Open Access Article
Open Access ArticleOne-step solvothermal synthesis of interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres with enhanced visible light photocatalytic properties†
Yongxing Zhang‡
a,
Xiangbo Zhou‡a,
Yuanyuan Zhaoa,
Zhongliang Liua,
Dong Maa,
San Chena,
Guangping Zhua and
Xuanhua Li *bc
*bc
aCollaborative Innovation Center of Advanced Functional Composites, Huaibei Normal University, Huaibei 235000, P. R. China
bState Key Laboratory of Solidification Processing Center of Nano Energy Materials, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an 710072, P. R. China. E-mail: lixh32@nwpu.edu.cn
cKey Laboratory of Materials Physics, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, P. R. China
First published on 20th January 2017
Abstract
Three-dimensional (3D) micro/nano hierarchical structure semiconductor catalysts not only inherit the excellent properties of single nanometer-size building blocks resulting from their high specific surface area and high activity but also provide the merits of high dispersion and easy recovery of microstructures. Herein, the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres have been prepared via a one-step, environmentally friendly solvothermal method. Such interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres show an excellent photocatalytic activity, which is 121 times faster than that of the commercial Cu2O powders, and is one of the highest reported to date in the Ag/Cu2O composite materials for the degradation of Methyl Orange (MO) under visible light illumination. Our experimental results for photodegradation of MO also indicate that the existence of Cu in the products does not favor high photocatalytic activity for Ag/Cu2O composites, which may be due to the existence of excess metal in the products which provides the recombination centers for electron–hole pairs. After five consecutive cycles, the photocatalytic activity of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres almost remains unchanged, indicating that they are photostable during the photodegradation of MO. Most importantly, such micrometer-sized overall structures of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres enable them to be recycled and reused easily from solution by natural settlement in a short time. These results suggest that the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres had potential applications in visible light photocatalysis for environmental remediation.
1 Introduction
It is well known that semiconductor photocatalysts with nanometer size display excellent catalytic activity resulting from their high specific surface area and high activity.1–5 However, easy agglomeration is one of the biggest drawbacks of these materials, which causes a sharp decline in the photocatalytic activity.6 In addition, finely nanometer-sized catalysts are very difficult to recycle from the liquid system, resulting in low utilization and recontamination. Considering these shortcomings of nanometer-size catalysts, more attention has been paid to assemble low-dimensional nanometer-size building blocks (such as nanoparticles, nanowires, nanotubes, and nanoflakes) into three-dimensional (3D) micro/nano hierarchical structure semiconductor catalysts.7–9 Such 3D micro/nano hierarchical structure semiconductor catalysts not only inherit the excellent properties of the single nanometer-size building blocks resulting from their high specific surface area and high activity but also provide the merits of high dispersion and easy recovery of microstructures.10–13Cuprous oxide (Cu2O) is a p-type metal oxide semiconductor with a direct band gap of 2.0–2.2 eV, which makes it a potentially promising photocatalyst for the photochemical decomposition of water into O2 and H2 and photocatalytic degradation of organic pollutants under visible light illumination.14–17 However, pure Cu2O exhibits very poor quantum efficiency because of the easy recombination between electron and hole.18 To overcome this limitation, modification of Cu2O with noble metals (Ag, Au, Pt or Pd) is one of the most efficient ways.19–22 This is mainly due to the fact that the noble metals can act as electron sinks due to the schottky barrier at the metal–semiconductor interface, while the photo induced holes can remain on the semiconductor surface.23,24 Therefore, the recombination of the electrons and holes can be prevented, and the photocatalytic efficiency of semiconductor is improved. In addition, compared with other noble metals (Au, Pt and Pd), Ag is more attractive because of its high electrical, low cost, and nontoxicity.25–29 It is widely accepted that Ag nanostructures exhibit a wealth of optical and photoelectrochemical properties directly related to their geometry dependent surface plasmon resonances, which make them great potential candidates in the field of photocatalysis.30 So, there have been many outstanding studies on the preparation of Ag/Cu2O composites for photocatalytic degradation of organic pollutants in wastewater. Li et al. reported the synthesis of Ag nanodisk–Cu2O hybrid nanoconcaves through site-selective growth of noble metals on Cu2O seeds for an effective synergistic catalyst.30 Xiong et al. presented a method for rapid fabricating 1D Ag@Cu2O core–shell heteronanowires for photodegradation of organic dyes.31 Wu et al. successfully prepared the Ag core@Cu2O shell architecture exhibiting the plasmonic enhancement of visible-light photocatalytic activity.32 Wang et al. presented the M/Cu2O (M = Ag, Au) octahedral nanocrystals with enhanced photocatalytic efficiency.33 However, most of these methods suffer from the limitations of requiring longer reaction times, two reaction steps, presence of toxic reducing agents—N2H4·3H2O, for example. Thus, it still remains a challenge to develop a one-step, environmentally friendly solvothermal method for obtaining interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres with enhanced visible light photocatalytic properties.
