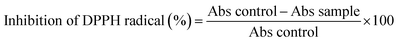Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards the development of an effective in vivo wound healing agent from Bacillus sp. derived biosurfactant using Catla catla fish fat†
Santanu Sana a,
Asit Mazumderb,
Sriparna Datta*c and
Dipa Biswasd
a,
Asit Mazumderb,
Sriparna Datta*c and
Dipa Biswasd
aDept. of Chemical Technology, University of Calcutta, 92, A.P.C. Road, Kolkata – 700 009, India. E-mail: sanasantanu@gmail.com
bDept. of Chemical Technology, University of Calcutta, 92, A.P.C. Road, Kolkata – 700 009, India. E-mail: amaz234@gmail.com
cDept. of Chemical Technology, University of Calcutta, 92, A.P.C. Road, Kolkata – 700 009, India. E-mail: sriparnadatta2014@gmail.com; Fax: +91-033-23519755; Tel: +91-9830695346 Tel: +91-33-2350-8386
dDept. of Chemical Technology, University of Calcutta, 92, A.P.C. Road, Kolkata – 700 009, India. E-mail: dipa.b@yahoo.com
First published on 28th February 2017
Abstract
The aim of the present study was to investigate the excisional wound healing activity of a biosurfactant isolated from Bacillus stratosphericus sp. A15 using Catla catla fish fat as economic substrate. The organism was screened on the basis of blood haemolytic activity, cell surface hydrophobicity, oil displacement test and its promising capacity of reducing surface tension (ST). The biosurfactant was identified as surfactin lipopeptide using IR, 1H NMR and mass spectrometric analysis. Mass spectrometric data indicated the presence of amino acid sequence as Val/Asp/Val/Leu/Leu/GluOMe linked with β-hydroxy fatty acid moiety containing 14 carbons in normal, iso or anteiso forms. An ointment of the test biosurfactant enhanced the wound closure (97%) in 10 days compared to the untreated control group (72%) (P < 0.05). Histopathological study confirmed the healing effect of the test biosurfactant in terms of well developed keratinocyte, presence of hair follicles, vacuoles, higher number of intact cells in dermis layer and a thick epidermal layer. The biosurfactant also showed anti-oxidant and antibacterial activity against Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 8739 which established its' additional advantage in wound protection.
1 Introduction
Skin is the largest tissue of our body that protects from infections, ultraviolet irradiation, chemical and mechanical injury. Wound healing is a connective tissue regenerated complex procedure which involves the interaction of cells and extracellular matrix.1 The healing comprised of sequential and overlapped biological events involving inflammation, proliferation and migration of different cell types.2 After an injury, the wound should be repaired without delay with minimum pain, inflammation and discomfort excluding microbial infection.3 Antibiotics are generally used for treatment of infectious wounds. Unfortunately resistance development is of serious concern apart from side effects of these antibiotics. Hence the need for new wound healing agents with antimicrobial properties is on the rise.Oxygen and nitrogen containing reactive species present at the wound site delay the healing process by lipid per-oxidation, DNA damage and free radical scavenger enzyme inactivation. Therefore new safe and effective wound healing agent with potential antioxidant activity has additional advantage to accelerate the healing process.4
Biosurfactants are microbiologically derived surface active agents containing both hydrophilic and hydrophobic substances5 which are preferred due to their biodegradable and environment friendly nature, biocompatibility, activity in extreme conditions of temperature, pH and salinity.6 Beside physicochemical properties, biosurfactant finds potential applications in pharmaceutical and cosmetic field for its antibacterial, antiviral and antifungal properties.7 The use of biosurfactant is restricted due to its production cost which can be reduced by using cheap substrate. We are pioneer in using waste fish fat as a cheap substrate, which is very much available in the local market of West Bengal. Till date only Deshpande M. and Daniels L. et al.8 have used animal fat to produce sophorolipid with Candida bombicola.
Accordingly, the present study is focused on the isolation, identification and pharmaceutical application in terms of wound healing activity of an efficient biosurfactant produced by a Bacillus sp. isolated from oil contaminated fertile soil.
