4·2H2O (6 g, 24.8 mmol) dissolved in 75 mL H2O at room temperature. The mixture was acidified to pH 2.0 with H2SO4 (2 M; 14 mL) and then treated with N2H6SO4 (0.9 g, 6.9 mmol). The solution turned dark violet and the pH increased to 2.8. After stirring for 3 h a solution of KCl (3 g, 40.2 mmol) in 20 mL H2O was added to the mixture. Finally, the solution was filtered and the filtrate was left standing at room temperature. Black-purple hexagonal plates formed overnight. The FT-IR, UV-Vis spectra and XRD of as-prepared {Mo72V30} are given in ESI (Fig. S1–S3†). The synthesis and characterization of as-prepared ionic liquid polyoxometalates {C18mim–Mo72V30} and {DODA–Mo72V30} are given in ESI (Fig. S4–S11†).2.3. General procedure for oxidation of alcohols
To a mixture of alcohol (0.2 mmol) and 2 mg {Mo72V30} nanocapsule (0.1 μmol, 0.05 mol%) in 0.4 mL EtOAc was added 3 mg NHPI (0.02 mmol, 10 mol%) and the reaction mixture was stirred under 1 atm O2 (balloon) at 70 °C for the required time. The reaction progress was monitored by TLC and GC. After completion of the reaction EtOAc was added and the mixture was filtered (or centrifuged) to remove the catalyst. The products were purified by plate silica chromatography using n-hexane/ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
2.4. General procedure for oxidation of benzylic hydrocarbons
To a mixture of benzylic hydrocarbon (0.2 mmol) and 2 mg {Mo72V30} nanocapsule (0.1 μmol, 0.05 mol%) in 0.4 mL EtOAc was added 4.5 mg NHPI (0.03 mmol, 15 mol%) and the reaction mixture was stirred under 1 atm O2 (balloon) at 70 °C for the required time. The reaction progress was monitored by TLC and GC. After completion of the reaction EtOAc was added and the mixture was filtered (or centrifuged) to remove the catalyst. The products were purified by plate silica chromatography using n-hexane/ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
2.5. Recycling procedure for oxidation of benzyl alcohol
To a mixture of benzyl alcohol (0.2 mmol) and 2 mg {Mo72V30} nanocapsule (0.1 μmol, 0.05 mol%) in 0.4 mL EtOAc was added 3 mg NHPI (0.02 mmol, 10 mol%) and the reaction mixture was stirred under 1 atm O2 (balloon) at 70 °C for the required time. After completion of the reaction, EtOAc was added and the mixture was filtered (or centrifuged) to remove the catalyst followed by washing with EtOAc or ethanol as safe solvents. It was dried under vacuum and weighted before using in the next run to avoid errors resulting from loss of the catalyst during work up.3. Results and discussion
The catalytic activity of {Mo72V30} nanocapsule was examined in the oxidation of benzyl alcohol using molecular oxygen (1 atm, balloon) as oxidant. Blank reaction showed no benzyl alcohol conversion at different conditions. The reaction was then carried out by using {Mo72V30} as catalyst in different solvents, at various temperatures and different amounts of NHPI or catalyst. Data for benzaldehyde yield can be found in Table 1. Data in entries 1–5 reflect solvent effects on catalytic performance: EtOAc and CH3CN-mediated reactions resulted in the 89 and 78% benzaldehyde yields, respectively under heterogeneous conditions at 70 °C, whereas EtOH, and water only gave a low conversion and yield. Considering the environmentally impacts, EtOAC was applied as solvent for further studies. The reaction at 70 °C exhibits the highest benzaldehyde yield (89%, entry 5), and the corresponding yield decreased at lower temperatures (entries 6–9).| Runs | Solvent | T/°C | Yield% |