In this work, we designed a one-step, environmentally friendly solvothermal method to synthesize a interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres at 180 °C for 1 h, employing ethylene glycol (EG) as a solvent, Cu(NO3)2·3H2O and AgNO3 as reagents in the presence of polyvinylpyrrolidone (PVP) as surfactant. To the best of our knowledge, this is the first report on the fabrication and photocatalytic capacity of interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres via a one-step, environmentally friendly solvothermal method. Most importantly, the preparation of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres in the efficient photocatalytic degradation of organic pollutants from water has the following features: (1) both Ag+ and Cu2+ are reduced simultaneously by EG in our reaction system to form Ag/Cu2O composites; (2) the existence of Cu in the products does not favor high photocatalytic activity for Ag/Cu2O composites under visible light illumination. And the photocatalytic activity gradually decreases with increasing Cu content; (3) such micrometer-size overall structures of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres enable them to be recycled and reused easily from solution by natural settlement in a short time.
2 Experimental sections
2.1 Synthesis of interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres
All reagents are of analytical grade and used without further purification. Cu(NO3)2·3H2O (99.0–102.0%), AgNO3 (≥99.8%), polyvinylpyrrolidone (PVP, Mw ≈ 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–58
000–58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, water content ≤ 5.0%), ethylene glycol (EG, water content ≤ 0.1%), absolute ethanol (water content ≤ 0.3%) and hydrogen peroxide (H2O2, ≥30.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Methyl Orange (MO) was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd., China. Deionized water was made in our lab.
000, water content ≤ 5.0%), ethylene glycol (EG, water content ≤ 0.1%), absolute ethanol (water content ≤ 0.3%) and hydrogen peroxide (H2O2, ≥30.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Methyl Orange (MO) was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd., China. Deionized water was made in our lab.
The sample was synthesized via a one-step solvothermal method, and the details are as follows: Cu(NO3)2·3H2O (1.5 mmol), AgNO3 (0.252 mmol) and polyvinylpyrrolidone (PVP, 0.2 g) were added into ethylene glycol (30 mL) under constant magnetic stirring (500 rpm) at room temperature for 1 h. The color of the solution was turned to pure blue. Then, the pure blue solution was transferred into a Teflon-skinned autoclave (50 mL capacity) and heated at 180 °C for 1 h. After the reaction system was naturally cooled to room temperature, the brownish black precipitates was separated from solution and thoroughly washed for several times with deionized water and absolute ethanol to remove the impurities, and then dried in a vacuum oven (pressure (relative vacuum degree) = −0.085 MPa) at 60 °C for 12 h.