2 Materials and methods
2.1 Chemicals
All the used reagents were of analytical grade. The 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide (H2O2), bovine serum albumin (BSA), trichloroacetic acid (TCA), ciprofloxacin (Albert David Ltd., India), D-(+)-glucosamine hydrochloride, acetyl acetone and Ehrlich's reagent were purchased from Sigma-Aldrich (India). Sodium hydroxide and hydrochloric acid were purchased from Merck (India). The fat of Catla catla fish, goat blood and waste frying oil were procured from local market. Bushnell-Haas (BH) media, peptone, beef extract and agar were from Hi-Media Laboratories Pvt. Ltd. (India).2.2 Culture medium components
The medium used for isolation and biosurfactant production was BH media containing KH2PO4 1 g l−1, K2HPO4 1 g l−1, NH4NO3 1 g l−1, MgSO4 0.2 g l−1, CaCl2 0.2 g l−1, FeCl3 0.05 g l−1 and autoclaved at 121 °C for 15 min. Enrichment medium was prepared by adding 20 g l−1 Catla catla fish fat (FF) as sole carbon source in sterile BH medium. The bacterial culture was maintained in nutrient agar (NA) media and sub-cultured after every two weeks. The blood agar media containing 5% (v/v) goat blood was prepared, following the method described by Qingmei L. et al.92.3 Isolation, identification and screening of the biosurfactant producing bacteria
Oil contaminated fertile soil samples were collected at late monsoon from Nadia district, West Bengal, India. The soil samples were inoculated in a 250 ml Erlenmeyer flask containing 100 ml enriched media and incubated at 35 °C in an orbital shaker incubator (ORBITEK-LJE, Scigenics Biotech Pvt. Ltd., Chennai, India) at 125 rpm for 7 days. Single colony was isolated using serial dilution (up to 10−6 times) in sterile 0.85% saline solution, and finally streaked in NA plates. After confirming the purity of the isolated strain through Gram staining and examining its morphological, cultural, biochemical and physiological characteristics it was finally confirmed by 16S rRNA gene sequence analysis (IMTECH, Chandigarh, India) and the sequence was submitted to NCBI/GenBank.Haemolytic assay10 and oil displacement test11 were used to select the efficient biosurfactant producer. Bath assay12 was performed to estimate bacterial cell surface hydrophobicity using the following equation.
| Cell surface hydrophobicity = 100 × [1 − (OD of the aqueous phase/OD of the cell suspension)] |
2.4 Surface tension and rheological property
The ST of the cell free supernatant was measured by the Du Nouy method using an electronic tensiometer (Data physics DCAT 11, Germany) at 25 °C taking ST of distilled water as control. Percentage reduction of ST was estimated using the following equation.13| Percentage ST reduction = {(γm − γc)/γm} × 100 |
Where γm and γc were the ST of initial medium and ST of cell free supernatant respectively.
2.5 Isolation and purification of biosurfactant
A bacterial suspension (5 × 105 cfu ml−1) in 100 ml sterilized BH media containing 2 g FF was placed in shaker. Samples were removed after every 24 h interval and the ST was measured after centrifugation (7000 rpm for 15 min) to determine the minimum time required for maximum ST lowering. The cell free supernatant was acidified by HCl up to pH 2.00 and kept overnight at 4 °C which was further centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C and the precipitate was dissolved in methanol. Finally, methanol was evaporated in rotary evaporator (Eyela, Rikakikai Co. Ltd., Tokyo, Japan) at 40 °C under reduced pressure.
000 rpm for 15 min at 4 °C and the precipitate was dissolved in methanol. Finally, methanol was evaporated in rotary evaporator (Eyela, Rikakikai Co. Ltd., Tokyo, Japan) at 40 °C under reduced pressure.
2.6 Estimation of critical micelle concentration (CMC)
CMC of the crude biosurfactant was examined by preparing a series of solution of biosurfactant from 1 mg l−1 to 200 mg l−1 in deionized water and the ST was estimated.2.7 Chemical characterization of the biosurfactant by FT-IR, 1H NMR and mass spectrometric analysis
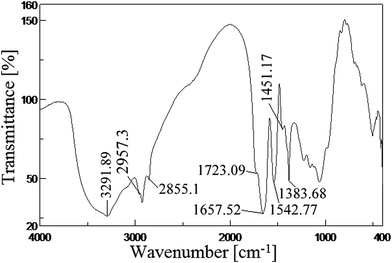
The IR spectra of the biosurfactant were recorded on FTIR-spectrophotometer (Jasco, FT/IR-6300, USA) in 4000–400 cm−1 region at a resolution of 2 cm−1 using a KBr pellet. The pellet was prepared by grinding biosurfactant and dry KBr in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio followed by pressing under 7500 kg weight for 30 s to attain a translucent pellet.
9 ratio followed by pressing under 7500 kg weight for 30 s to attain a translucent pellet.
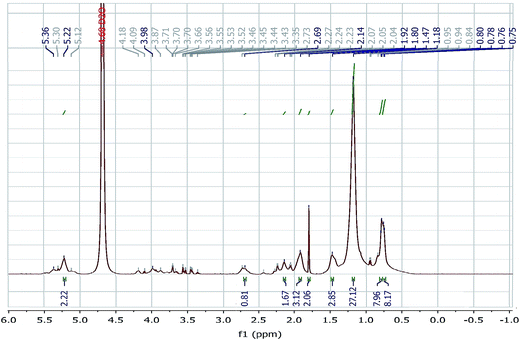
Proton nuclear magnetic resonance (1H NMR) of the purified biosurfactant was recorded on Bruker Avance 400 MHz spectrometer using tetramethyl silane (TMS) as internal standard and deuterated water as solvent.