|---|---|---|---|
| a The reactions were stirred for 3 h under 1 atm O2 (balloon) in 0.4 mL solvent containing 0.2 mmol benzyl alcohol, 3 mg NHPI (0.02 mmol, 10 mol%) and 2 mg (0.1 μmol) catalyst. | |||
| 1 | Solvent free | 70 | 8 |
| 2 | Water | 70 | 13 |
| 3 | EtOH | 70 | 22 |
| 4 | Acetonitrile | 70 | 78 |
| 5 | EtOAc | 70 | 89 |
| 6 | EtOAc | 60 | 57 |
| 7 | EtOAc | 50 | 31 |
| 8 | EtOAc | 40 | 12 |
| 9 | EtOAc | 25 | 0 |
Screening of the catalyst and NHPI amount on the catalytic performance was further set of experiments. Data in entries 1–6 in Table 2 shows the effect of the applied catalyst amount on the catalytic performance. The higher the amount of catalyst loading, the higher are conversion and yield. After 3 h, the reaction reaches almost quantitative conversion of benzyl alcohol and 89% benzaldehyde was obtained using 0.05 mol% of catalyst (entries 1–4). Nevertheless, increasing the catalyst loading upto 0.5 and 1 mol% led to a significant decrease in the yields to 40 and 18%, respectively (entries 5, 6). The formation of the blackberry structures in concentrated solution of {Mo72V30} macoanion reduces remarkably the active sites.23
| Runs | Catalyst mol% | NHPI mol% | Oxidant | Yield% (3 h) |
|---|---|---|---|---|
| a The reactions were stirred at 70 °C in 0.4 mL ethyl acetate containing 0.2 mmol benzyl alcohol.b The amount of TEMPO was 10 mol%.c 0.4 mmol of oxidants were used. | ||||
| 1 | 0 | 10 | O2 | 0 |
| 2 | 0.01 | 10 | O2 | 54 |
| 3 | 0.02 | 10 | O2 | 67 |
| 4 | 0.05 | 10 | O2 | 89 |
| 5 | 0.5 | 10 | O2 | 40 |
| 6 | 1 | 10 | O2 | 18 |
| 7 | 0.05 | 0 | O2 | 0 |
| 8 | 0.05 | 5 | O2 | 24 |
| 9 | 0.05 | 7.5 | O2 | 46 |
| 10 | 0.05 | 12.5 | O2 | 60 |
| 11 | 0.05 | 15 | O2 | 47 |
| 12 | 0.05 | 10 | Air | 68 |
| 13 | 0.05 | 0 | TEMPO/O2b | 27 |
| 14 | 0.05 | 0 | H2O2c | 15 |
| 15 | 0.05 | 0 | UHPc | 46 |
| 16 | 0.05 | 0 | TBHPc | 22 |
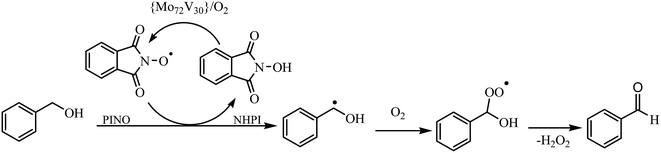
Knowing that the reaction does not proceed in the absence of NHPI, the effect of NHPI amount on the catalytic performance was studied. The data in entries 4, 7–11 in Table 2 revealed that the efficiency of oxidation was dependent on the NHPI amount. Increasing the NHPI loading to 10 mol% led to an increase in benzaldehyde yield to 89% demonstrating a co-catalyst role for NHPI in combination with {Mo72V30} (entry 4). Considering the free radical oxidation activity of NHPI,24,25 a radical pathway may be prevailed for title oxidation system using O2. The time course study of benzyl alcohol oxidation was investigated under optimized conditions. As shown in Fig. 1, benzyl alcohol conversion sharply increased with an increase in the reaction time from 30 to 180 min and, thereafter, it was mostly invariable which matches the expected chain propagation process during radical oxidation. To prove this claim, a radical scavengers such as 2,6-di-tert-butyl-4-methylphenol was added to the reaction mixture which led to a sluggish progress in the oxidation of benzyl alcohol under the same conditions.