2.2 Photocatalytic properties
The catalytic activity experiments of the obtained products for the oxidation and decoloration of the Methyl Orange (MO) dye under visible light illumination were carried out at ambient temperature. In our experiment, 40 mg of the obtained products were dispersed in 40 mL of MO aqueous solution (30 mg L−1). The catalyst/substrate ratio is 33.3 mg catalyst per mg substrate in our model reactions. Before illumination, the mixed solution was magnetically stirred in the dark for 20 min to ensure the establishment of an adsorption–desorption equilibrium and then followed by standing for 10 min. Then, the dispersion was irradiated by a 500 W xenon lamp under magnetic stirring (500 rpm, the distance between the dispersion and xenon lamp is 10 cm). At given time intervals, the dispersion was sampled and centrifuged to separate the catalyst. The concentration of MO in the solution was determined at different intervals using a UV/vis/NIR spectrophotometer (Hitachi U-4100). In addition, we have also studied the effect of the hydrogen peroxide solution (H2O2, 30% wt) on the photocatalytic degradation of MO aqueous solution.2.3 Characterization
Powder X-ray diffraction (XRD) patterns were obtained in the 2θ range of 15–85° using a Philips X'Pert Pro X-ray diffractometer with Cu Kα radiation (1.5418 Å). The scanning electron microscope (SEM) images were taken on a SEM (JEOL-6610-LV), equipped with X-ray energy dispersive spectroscopy (EDS, X-Max, Oxford Instruments) capabilities, working at an accelerating voltage of 10.0 kV. The transmission electron microscope (TEM) and high resolution transmission electron microscope (HRTEM) analysis images were performed on a JEOL JEM-2100 transmission electron microscope operating at an accelerating voltage of 200 kV. The X-ray photoelectron spectroscopy (XPS) was collected on the ESCALab MKII X-ray photoelectron spectrometer (K-Alpha 1063). Photoluminescence (PL) spectra were tested on a F280 fluorescence spectrophotometer. The UV-vis spectra were measured by a UV/vis/NIR spectrophotometer (Hitachi U-4100).3 Results and discussion
3.1 Structure and morphology of interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres
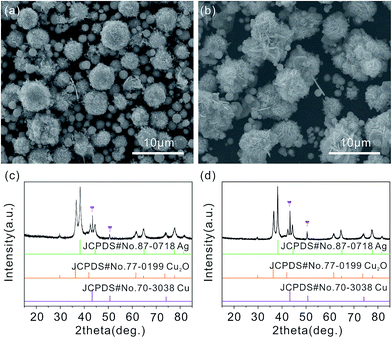
The composition and phase purity of the as-prepared product are carried out by X-ray diffraction (XRD). The obtained pattern is identified through comparison with standard Cu2O and Ag patterns. As shown in Fig. 1(a), the diffraction peaks center at 2θ values of 29.64, 36.52, 42.42, 61.55, 69.78, 73.73 and 77.60° are agree with (110), (111), (200), (220), (310), (311) and (222) crystal planes of the cubic Cu2O (JCPDS card no. 77-0199) phase, and the other diffraction peaks at 2θ = 38.26, 44.47, 64.71, 77.73 and 81.90 are corresponded to (111), (200), (220), (311) and (222) crystal planes of cubic Ag (JCPDS card no. 87-0718) phase. The XRD pattern of the as-prepared Ag/Cu2O product shows the coexistence of Ag and Cu2O, and no additional peaks are detected. The results clearly illustrate the high purity of the as-prepared Ag/Cu2O product. Scanning electron microscopy (SEM) image of the as-prepared Ag/Cu2O product (Fig. 1(b) and (c)) shows that it consists of many monodispersed and uniform Ag/Cu2O composite microspheres with a diameter of approximately 4–5 μm. The detailed morphology of the microspheres is shown in Fig. 1(d), which reveals that it has interlaced nanoflake-assembled flower-like hierarchical structure. In addition, the flower-like hierarchical structure is composed of interlaced nanoflakes with a thickness of 80–90 nm. The as-prepared microspheres are monodispersed and can be well dispersed in the solution without large aggregation (Fig. 1(a) and (b)).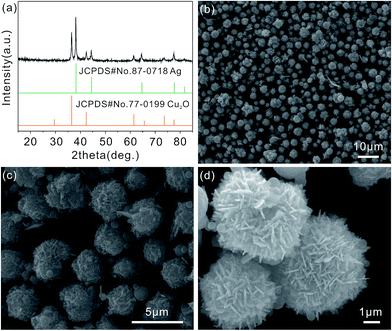 | ||