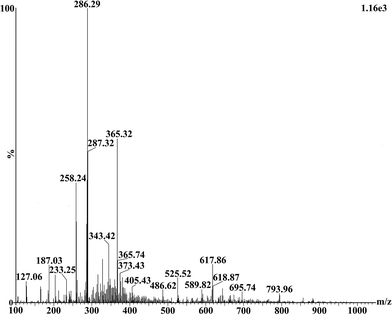
Mass spectrometric analysis of purified biosurfactant was performed on JEOL MS station (JMS-700) after dissolving in deionized water.
2.8 Stability study in different conditions
Stability of the biosurfactant was performed under wide range of temperature, pH and salinity at its CMC. Thermal stability was checked by keeping it at 100 °C for different time periods (0, 5, 10, 20, 40 and 60 min) and autoclaving at 121 °C for 15 min. Stability at pH values (2 to 13) of the biosurfactant was examined. Effect of salinity on the stability of surfactant property was examined by treating it with different concentrations of NaCl (0–6% w/v) and the ST was examined after keeping it for 5 days at room temperature.142.9 Evaluation of in vitro antioxidant activity
Where, Abs control was the absorbance of the control reaction and Abs sample was the absorbance of the test biosurfactant. Ascorbic acid was used as the standard. The concentration of sample at which DPPH radicals were scavenged by 50% was taken as the IC50.
| % scavenged H2O2 = [(1 − absorbance of control/absorbance of sample) × 100] |
2.10 Determination of antimicrobial activity
The antimicrobial activity of the produced biosurfactant was found against a common Gram positive bacterium, Staphylococcus aureus ATCC 25923 and a Gram negative bacterium, Escherichia coli ATCC 8739 collected from Institute of microbial technology, Chandigarh, India. The antimicrobial activity and minimum inhibitory concentration (MIC) were determined by broth dilution technique as per the guideline of European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Disease (ESCMID).17 A broth without drug was regarded as negative control and ciprofloxacin as positive control.2.11 Wound healing activity
Keeping in view the antioxidant and antimicrobial activity of the biosurfactant BS15, an endeavour was taken to investigate its potential for excisional wound healing after preparing water based ointment with it.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light and dark circle. Standard laboratory diet and water were provided ad libitum. The experimental protocol followed in this study was described by Pople P. V. et al.19 The dorsal part of each rabbit was shaved prior to the experiment and divided in four marked areas for topical application. The marked areas were treated with test biosurfactant ointment, ointment base as control, 10% w/v formalin solution as positive control and framycetin sulphate ointment (Sanofi India Ltd., Mfg. Lic. no. 439/L, Batch no. E6363, Mfg. date-10/2016; Exp. date-03/2019) as standard drug. A careful observation was followed up to 24 h to notice any erythema and edema as indication of irritation.
12 h light and dark circle. Standard laboratory diet and water were provided ad libitum. The experimental protocol followed in this study was described by Pople P. V. et al.19 The dorsal part of each rabbit was shaved prior to the experiment and divided in four marked areas for topical application. The marked areas were treated with test biosurfactant ointment, ointment base as control, 10% w/v formalin solution as positive control and framycetin sulphate ointment (Sanofi India Ltd., Mfg. Lic. no. 439/L, Batch no. E6363, Mfg. date-10/2016; Exp. date-03/2019) as standard drug. A careful observation was followed up to 24 h to notice any erythema and edema as indication of irritation.Group I: untreated (negative control) group (NC).
Group II: ointment base treated control group (OB).
Group III: BS15 (5 mg ml−1 dose) treated group (BS).
Group IV: framycetin sulphate ointment treated group (FS).
The medications were administered topically to cover the wound completely. Treatment was continued for 10 days at a fixed time, each day.
Wound index was calculated following an arbitrary scoring system20 as mentioned in Table 1.
| Gross change | Wound index |
|---|---|
| Complete healing of wound | 0 |
| Incomplete but healthy healing of wound | 1 |
| Delayed but healing | 2 |
| No instigation of healing but the environment is healthy | 3 |
| Pus formation: proof of necrosis | 4 |
| Total | 10 |
Hexosamine content was measured from approximately 40 mg of dried granulated tissue samples. The tissue was hydrolyzed and hexosamine was estimated following the method described by Dische Z. et al.26 using a standard curve of D-(+)-glucosamine hydrochloride.