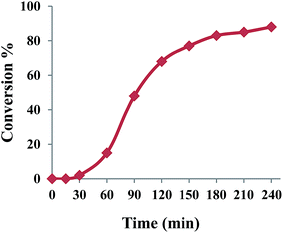 | ||
| Fig. 1 Reaction profile of the aerobic oxidation of benzyl alcohol co-catalyzed by {Mo72V30} and NHPI. | ||
The reaction performance was also decreased under “air” producing benzaldehyde in 68% yield after 3 h (entry 12).21 As expected, conducting the reaction under anoxic conditions (Ar atmosphere) did not yield any oxidation product at any conditions. Moreover, replacing the NHPI with TEMPO ((2,2,6,6-tetramethylpiperidin-1-yl)oxyl) under the same conditions, reduced the benzyl alcohol conversion to less than 30% (entry 13) due to steric hindrance associated with the methyl groups of TEMPO.5,26 Other common terminal oxidants, including H2O2 (30%), tert-butyl hydroperoxide (TBHP, 70%) and urea–hydrogen peroxide (UHP), were inferior producing benzaldehyde in 15, 22, and 46% yields, respectively after 3 h (entries 14–16). With respect to the above results, a radical mechanism involving both NHPI and Keplerate catalyst may be postulated (Fig. 2). The use of redox-active {Mo72V(IV)30}, in combination with O2 enable efficient conversion of NHPI into the phthalimido-N-oxyl (PINO) radical, abstracting H from the alcohol to produce an organic radical. Once the radical trapped by O2, leads to the formation of the carbonyl compounds after elimination of H2O2.9,27–29
The ability of other catalysts was probed to promote the oxidation of benzyl alcohol under optimized conditions (Table 3). Simple Mo and V salts (entry 1–4) exhibited poor activity under this condition (0–42%). Inspection of data in entries 5–10 in Table 3 indicates the activity rate of other Keplerate-type POMs. Fe-Containing polyoxomolybdate, {Mo72Fe30},30 was actually inactive catalyst for this transformation (entry 6). However, {Mo132},31 {Mo72Cr30},32 and {W72V30},33 exhibited low/moderate activity at the same conditions (entries 5–8). Further, modification of {Mo72V30} by organic cations such as 1-methyl-3-octadecaneimidazolium (C18mim, see ESI for the synthesis and characterization Fig. S4–S7†) and dioctadecyldimethylammonium (DODA, see ESI for the synthesis and characterization Fig. S8–S11†) producing organic–inorganic nanohybrides reduced the yield of benzaldehyde to 67 and 45%, respectively. Protection of active sites by the long chains of organic counter ions of title surfactant-encapsulated cluster may be a good reason for reduced efficiency.34
| Runs | Catalyst | Catalyst mol% | Yield% |
|---|---|---|---|
| a The reactions were stirred at 70 °C for 3 h under 1 atm O2 (balloon) in 0.4 mL ethyl acetate containing 0.2 mmol benzyl alcohol and 10 mol% NHPI (3 mg).b The mol% of simple salts are the same with metal concentration in Keplerate POMs, i.e. 72 times Mo and 30 times V. | |||
| 1 | NaVO3 | 1.5b | 36 |
| 2 | NaMoO4·2H2O | 3.6b | 0 |
| 3 | NaVO3 + NaMoO4·2H2O | 1.5% V + 3.6% Mob | 24 |
| 4 | VOSO4 | 1.5b | 42 |
| 5 | {Mo132} | 0.05 | 16 |
| 6 | {Mo72Fe30} | 0.05 | 0 |
| 7 | {Mo72Cr30} | 0.05 | 62 |
| 8 | {W72V30} | 0.05 | 48 |
| 9 | {Mo72V30} | 0.05 | 89 |
| 10 | {C18mim–Mo72V30} | 0.05 | 67 |
| 11 | {DODA–Mo72V30} | 0.05 | 45 |
Thus, with respect to the above results, to reach the best performance in the oxidation of benzyl alcohol, 0.05 mol% {Mo72V30} and 10 mol% NHPI should be stirred at 70 °C in EtOAC under molecular oxygen (1 atm, balloon) for 3 h, leading to 89% benzaldehyde. Similar to benzyl alcohol, a number of primary and secondary substituted benzylic alcohols were oxidized smoothly to their corresponding aldehyde (Table 4, entries 2–9) and ketones products (entries 10–13) regardless of the electronic and steric nature of the arene substituent. The formation of benzoic acids and esters by-products was completely controlled and desired aldehyde and ketones were obtained in yields ranging from 70% to quantitative. Nevertheless, aryl ring substituted with electron-withdrawing nitro group (entries 8, 9) did reduce the conversion of the starting material resulting from decreasing in the spin density on radical center.29 Further, treatment of cyclohexanol under the optimized conditions for 12 h gave only 2% cyclohexanone indicating that our conditions are not amenable for aliphatic C–H activation. However, the catalytic system oxidized 2-adamantanol moderately to the pertinent ketone under the same condition (entry 14).