| Fig. 1 XRD pattern (a) and SEM images (b–d) of the flower-like hierarchical Ag/Cu2O composite microspheres. | ||
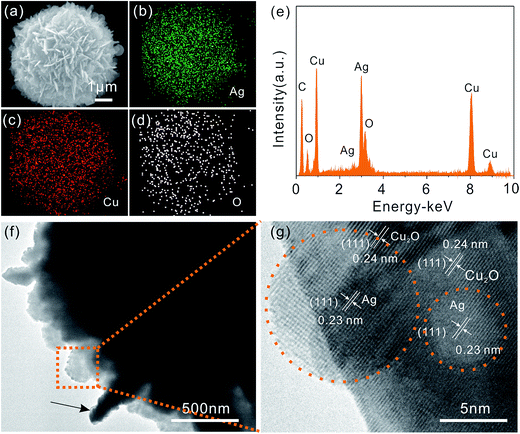
In order to obtain detailed information about Ag and Cu2O distributions in the flower-like hierarchical Ag/Cu2O composite microspheres, the product is also analyzed by electron mapping image analysis (Fig. 2(a–d)). The images are acquired by visualizing the inelastically scattered electrons in the energy loss windows for elemental Cu, Ag, and O. The different color areas shown in parts (b–d) of Fig. 2 indicate Cu-, Ag-, and O-enriched areas of the product, respectively. The images show that the elemental Cu and Ag are well dispersed on the surface of the flower-like hierarchical Ag/Cu2O composite microspheres. Fig. 2(e) shows EDS spectrum of the product, in which the Cu, Ag, and O elements is observed. Transmission electron microscopy (TEM) is also used to characterize the flower-like hierarchical Ag/Cu2O composite microspheres. The nanoflakes, constructing the hierarchical composite microsphere, are transparent to electron beam (Fig. 2(f)). In some areas, the nanoflake is oriented approximately perpendicular to the plane of the supporting copper TEM grid (marked by black arrow in Fig. 2(f)), from which the thickness of the nanoflake is estimated to be around 110 nm. A typical high resolution transmission electron microscopy (HRTEM) image of Ag/Cu2O heterostructures (as shown in Fig. 2(g)) reveal that the metallic Ag nanoparticles with a diameter of 5–10 nm (indicated by orange dotted circles in Fig. 2(g)) attach on the surface of Cu2O nanoflakes. The HRTEM image also shows the interplanar spacing of 0.24 nm and 0.23 nm (marked by white lines and arrows), which correspond to (111) lattice planes of Cu2O and (111) lattice planes of Ag, respectively.
The surface components and chemical states of the flower-like hierarchical Ag/Cu2O composite microspheres are investigated by XPS analysis and the corresponding results are shown in Fig. 3. The survey spectrum shows the silver peaks (Ag 3d), the copper photoelectron peaks (Cu 3s, Cu 2p, Cu 3p, and its Cu LMM Auger), the oxygen peaks (O 1s) and the photoelectron peak of the adventitious carbon (C 1s). The appearance of C peak mainly comes from pump oil due to vacuum treatment before the XPS test. No other peaks can be observed, indicating that the composite microspheres are composed of Cu, Ag and O.
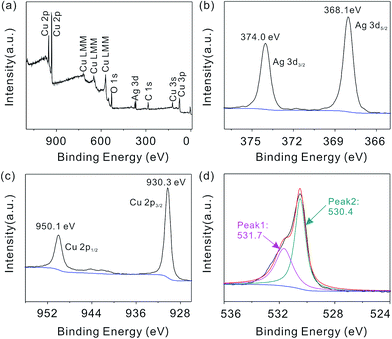 | ||
| Fig. 3 XPS spectrum of the flower-like hierarchical Ag/Cu2O composite microspheres (a); and the high-resolution spectra of Ag 3d (b), Cu 2p (c) and O 1s (d). | ||
High-resolution spectra of Ag, Cu and O species are shown in Fig. 3(b–d), respectively. Fig. 3(b) shows the Ag 3d XPS spectrum for the composite microspheres. Two peaks located at 374.0 eV and 368.1 eV are attributed to Ag 3d3/2 and Ag 3d5/2, respectively. Peak positions of Ag 3d are close to that of pure metallic Ag.34 In addition, the binding energy of Ag 3d shifts to a lower binding energy compared with the corresponding value of the bulk Ag (368.2 and 374.2 eV, respectively), due to the interaction between the Ag and Cu2O microcrystals. The XPS spectrum shown in Fig. 3(c) demonstrates the Cu 2p1/2 and Cu 2p3/2 spin–orbital photoelectrons located at binding energies of 950.1 eV and 930.3 eV, respectively, which are in good agreement with the reported values of Cu2O.35,36 From Fig. 3(d), the O 1s profile is asymmetric and can be fitted into two symmetrical peaks located at 531.7 (Peak 1) and 530.1 eV (Peak 2). The fitted peaks are originated from the lattice oxygen of adsorbed oxygen and Cu2O, respectively.37,38 The XRD, EDS and XPS (as shown in Fig. 1(a), 2(e) and 3) results consistently prove the formation of Ag and Cu2O.