2.12 Statistical analysis
Statistical data analysis was performed and shown as mean ± standard error of mean (SEM). The statistical significance was considered using one-way analysis of variance (ANOVA) using GraphPad Prism version 5.01. (California, USA) and were considered significant when P was less than 0.05. All the experiment was performed thrice.3 Results and discussion
3.1 Isolation and identification of the organism
After screening from a large number of isolates an effective biosurfactant producer (A15) was selected on the basis of ST lowering capability. The cell free supernatant showed highest reduction of ST from 71.02 mN m−1 to 32.2 mN m−1 i.e. 54.64% as compared to other isolates.As per the biochemical results the selected strain was an aerobic, rod shaped, endospore forming Gram positive bacteria and the colonies were of irregular shaped. It expressed positive response for catalase activity and negative for oxidase activity. Though the strain could not produce amylase but it produced protease as examined by hydrolysis of skim milk. All these properties indicated the test strain as a member of Bacillus genus. In addition to the biochemical tests 16S rRNA gene sequence (1441 bp) analysis shown 97.8% homology with Bacillus stratosphericus 41KF2a(T). The phylogenetic tree constructed with 16S rRNA gene sequence as presented in Fig. S1.† The NCBI GeneBank accession number of the test bacteria was KU644139 and it should be classified as a strain of Bacillus sp. A15. Bacillus stratosphericus has been reported to produce extracellular alkaline protease.27 But our study focused on biosurfactant production of Bacillus stratosphericus using Catla catla FF.
3.2 Screening of biosurfactant producer
3.3 Kinetics of biosurfactant production
After 48 h of growth, the lowering of ST started which continued till 120 h (lowest value of 32.2 mN m−1) along with a similar profile of increase in biomass. Thus it indicated that the biosurfactant production from Catla catla FF occurred in the exponential growth phase of the Bacillus sp. and it followed growth associated kinetics31 (Fig. 1).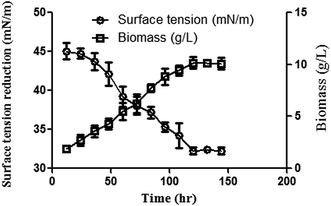 | ||
| Fig. 1 Increase in biomass and ST reduction profiles of Bacillus sp. mean ± SEM of three individual experiments. | ||
3.4 Critical micelle concentration
The curve for showing CMC of the biosurfactant (Fig. S3†) derived from A15 strain expressed a CMC value of 46.8 mg l−1 at 25 °C.Efficient surfactants show very low CMC value. The CMC value of BS15 was significantly lower than similar lipopeptide type biosurfactants as reported by others.32,33 The fatty acid part of lipopeptide plays an important role in controlling its surface active properties and the number of carbon atom in fatty acid chain is inversely related to the CMC.34,35
3.5 Characterization of BS15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Asp
Asp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Val
Val![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Leu was 1
Leu was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respectively. Still the result of dissociation was not able to differentiate Ile from Leu as these are isomeric in nature. Nearly similar type of C12 lipopeptide containing Asp, Glu, Val and Leu in molar ratio of 1
2 respectively. Still the result of dissociation was not able to differentiate Ile from Leu as these are isomeric in nature. Nearly similar type of C12 lipopeptide containing Asp, Glu, Val and Leu in molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was produced by Bacillus subtilis HSO according to the report of Liu X. Y. et al.43 Apart from few differences, the isolated lipopeptide type biosurfactant produced by Bacillus stratosphericus A15 showed many similarities with previously reported structure of surfactin.37
4 was produced by Bacillus subtilis HSO according to the report of Liu X. Y. et al.43 Apart from few differences, the isolated lipopeptide type biosurfactant produced by Bacillus stratosphericus A15 showed many similarities with previously reported structure of surfactin.37
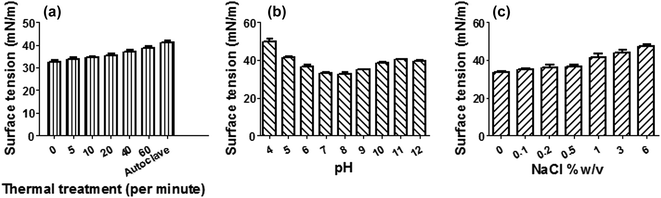
3.6 Stability study
The application of biosurfactant depends on its stability (Fig. 5) at extreme conditions of thermal treatment, pH and salinity. The Bacillus sp. derived biosurfactant showed to be thermo-stable by exhibiting stability up to 100 °C for 60 min and also autoclaving without changing the surface active property. It also showed stability over a pH range of 4 to 12 with maximum stability at alkaline pH 8 which might signify a better stability of fatty acid surfactant micelles in the presence of alkali. The solution turned turbid at acidic pH due to partial precipitation. The biosurfactant derived from the Bacillus sp. was also found to be stable within a wide range of NaCl concentration. Hence BS15 can be safely applied in food, pharmaceutical, cosmetic, petroleum and ore handling industries.3.7 Antioxidant activity of the biosurfactant
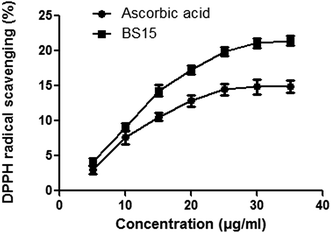
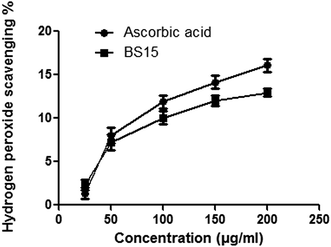
BS15 could donate hydrogen to free radicals, particularly to the lipid peroxides or hydrogen peroxide radicals, the major propagators of the chain autoxidation of lipids, to form non-radical species, resulting in the inhibition of lipid peroxidation.46 Thus BS15 might act as a potential wound healing agent by preventing oxidative damage at the wound site.