| Entry | Substrate | Time (h) | Productb | Yieldc% | TOFd |
|---|---|---|---|---|---|
| a The reactions of entries 1–14 were run using 0.2 mmol substrate at 0.4 mL EtOAc under O2 (1 atm, balloon) at 70 °C with 0.05 mol% catalyst (2 mg) and 10 mol% NHPI (3 mg) except for entry 10 which was run at a 0.5 mmol scale (0.5 mmol 1-phenyl ethanol, 5 mg catalyst, 7.5 mg NHPI at 1 mL EtOAc, see Fig. S12 for GC analysis). Entries 15–18 use 15 mol% NHPI (4.5 mg).b The selectivity of products were >99% based on GC analysis.c GC yield based on benzyl chloride as internal standard (see ESI of ref. 35 for GC conditions). The yields in parenthesis refer to the isolated products.d TOF were calculated as moles of consumed substrate/moles of catalyst after 1 h. | |||||
| 1 |  |
3 | 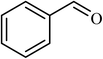 |
89 (85) | 300 |
| 2 | 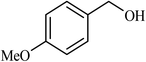 |
3 | 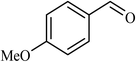 |
90 | 340 |
| 3 | 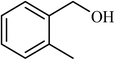 |
3.5 | 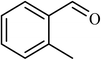 |
89 | 200 |
| 4 | 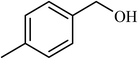 |
3 | 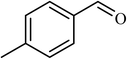 |
83 | 230 |
| 5 | 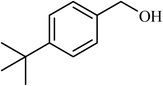 |
5 | 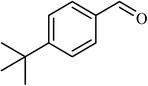 |
82 | 185 |
| 6 | 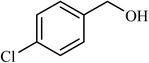 |
5 | 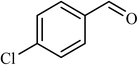 |
77 | 140 |
| 7 | 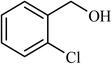 |
5 | 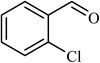 |
84 | 100 |
| 8 | 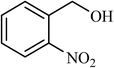 |
6 | 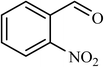 |
50 | 60 |
| 9 | 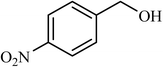 |
6 | 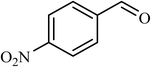 |
40 | 43 |
| 10 | 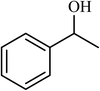 |
2.5 | 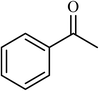 |
88 (85) | 620 |
| 11 | 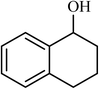 |
3 | 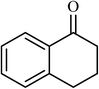 |
90 | 400 |
| 12 | 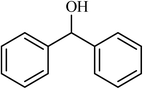 |
4 | 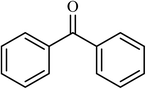 |
(78) | 180 |
| 13 | 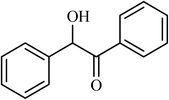 |
4.5 | 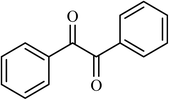 |
(66) | 80 |
| 14 | 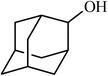 |
5 | 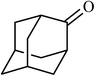 |
43 | 40 |
| 15 | 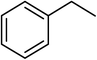 |
3 | 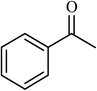 |
94 | 440 |
| 16 | 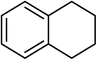 |
2.5 | 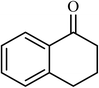 |
90 (82) | 400 |
| 17 | 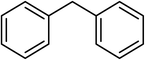 |
7 | 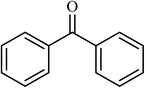 |
(83) | 140 |
| 18 | 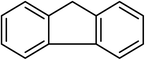 |
10 | 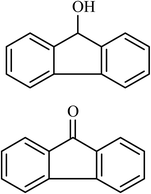 |
(24) (45) | 80 |