3.2 Effect of the molar ratio of Cu(NO3)2·3H2O to AgNO3 on the phase composition of the final products
The effect of the molar ratio of Cu(NO3)2·3H2O to AgNO3 on the phase composition of the final products has been conducted in our experiments. During these experiments, the molar ratios of Cu(NO3)2·3H2O to AgNO3 are tuned from 2.0 mmol/0.376 mmol (named Sample 1), 2.0 mmol/0.500 mmol (named Sample 2), to 1.5 mmol/0.252 mmol (named Sample 3), while the volume of EG, the amount of PVP, reaction temperature and reaction time is fixed at 30 mL, 0.2 g, 180 °C and 1 h, respectively. As shown in Fig. 4(a), the flower-like hierarchical microspheres with a diameter about 3–4 μm are obtained when the molar ratio of Cu(NO3)2·3H2O to AgNO3 is 2.0 mmol/0.376 mmol. Under the molar ratio of 2.0 mmol/0.5 mmol, the flower-like hierarchical microspheres are still able to be prepared (Fig. 4(b)). When the molar ratio is 1.5 mmol/0.252 mmol, the flower-like hierarchical microspheres are also prepared successfully (Fig. 1). Thus, the molar ratio of Cu(NO3)2·3H2O to AgNO3 is not obviously effect on the morphology. The corresponding XRD patterns of the Sample 1 and 2 are shown in Fig. 4(c) and (d), respectively. From the figures, it is clear that the coexistence of Ag and Cu2O. In addition, impure diffraction peaks are also detected (marked by purple inverted triangles). After careful analysis, these impure diffraction peaks can be indexed to the pattern of copper (Cu) according to JCPDS card no. 70-3038, as shown in Fig. 4(c) and (d). In addition, the exact contents of Cu in Sample 1 and 2 are 1 wt% and 3 wt%, respectively. In our experiments, we have also systematically studied the effect of the reaction time on the formation of the flower-like hierarchical Ag/Cu2O composite microspheres. The results indicate that the morphologies and phase purities of the obtained products can be regulated by the reaction time. And the detailed contents are shown in ESI (Fig. S1†).3.3 Optical properties of Ag/Cu2O composites
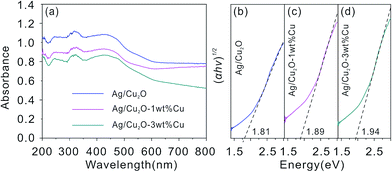
The UV-vis diffuses reflectance spectra of the flower-like hierarchical Ag/Cu2O composite microspheres, the Ag/Cu2O-1 wt% Cu and the Ag/Cu2O-3 wt% Cu are shown in Fig. 5(a). According to the Kubelka–Munk equation: α = (1 − R)2/2R, where R is the reflection coefficient of the product, R = 10−A, A is an optical absorption. The optical band gap (Eg) can be related to absorption coefficient (α) by the following equation:39| αhν = const(hν − Eg)2 |
Fig. 5(b)–(d) plots the relationship of (αhν)1/2 versus energy (hν) of the products and the optical band gap is the extrapolated value. Obviously, the band gap of Ag/Cu2O composites is 1.81 eV, whereas the band gaps of the Ag/Cu2O-1 wt% Cu and Ag/Cu2O-3 wt% Cu composites has been slightly increased to 1.89 and 1.94 eV, respectively. The flower-like hierarchical Ag/Cu2O composite microspheres show a broad and strong absorbance in the visible light region due to its narrow band gap (1.81 eV). However, when there are some Cu contents in the Ag/Cu2O composites, the Ag/Cu2O-1 wt% Cu and Ag/Cu2O-3 wt% Cu exhibit not only a blue shift in the absorption edge but also a weak absorption in the visible light range. This indicates that the appearance of Cu in the products obviously inhibit the light absorption properties of Ag/Cu2O composites. Furthermore, the absorption intensity is also found to gradually decrease with increasing Cu content. Therefore, good photocatalytic activity of the flower-like hierarchical Ag/Cu2O composite microspheres under visible light illumination is expected.