3.8 Antimicrobial activity
Lipopeptide biosurfactants like surfactin, iturin, lichenysin can act as effective antibacterial agent against a wide range of Gram positive and Gram negative bacteria in comparison to other classes of biosurfactants.47 The isolated biosurfactant showed MIC value of 600 μg ml−1 and 100 μg ml−1 against Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 8739 respectively. This antimicrobial activity of the isolated biosurfactant may help in faster healing by preventing bacterial infection at the wound site.3.9 Skin irritation
The BS15 ointment was free from skin irritation by showing no sign of erythema and edema following 24 h dermal exposure when applied to rabbit skin.3.10 Wound healing activity of BS15
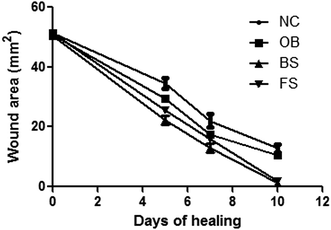
The visual observation (Fig. 8) revealed the progress of healing of NC, OB, BS and FS groups respectively. The rate of healing was significantly higher (P < 0.05) in BS and FS groups in comparison to other two groups in terms of wound closure. No significant changes were observed on first 2 days of treatment for all the groups.
From day 3 the wound turned dark red in colour which indicated the beginning of healing. The number of new blood vessels and the depth of dermal tissues at the wound site were significantly increased in BS and FS groups from the 5th day of treatment. Also swelling, redness and microbial attack was absent in these groups. Finally, complete wound closure and granulation tissue formation were achieved on 10th day of treatment. Conversely the NC and OB groups were followed by pus formation, microbial attack and the wound remained open till the end of treatment. This finding was supported by literature48 as natural contraction of wound occurred in 21 days.
The wound closure was monitored and expressed as in Fig. 8 where the BS group exhibited best wound closure (1.17 mm2) in comparison to NC group (14.17 mm2), OB group (10.67 mm2) and FS group (1.83 mm2) after 10 days treatment.
The percentage wound closure of BS15 group after 10 days treatment established a promising result of 97.70%, which was even better than FS group (96.43%) (P < 0.05). Whereas at the end of treatment, percentage wound contraction of OB (79.02%) group was near to NC (72.40%) group but much lower than the BS and FS group. So, significant healing effects in terms of faster wound contraction were observed in BS and FS treated groups in comparison to other groups.
The mean wound indices of each group on 3rd, 5th, 7th and 10th days of treatment were estimated. Mean wound index of BS and FS group on 10th day showed a value of 1.17 and 1.00 respectively (P < 0.05) in comparison to NC group (3.17). The mean wound index reduction of OB group (2.33) was also significant (P < 0.05) as compared to NC group.
The tensile strength of the regenerated tissue at wound site is expressed in Table 2. The result showed that the tissue tensile strength of BS (409.83 g) and FS (404.67 g) groups were much higher than the other NC (230.5 g) and OB (312.17 g) group. The tissue tensile strength and stability increases with collagen maturation by the help of enhancing intramolecular and intermolecular cross linking.49
| Treatment groups | Percentage wound contraction | Wound index | Tensile strength (g) |
|---|---|---|---|
| a Values were mean ± SEM, n = 6 in each group.b P < 0.05 compared to NC group.c P < 0.05 compared to OB group.d P < 0.05 compared to FS group. | |||
| NC | 72.40 ± 2.41 | 3.17 ± 0.98 | 230.5 ± 10.82 |
| OB | 79.02 ± 3.62b | 2.33 ± 1.21b | 312.17 ± 12.20b |
| BS15 | 97.70 ± 1.53b,c,d | 1.17 ± 0.75b,c,d | 409.83 ± 12.56b,c,d |
| FS | 96.43 ± 1.81b,c | 1.00 ± 0.63b,c | 404.67 ± 17.67b,c |
| Treatment groups | DNA (mg per g tissue) | Total protein (mg per g tissue) | Hexosamine (μg per mg tissue) |
|---|---|---|---|
| a Values were mean ± SEM, n = 6 in each group.b p < 0.05 compared to NC group.c p < 0.05 compared to OB group.d p < 0.05 compared to FS group. | |||
| NC | 1.135 ± 0.04 | 14.98 ± 0.63 | 0.296 ± 0.033 |
| OB | 1.235 ± 0.14b | 16.87 ± 0.55b | 0.336 ± 0.022 |
| BS15 | 1.80 ± 0.21b,c,d | 20.66 ± 1.68b,c,d | 0.437 ± 0.023 |
| FS | 1.76 ± 0.17b,c | 20.66 ± 2.00b,c | 0.436 ± 0.013 |
Hexosamine is one of the components of glycosaminoglycan family. It is the prime element for synthesis of new extracellular matrix.50 The glycosaminoglycan improves electrostatic and ionic interaction to alleviate the collagen fibres and its characteristic size. Table 3 clearly expressed that BS15 (47.4%) treatment significantly increased the hexosamine content, compared to control (P < 0.05) at the end of 10 days treatment, thereby it helped in the progress of wound healing in terms of collagen content regeneration and stabilization of collagen fibres. FS (46.8%) treatment also expressed similar type result.