Next, we checked the potential of title catalytic method for the oxidation of benzylic hydrocarbons. Under the above optimized conditions, tetralin converted selectively to tetralone in 70% yield within 3 h and after which it remained almost unchanged. By increasing the catalyst loading up to 0.1 mol%, the conversion was almost the same (75%), nevertheless the reaction selectivity decreased markedly by producing an approximately equal yield of the pertinent alcohol (40%) and ketone (35%). Then, NHPI amount was increased to 15 mol%, in which the conversion reached to 90% and tetralone was produced as sole product within 2.5 h. Under this new condition, ethylbenzene and diphenylmethane oxidized smoothly and the related ketones were achieved quantitatively after 3 and 7 h, respectively. Nevertheless, oxidation of fluorene proceeded slowly and a mixture of the pertinent alcohol (24%) and ketone (45%) was achieved after 10 h.
Finally, we sought to evaluate the catalyst activity and durability. The TOF of catalyst for oxidation of different substrates summarized in Table 4, demonstrated the desired activity for title nanocluster. Moreover, the durability of {Mo72V30} nanocluster was established by TONs of 4920 and 5850 after 12 h oxidation of benzyl alcohol and 1-phenyl ethanol respectively, using 0.01 mol% catalyst. More evidences for stability of catalyst were achieved using FT-IR and UV-vis spectra as well as XRD pattern (Fig. 3). No vibrational changes were observed after recycling, indicating that the nanocluster maintains its integrity during oxidation reaction (Fig. 3A). There were also no noticeable changes in UV-Vis spectra and the XRD pattern (Fig. 3B and C) which further reveals that the reaction had no effect on the structure and properties of catalyst. Comparing the UV-Vis characteristic band (510 nm, Fig. 3B) of recovered and fresh catalysts substantiated remarkable durability of title catalyst. Considering the possible loss of catalyst during steps of work up procedure, only a 24% reduction in the absorbance was observed. These results demonstrated that {Mo72V30} rather than the products of its destruction acts as an active catalyst form in the oxidation process.
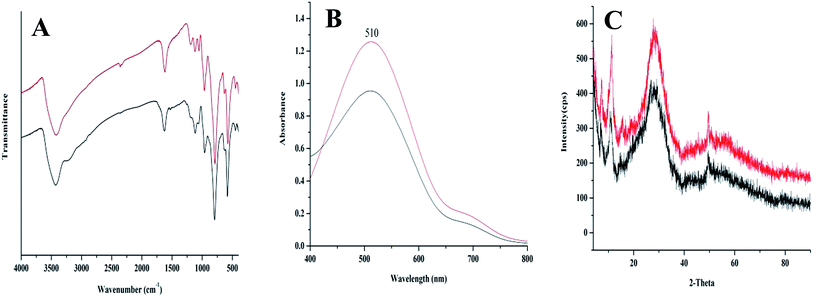 | ||
| Fig. 3 FT-IR (A), UV-Vis spectra (B) and XRD pattern (C) of fresh (red) and recovered {Mo72V30} (black) from the aerobic benzyl alcohol oxidation reaction. | ||
These promising results for survival of {Mo72V30} as a heterogeneous catalyst encouraged us to screen its recyclability in the oxidation of benzyl alcohol. The solid catalyst could be readily recovered after a simple filtration step and recycled up to five times in the oxidation of benzyl alcohol. Only a 9% reduction in the yield of benzaldehyde product was observed after five runs (Fig. S13†). Standard leaching experiments using the hot filtration method and AAS provided negligible quantities of Mo or V within the limit of detection (<1 ppm). Only 3% conversion evolution was observed for solid-free filtrate after 12 h. These advantages make the method amenable to scalability readily. {Mo72V30} could catalyze oxidation of benzyl alcohol in a semi-scaled up procedure (10.0 mmol) and the benzaldehyde was secured in 83% yield after 3 h.