3.4 Photocatalytic properties of Ag/Cu2O composites
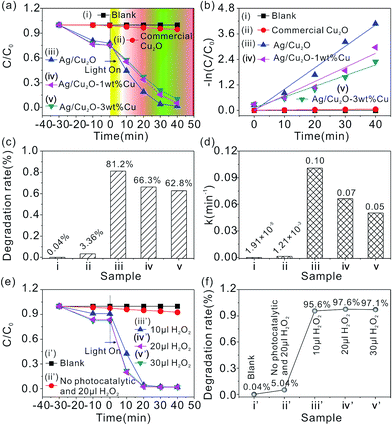
In the current work, the photocatalytic decomposition of MO is used to evaluate the photocatalytic activity of the flower-like hierarchical Ag/Cu2O composite microspheres under visible light illumination at room temperature. A control experiment without any catalyst (blank experiment), commercial Cu2O, the Ag/Cu2O-1 wt% Cu and Ag/Cu2O-3 wt% Cu composites are used for comparison. Absorbance, an indication of MO concentration, is used to measure the extent of MO dye decomposition. The characteristic absorption peak at 464 nm of MO is monitored to follow the catalytic degradation process. The ratio C/C0 is used to describe the degradation, which stands for the concentration ratio after and before a certain reaction time.Fig. 6(a) summarizes the activities of the photocatalyst toward MO degradation through monitoring the adsorption intensity at 464 nm versus time. Before visible light illumination, the mixed solution containing the catalyst and MO is stirred in the dark for 20 min to ensure that MO is adsorbed to saturation on the surface of catalysts. In the control experiment without any catalyst (blank experiment, curve (i) in Fig. 6(a)), C/C0 (relative concentration) of MO is degraded by only 0.04% after visible light illumination for 20 min (Fig. 6(c), Panel i). A photocatalytic test is also conducted using commercial Cu2O under the same conditions as aforementioned (curve (ii) in Fig. 6(a)). It can be seen that about 3.36% of the MO is degraded after 20 min (Fig. 6(c), Panel ii). Further experiment is carried out to compare the photocatalytic properties of the flower-like hierarchical Ag/Cu2O composite microspheres, the Ag/Cu2O-1 wt% Cu and Ag/Cu2O-3 wt% Cu. Under the same conditions, the flower-like hierarchical Ag/Cu2O composite microspheres illustrate a higher photocatalytic activity (curve (iii) in Fig. 6(a)) than the Ag/Cu2O-1 wt% Cu (curve (iv) in Fig. 6(a)) and the Ag/Cu2O-3 wt% Cu (curve (v) in Fig. 6(a)). About 81.2% of the MO is degraded by the flower-like hierarchical Ag/Cu2O composite microspheres (Fig. 6(c), Panel iii) after 20 min; by contrast, the Ag/Cu2O-1 wt% Cu decompose the MO to 66.3% after 20 min (Fig. 6(c), Panel iv) and the Ag/Cu2O-3 wt% Cu decompose the MO to 62.8% after 20 min (Fig. 6(c), Panel v). From experimental results, this indicates that the existence of Cu in the products does not favor high photocatalytic activity for Ag/Cu2O composites. The photocatalytic activity gradually decreases with increasing Cu content.