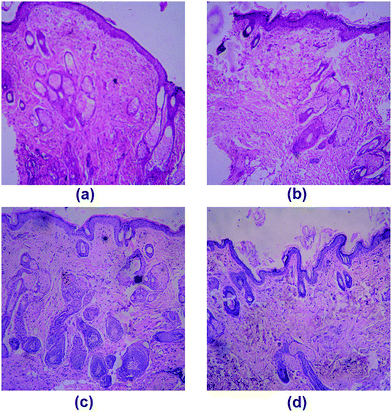
The histological examination of BS (Fig. 10c) and FS (Fig. 10d) groups showed similarity of healing by expressing well organized granular tissue reepithelialisation, satisfactory formation of thick layered keratinocyte and wide epidermal layer with clear periphery between dermal and epidermal region. The presence of hair follicles, sebaceous glands and higher number of intact cells in dermis layer at the end of scheduled treatment period showed well organised regeneration process. The dermis papillae were also apparently perceived. The topical application of BS15 significantly minimized the wound closure time with potent healing effect on full thickness excision wound. Among the various sequential stages of wound healing, proliferation and migration of epithelial cells are the most important phase for healing.53 Topical application of BS15 markedly enhanced the in vivo excision wound by both migration and proliferation of keratinocytes to cover the wound space and re-epithelialization by newly formed tissue cells at wound site. Contraction of myofibroblasts in granular tissue played advantageous role towards healing process.54 The excellent formation of scar tissue with systematically arranged collagen fibres contributed towards healing process by BS and FS.
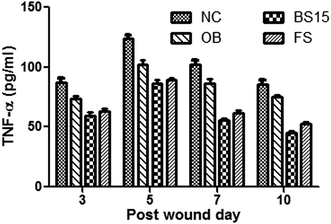
BS15 lipopeptide thus found to exhibit significant improvement of scar quality both by recovery of organised collagen regeneration and maturation. Lipoprotein expressed wound healing activity by its anti-inflammatory and immunomodulatory effect55 which was supported by decrement of TNF-α level in blood serum. It has been shown that TNF-α influenced the inflammatory response, angiogenesis, reepithelialization, extracellular matrix deposition and remodelling.56–58 The healing effect of our study can be illustrated by enhancement of granulation tissue formation through increment of endothelial progenitor cells and its paracrine effect which resulted the acceleration of tissue re-epithelialization at the wound site.
4 Conclusions
The Catla catla fish fat was chosen as an interesting and cost-effective alternative hydro carbon source for both fat degradation and efficient biosurfactant production. It can serve as an excellent model for waste management by protecting the environment and reutilising the animal fat. The physical parameters related to wound closure and biochemical parameters showed significant wound healing of the biosurfactant, BS15 produced by Bacillus stratosphericus A15. Histological studies established a noticeable healing activity in terms of rapid wound closure by connective tissue regeneration, thick epidermal layer and keratinocyte formation at the wound site. In vitro antimicrobial activity and anti-oxidant activity of BS15 was an additional advantage. Hence BS15 could be established as an alternative to antibiotic for application in near future.Acknowledgements
We would like to thank University Grants Commission, India for providing financial support and CRNN – University of Calcutta for providing instrumental facilities.References
- T. Velnar, T. Bailey and V. Smrkolj, J. Int. Med. Res., 2009, 37, 1528–1542 CrossRef CAS PubMed.
- G. S. Sidhu, H. Mani, J. P. Gaddipati, A. K. Singh, P. Seth, K. K. Banaudha, G. K. Patnaik and K. Maheshwari, Wound Repair Regen., 1999, 7, 362–374 CrossRef CAS PubMed.
- H. R. P. Naik, H. S. B. Naik, T. R. R. Naik, H. R. Naika, K. Gouthamchandra, R. Mahmood and B. M. Ahamed, Eur. J. Med. Chem., 2009, 44, 981–989 CrossRef CAS PubMed.