Ultimately, we compared the result and conditions used in this work for oxidation of benzyl alcohol (Table 5) as well as ethyl benzene (Table 6) with our previous works using Co–Schiff base complexes,19,20 and some reports using V-containing catalysts. The superiority of {Mo72V30} is established with respect to catalyst mol%, solvent nature, temperature and reaction time.
| Entry | Catalyst | Catalyst mol% | Conditions | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | {Mo72V30} | 0.05 | EtOAc/O2/NHPI/70 °C | 3 | 89 | This work |
| 2 | CoL2@SMNP | 0.5 | CH3CN/O2/NHPI/70 °C | 16 | 97 | 19 |
| 3 | CoL2@TiO2 | 0.5 | CH3CN/O2/NHPI/70 °C/visible light | 3 | 91 | 20 |
| 4 | H5PVMo10O40 | 2 | PEG200/O2/100 °C | 16 | 100 | 36 |
| 5 | H5PVMo10O40 | 1 | ScCO2/O2/100 °C | 16 | 81 | 37 |
| 6 | H5PV2Mo12O40 | 0.01 | DMSO/O2/135 °C | 4 | 85 | 38 |
| 7 | [C4mim]5+[PMo10V2O40]5−SBA-15 | 40 | CH3CN/O2/100 °C | 12 | 98 | 39 |
| 8 | (DODA)4PMo11VO40 | 0.05 | Solvent-free/H2O2/90 °C | 6 | 53 | 40 |
| 9 | VOSO4/NaNO2 | 5 | CH3CN/O2/H2O/80 °C | 2 | 99.5 | 41 |
| Entry | Catalyst | Catalyst mol% | Conditions | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | {Mo72V30} | 0.05 | EtOAc/O2/NHPI/70 °C | 3 | 89 | This work |
| 2 | CoL2@SMNP | 0.5 | CH3CN/O2/NHPI/70 °C | 9 | 97 | 19 |
| 3 | CoL2@TiO2 | 0.75 | CH3CN/O2/NHPI/70 °C/visible light | 6 | 97 | 20 |
| 3 | VO(acac)2 | 0.5 | PhCN/NHPI/O2/90 °C | 12 | 92 | 42 |
| 4 | NH4VO3 | 0.5 | PhCN/NHPI/O2/90 °C | 12 | 90 | 42 |
| 5 | CPS-[VO(SAAM)2] | 0.1 | O2/110 °C | 14 | 98 | 43 |
| 6 | [Bu4N]VO3 | 0.2 | CH3CN/NHPI/O2/75 °C | 20 | 79.5 | 44 |
| 7 | V-MCM-41 | 1.67 | Acetone/TBHP/60 °C | 8 | 97 | 45 |
| 8 | [V3O3(OEt)(ashz)2(μ-OEt)]2 | 0.1 | PCA/MeCN/H2O2/50 °C | 2.5 | 16.4 | 46 |
4. Conclusion
In conclusion, for the first time we demonstrated the use of the {Mo72V30} Keplerate polyoxometalate as an efficient catalyst for the selective aerobic benzylic C–H oxidation of primary and secondary benzylic alcohols as well as hydrocarbons. The high activity of catalyst provides high yielding methods for producing different carbonyl compounds. The survival of catalyst was demonstrated by spectral data as well as leaching experiments. The title methodology uses molecular oxygen as an oxygen source, ethyl acetate as a safe reaction media and shows great potential in scalability with relatively low catalyst loading. These benefits plus excellent reusability of the catalyst render the present strategy practical to address the environmental and industrial issues.Acknowledgements
Support for this work by Research Council of University of Birjand and “Iran Science Elites Federation” is highly appreciated. We also thank Dr F. Feizpour for GC analysis.References
- D. H. Pybus and C. S. Sell, The chemistry of fragrances, Royal Society of Chemistry, 1999 Search PubMed.