The photoluminescence (PL) technique is an effective way to study the efficiency of charge carrier trapping, immigration and transfer. A lower PL intensity usually implies a lower electron–hole recombination rate and higher photocatalytic activity.40 Fig. 7 shows the measured PL emission spectra of the as-prepared structures at an excitation wavelength of 325 nm, which is higher than the band gap of Cu2O. It is clearly observed that the commercial Cu2O display a strong PL intensity at around 650 cm−1, which should be the near band emission of Cu2O.41 After the Ag is combined with the Cu2O, the PL intensity shows an apparent decrease, indicating that the recombination of the excitons in Cu2O is efficiently hampered. However, in the case of Ag/Cu2O-1 wt% Cu, evidently the PL intensity is increased compared to Ag/Cu2O, suggesting that Cu inhibits the photogenerated electrons trapped by Ag, thereby resulting in the PL intensity increasing. Moreover, it is noted that the PL intensity of Ag/Cu2O-1 wt% Cu is also lower than Ag/Cu2O-3 wt% Cu. Based on the above results, it is concluded that the existence of excess Cu in the products provides the recombination centers for electron–hole pairs42,43 and thereby the Ag/Cu2O shows more effective charge separation. As displayed in Fig. 7, the PL intensity follows the order: Ag/Cu2O < Ag/Cu2O-1 wt% Cu < Ag/Cu2O-3 wt% Cu < commercial Cu2O, which is greatly consistent with the photocatalytic results.
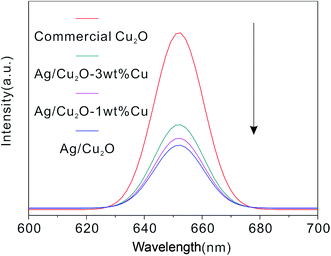 | ||
| Fig. 7 PL spectra of commercial Cu2O, Ag/Cu2O, Ag/Cu2O-1 wt% Cu and Ag/Cu2O-3 wt% Cu with an excitation wavelength of 325 nm. | ||
The photodegradation of MO can be described as pseudo first order: ln(C/C0) = −kt, where C0 and C are MO concentrations initially and after time t, respectively, and the rate constants (k, min−1) are determined from plots of ln(C/C0) versus illumination time. Fig. 6(b) shows the linear relationship represented by the ln(C/C0) versus reaction time t for different catalysts employed in our work. The apparent reaction rate constant (k) can be calculated from the rate equation ln(C/C0) = −kt. The reaction rate constants for the photodegradation of MO are ki = 1.91 × 10−5, kii = 1.21 × 10−3, kiii = 0.1, kiv = 0.07 and kv = 0.05 min−1 for the blank experiment, the commercial Cu2O, the flower-like hierarchical Ag/Cu2O composite microspheres, the Ag/Cu2O-1 wt% Cu and Ag/Cu2O-3 wt% Cu composites, respectively (Fig. 6(d)). From the results, the kinetic reaction constants k of MO photodegradation in the presence of the flower-like hierarchical Ag/Cu2O composite microspheres is 121, 1.43 and 2 times faster than that of the reaction in the presence of the commercial Cu2O, the Ag/Cu2O-1 wt% Cu and the Ag/Cu2O-3 wt% Cu composites, respectively. Table 1 summarizes the photocatalytic degradation capacity of various photocatalysts for MO. It is found that the photocatalytic degradation capacity of the flower-like hierarchical Ag/Cu2O composite microspheres is lower than that of nanoscale Cu2O. Even though, the photocatalytic degradation capacity of the flower-like hierarchical Ag/Cu2O composite microspheres for MO is still better than that of other previously reported Ag/Cu2O composites and some other related semiconductor photocatalyst materials.