- P. J. Houghton, P. J. Hylands, A. Y. Mensah, A. Hensel and A. M. Deters, J. Ethnopharmacol., 2005, 100, 100–107 CrossRef CAS PubMed.
- A. M. Abdel-Mawgoud, F. Lepine and E. Deziel, Appl. Microbiol. Biotechnol., 2010, 86, 1323–1336 CrossRef CAS PubMed.
- I. M. Banat, R. S. Makkar and S. S. Cameotra, Appl. Microbiol. Biotechnol., 2000, 53, 495–508 CrossRef CAS PubMed.
- L. Rodrigues, I. M. Banat, J. Teixeira and R. Oliveira, J. Antimicrob. Chemother., 2006, 57, 609–618 CrossRef CAS PubMed.
- M. Deshpande and L. Daniels, Bioresour. Technol., 1995, 54, 143–150 CrossRef CAS.
- L. Qingmei, Y. Hang, W. Jun, G. Guohong, Z. Wei, F. Yonghong, W. Li, Y. Jianming and Y. Zengliang, Plasma Sci. Technol., 2006, 8, 491 CrossRef.
- G. A. Plaza, I. Zjawiony and I. M. Banat, J. Pet. Sci. Eng., 2006, 50, 71–77 CrossRef CAS.
- D. K. Jain, D. L. Thompson and H. Lee, J. Microbiol. Methods, 1991, 13, 271–279 CrossRef.
- M. Rosenberg, D. Gutnick and E. Rosenberg, FEMS Microbiol. Lett., 1980, 9, 29–33 CrossRef CAS.
- S. Pansiripat, O. Pornsunthorntawee, R. Rujiravanit, B. Kitiyanan, P. Somboonthanate and S. Chavadej, Biochem. Eng. J., 2010, 49, 185–191 CrossRef CAS.
- H. Amani, M. M. Muller, C. Syldatk and R. Hausmann, Appl. Biochem. Biotechnol., 2013, 170, 1080–1093 CrossRef CAS PubMed.
- P. Bersuder, M. Hole and G. Smith, J. Am. Oil Chem. Soc., 1998, 75, 181–187 CrossRef CAS.
- R. J. Ruch, S. J. Cheng and J. E. Klaunig, Carcinogenesis, 1989, 10, 1003–1008 CrossRef CAS PubMed.
- European Committee for Antimicrobial SusceptibilityTesting (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution, Clin. Microbiol. Infect., 2000, 6, 1–7 Search PubMed.
- L. Lachman, H. A. Liberman and J. L. Kanig, The Theory and Practice of Industrial Pharmacy, Varghese Publishing House, Bombay, 3rd edn, 1987, pp. 534–563 Search PubMed.
- P. V. Pople and K. K. Singh, AAPS PharmSciTech, 2006, 7, E63–E69 CrossRef.
- R. G. Auddy, M. F. Abdullah, S. Das, P. Roy, S. Datta and A. Mukherjee, BioMed Res. Int., 2013, 2013, 1–8 CrossRef PubMed.
- B. T. Kanti, A. Biswajit, P. B. Nitai, B. Subahshree and M. Biswapati, Acta Pharmacol. Sin., 2001, 22, 1113–1116 Search PubMed.
- T. K. Biswas, L. N. Maity and B. Mukherjee, Int. J. Lower Extremity Wounds, 2004, 3, 143–150 CrossRef PubMed.
- O. Ziv-Polat, M. Topaz, T. Brosh and S. Margel, Biomaterials, 2010, 31, 741–747 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- K. Burton, Biochem. J., 1956, 62, 315–323 CrossRef CAS PubMed.
- Z. Dische and E. Borenfreund, J. Biol. Chem., 1962, 4, 330–334 Search PubMed.
- D. A. Bindu and I. B. Reddy, Biochemistry, 2013, 2, 29–31 Search PubMed.
- T. Varadavenkatesan and V. R. Murty, ISRN Microbiol., 2013, 2013, 1–8 CrossRef PubMed.
- C. Mulligan, D. Cooper and R. Neufeld, J. Ferment. Technol., 1984, 62, 311–314 CAS.
- M. Morikawa, Y. Hirata and T. A. Imanaka, BBA-Mol. Cell Biol. L., 2000, 1488, 211–218 CAS.
- A. Persson, E. Osterberg and M. Dostalek, Appl. Microbiol. Biotechnol., 1988, 29, 1–4 CrossRef CAS.
- Y. Li, R. Q. Ye and B. Z. Mu, J. Surfactants Deterg., 2009, 12, 31–36 CrossRef CAS.
- K. V. Pathak and H. Keharia, 3 Biotech, 2014, 4, 41–48 CrossRef.