- R. Ciriminna, V. Pandarus, F. Béland, Y.-J. Xu and M. Pagliaro, Org. Process Res. Dev., 2015, 19, 1554–1558 CrossRef CAS.
- T. Fey, H. Fischer, S. Bachmann, K. Albert and C. Bolm, J. Org. Chem., 2001, 66, 8154–8159 CrossRef CAS PubMed.
- J.-E. Bäckvall, Modern oxidation methods, John Wiley & Sons, 2011 Search PubMed.
- F. Cardona and C. Parmeggiani, Transition Metal Catalysis in Aerobic Alcohol Oxidation, Royal Society of Chemistry, 2014 Search PubMed.
- E. Brulé and Y. R. de Miguel, Org. Biomol. Chem., 2006, 4, 599–609 Search PubMed.
- C. Baleizao and H. Garcia, Chem. Rev., 2006, 106, 3987–4043 CrossRef CAS PubMed.
- S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- M. Sutradhar, L. M. D. R. S. Martins, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Coord. Chem. Rev., 2015, 301–302, 200–239 CrossRef CAS.
- A. Müller and P. Gouzerh, Chem. Soc. Rev., 2012, 41, 7431–7463 RSC.
- A. Müller and P. Gouzerh, Chem.–Eur. J., 2014, 20, 4862–4873 CrossRef PubMed.
- A. Rezaeifard, R. Haddad, M. Jafarpour and M. Hakimi, J. Am. Chem. Soc., 2013, 135, 10036–10039 CrossRef CAS PubMed.
- A. Rezaeifard, R. Haddad, M. Jafarpour and M. Hakimi, ACS Sustainable Chem. Eng., 2014, 2, 942–950 CrossRef CAS.
- F. Jalilian, B. Yadollahi, M. R. Farsani, S. Tangestaninejad, H. A. Rudbari and R. Habibi, Catal. Commun., 2015, 66, 107–110 CrossRef CAS.
- F. Jalilian, B. Yadollahi, M. Riahi Farsani, S. Tangestaninejad, H. Amiri Rudbari and R. Habibi, RSC Adv., 2015, 5, 70424–70428 RSC.
- C. Yang, W. Zhao, Z. Cheng, B. Luo and D. Bi, RSC Adv., 2015, 5, 36809–36812 RSC.
- H. Haddadi, E. M. Korani, S. M. Hafshejani and M. R. Farsani, J. Cluster Sci., 2015, 26, 1913–1922 CrossRef CAS.
- E. Nikbakht, B. Yadollahi and M. R. Farsani, Inorg. Chem. Commun., 2015, 55, 135–138 CrossRef CAS.
- M. Jafarpour, A. Rezaeifard, V. Yasinzadeh and H. Kargar, RSC Adv., 2015, 5, 38460–38469 RSC.
- M. Jafarpour, H. Kargar and A. Rezaeifard, RSC Adv., 2016, 6, 25034–25046 RSC.
- M. Jafarpour, F. Feizpour and A. Rezaeifard, RSC Adv., 2016, 6, 54649–54660 RSC.
- B. Botar, P. Kögerler and C. L. Hill, Chem. Commun., 2005, 21, 3138–3140 RSC.
- P. Yin, D. Li and T. Liu, Chem. Soc. Rev., 2012, 41, 7368–7383 RSC.
- F. Recupero and C. Punta, Chem. Rev., 2007, 107, 3800–3842 CrossRef CAS PubMed.
- Y. Ishii, S. Sakaguchi and T. Iwahama, Adv. Synth. Catal., 2001, 343, 393–427 CrossRef CAS.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- P. J. Figiel, J. M. Sobczak and J. J. Ziolkowski, Chem. Commun., 2004, 244–245 RSC.
- F. Recupero and C. Punta, Chem. Rev., 2007, 107, 3800–3842 CrossRef CAS PubMed.
- F. Minisci, C. Punta and F. Recupero, J. Mol. Catal. A: Chem., 2006, 251, 129–149 CrossRef CAS.
- A. Müller, S. Sarkar, S. Q. N. Shah, H. Bögge, M. Schmidtmann, S. Sarkar, P. Kögerler, B. Hauptfleisch, A. X. Trautwein and V. Schünemann, Angew. Chem., Int. Ed., 1999, 38, 3238–3241 CrossRef.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann and F. Peters, Angew. Chem., Int. Ed., 1998, 37, 3359–3363 CrossRef.
- A. M. Todea, A. Merca, H. Bögge, J. van Slageren, M. Dressel, L. Engelhardt, M. Luban, T. Glaser, M. Henry and A. Mueller, Angew. Chem., Int. Ed., 2007, 46, 6106–6110 CrossRef CAS PubMed.
- A. M. Todea, A. Merca, H. Bögge, T. Glaser, L. Engelhardt, R. Prozorov, M. Luban and A. Müller, Chem. Commun., 2009, 3351–3353 RSC.
- D. Volkmer, A. Du Chesne, D. G. Kurth, H. Schnablegger, P. Lehmann, M. J. Koop and A. Muller, J. Am. Chem. Soc., 2000, 122, 1995–1998 CrossRef CAS.
- A. Rezaeifard, M. Jafarpour, A. Farrokhi, S. Parvin and F. Feizpour, RSC Adv., 2016, 6, 64640–64650 RSC.
- A. Haimov and R. Neumann, Chem. Commun., 2002, 876–877 RSC.
- G. Maayan, B. Ganchegui, W. Leitner and R. Neumann, Chem. Commun., 2006, 2230–2232 RSC.
- A. M. Khenkin and R. Neumann, J. Org. Chem., 2002, 67, 7075–7079 CrossRef CAS PubMed.
- A. Bordoloi, S. Sahoo, F. Lefebvre and S. B. Halligudi, J. Catal., 2008, 259, 232–239 CrossRef CAS.
- L. Jing, J. Shi, F. Zhang, Y. Zhong and W. Zhu, Ind. Eng. Chem. Res., 2013, 52, 10095–10104 CrossRef CAS.
- Z. Du, H. Miao, H. Ma, Z. Sun, J. Ma and J. Xu, Adv. Synth. Catal., 2009, 351, 558–562 CrossRef CAS.
- R. Neumann and A. M. Khenkin, Chem. Commun., 1996, 2643–2644 RSC.
- Q. Jinwei, F. Zaihui, L. Yachun, H. E. Xiangling, D. Zhang, W. U. Wenfeng, W. Yanlong, G. Xinglang, D. Xiaolin and W. U. Haitao, et al., Chin. J. Catal., 2011, 32, 1342–1348 CrossRef.
- B. Gao, Y. Li and N. Shi, React. Funct. Polym., 2013, 73, 1573–1579 CrossRef CAS.
- P. J. Figiel and J. M. Sobczak, New J. Chem., 2007, 31, 1668–1673 RSC.
- M. Sutradhar, M. V. Kirillova, M. F. C. da Silva, L. M. Martins and A. J. L. Pombeiro, Inorg. Chem., 2012, 51, 11229–11231 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR, UV-Vis and XRD of {Mo72V30}, the synthesis procedures and characterizations of as-prepared ionic liquid Keplerate polyoxometalates, the results for catalyst recycling and GC trace for acetophenone analysis (entry 10 in Table 4). See DOI: 10.1039/c6ra26942g |
| This journal is © The Royal Society of Chemistry 2017 |