- N. H. Youssef, K. E. Duncan and M. J. McInerney, Appl. Environ. Microbiol., 2005, 71, 7690–7695 CrossRef CAS PubMed.
- H. Razafindralambo, P. Thonart and M. Paquot, J. Surfactants Deterg., 2004, 7, 41–46 CrossRef CAS.
- Y. Al-Wahaibi, S. Joshi, S. Al-Bahry, A. Elshafie, A. Al-Bemani and B. Shibulal, Colloids Surf., B, 2014, 114, 324–333 CrossRef CAS PubMed.
- A. F. de Faria, D. S. Teodoro-Martinez, G. N. de Oliveira Barbosa, B. G. Vaz, I. S. Silva, J. S. Garcia, M. R. Totola, M. N. Eberlin, M. Grossman, O. L. Alves and L. R. Durrant, Process Biochem., 2011, 46, 1951–1957 CrossRef.
- N. K. Bordoloi and B. K. Konwar, Colloids Surf., B, 2008, 63, 73–82 CrossRef CAS PubMed.
- S. Joshi, C. Bharucha and A. J. Desai, Bioresour. Technol., 2008, 99, 4603–4608 CrossRef CAS PubMed.
- A. A. Bodour, K. P. Drees and R. M. Maier, Appl. Environ. Microbiol., 2003, 69, 3280–3287 CrossRef CAS PubMed.
- X. Y. Liu, S. Z. Yang and B. Z. Mu, Process Biochem., 2009, 44, 1144–1151 CrossRef CAS.
- M. Kowall, J. Vater, B. Kluge, T. Stein, P. Franke and D. Ziessow, J. Colloid Interface Sci., 1998, 204, 1–8 CrossRef CAS PubMed.
- X. Y. Liu, S. Z. Yang and B. Z. Mu, J. Pept. Sci., 2008, 14, 864–875 CrossRef CAS PubMed.
- O. Tabbene, D. Gharbi, I. B. Slimene, S. Elkahoui, M. N. Alfeddy, P. Cosette, M. L. Mangoni, T. Jouenne and F. Limam, Appl. Biochem. Biotechnol., 2012, 168, 2245–2256 CrossRef CAS PubMed.
- E. Yalcın and K. Cavusoglu, Turk. J. Biochem., 2010, 35, 243–247 Search PubMed.
- C. W. Bamforth, R. E. Muller and M. D. Walker, J. Am. Soc. Brew. Chem., 1993, 53, 79–88 Search PubMed.
- L. Rodrigues, I. M. Banat, J. Teixeira and R. Oliveira, J. Antimicrob. Chemother., 2006, 57, 609–618 CrossRef CAS PubMed.
- W. T. Lawrence, Clin. Plast. Surg., 1998, 25, 321–340 CAS.
- I. K. Hornstra, S. Birge, B. Starcher, A. J. Bailey, R. P. Mecham and S. D. Sapiro, J. Biol. Chem., 2003, 278, 14387–14393 CrossRef CAS PubMed.
- J. M. Trowbridge and R. L. Gallo, Glycobiology, 2002, 12, 117R–125R CrossRef CAS PubMed.
- R. P. Samy, M. Kandasamy, P. Gopalakrishnakone, B. G. Stiles, E. G. Rowan, D. Becker, M. K. Shanmugam, G. Sethi and V. T. K. Chow, PLoS One, 2014, 9, e80199 Search PubMed.
- Y. P. Han, T. L. Tuan, H. Wu, M. Hughes and W. L. Garner, J. Cell Sci., 2001, 114, 131–139 CAS.
- M. V. Plikus, D. L. Gay, E. Treffeisen, A. Wang, R. J. Supapannachart and G. Cotsarelis, in Seminars in Cell & Developmental Biology, Academic press, 2012, vol. 23, pp. 946–953 Search PubMed.
- B. Hinz, J. Invest. Dermatol., 2007, 127, 526–537 CrossRef CAS PubMed.
- S. C. Gordts, I. Muthuramu, R. Amin, F. Jacobs and B. D. Geest, Pharmaceuticals, 2014, 7, 419–432 CrossRef CAS PubMed.
- D. P. Poppas, J. M. Massicotte, R. B. Stewart, A. B. Roberts, A. Atala, A. B. Retik and M. R. Freeman, Lasers Surg. Med., 1996, 19, 360–368 CrossRef CAS PubMed.
- D. R. Edwards, G. Murphy, J. J. Reynolds, S. E. Whitham, A. J. Docherty, P. Angel and J. K. Heath, EMBO J., 1987, 6, 1899 CAS.
- M. F. Cordeiro, A. Mead, R. R. Ali, R. A. Alexander, S. Murray, C. Chen, C. York-Defalco, N. M. Dean, G. S. Schultz and P. T. Khaw, Gene Ther., 2003, 10, 59–71 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26904d |
| This journal is © The Royal Society of Chemistry 2017 |