| Catalysts | Degradation time | Degradation percentage | Ref. |
|---|---|---|---|
| Interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres | 20 min | 81.3% | This study |
| Ag/Cu2O-1 wt% Cu | 20 min | 66.3% | |
| Ag/Cu2O-3 wt% Cu | 20 min | 62.8% | |
| Commercial Cu2O | 20 min | 3.36% | |
| Hierarchical Cu2O nanospheres | 10 min | 98.1% | 46 |
| Porous Cu2O nanospheres | 20 min | 95.8% | 47 |
| Ag@Cu2O core–shell heteronanowires | 20 min | 75.1% | 31 |
| Ag–Cu2O composites | 20 min | 77.8% | 48 |
| Reduced graphene oxide supported Ag–Cu2O composites with hierarchical structures | 20 min | 71.5% | 49 |
| Ag–Cu2O nanocorncobs | 60 min | 68.8% | 50 |
| Cu2O/Ag composite nanospheres | 60 min | 78.9% | 51 |
| Copper(II)-based high-connected metal–organic frameworks | 20 min | 56.8% | 52 |
| Pd cocatalyst on Sm-doped BiFeO3 nanoparticles | 60 min | 62.2% | 53 |
| Novel tungstosilicic acid immobilized on zeolites catalyst | 120 min | 58.8% | 54 |
| Pine tree-like Cu/ZnO heterostructured film | 120 min | 68.5% | 55 |
| WO3/TiO2 nanowires | 160 min | 50.2% | 56 |
Fig. 6(e) shows the effect of H2O2 dosage on the photocatalytic activity of the flower-like hierarchical Ag/Cu2O composite microspheres. As shown in Fig. 6(e), the photodegradation rates (C/C0) of MO can be neglected after visible light illumination for 20 min in the blank experiment (curve (i′), and (Fig. 6(e), Sample i′)), and only 20 μL H2O2 (curve (ii′), and (Fig. 6(e), Sample ii′)). However, when the flower-like hierarchical Ag/Cu2O composite microspheres are added in this reaction system in the presence of H2O2, the photodegradation rates of MO are over 95% after visible light illumination for 20 min (Fig. 6(e), curve (iii′), curve (iv′) and curve (v′)). Compared with the condition without adding H2O2 (Fig. 6(c), Panel iii), the photocatalytic degradation rates of MO increase to over 14.4% after 20 min illumination. The results suggest that the addition of small amounts of H2O2 can accelerate the photodegradation rate of the flower-like hierarchical Ag/Cu2O composite microspheres.44 It is possible that more radicals are formed in the presence of H2O2, thus leading to an increase of the photodegradation rate.45
The degradation rate versus different initial dosage of H2O2 has been shown in Fig. 6(f). From the figure, when the initial dosage of H2O2 is 10 μL, the flower-like hierarchical Ag/Cu2O composite microspheres display the lowest photodegradation rate (about 95.6%). When the concentration of H2O2 is 20 μL (Sample iv′), the photodegradation rate reaches a maximum (about 97.6%). When the dosage of H2O2 is further increased to 30 μL (Sample v′), the photodegradation rate begins to decrease (about 97.1%). The results suggest that an optimum dosage of H2O2 can be 20 μL. At low dosages of H2O2, the number of hydroxyl radicals (˙OH radicals) increase when increasing the dosages of H2O2, so the degradation rate of MO enhances correspondingly. However, excess H2O2 can result in a self-consumption and scavenging effect of ˙OH radical through the reaction described by eqn (1) and (2); at the same time, ˙OH radicals can also dimerize to H2O2 by eqn (3) at high dosage.57–61
| H2O2 + ˙OH → ˙HO2 + H2O | (1) |
| ˙HO2 + ˙OH → O2 + H2O | (2) |
| ˙OH + ˙OH → H2O2 | (3) |
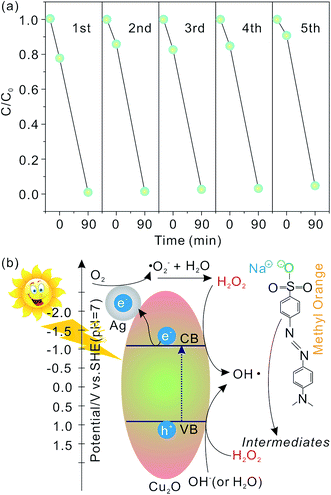
Catalyst stability and reusability are of vital importance in practical applications. The photocatalytic activity for the flower-like hierarchical Ag/Cu2O composite microspheres in degradation of MO is studied in consecutive cycles (90 min illumination for each cycle) and shows in Fig. 8(a). From the results obtained, it is observed that over 98.97% of MO is photodegraded when the flower-like hierarchical Ag/Cu2O composite microspheres are used for the first time. After five consecutive cycles, 95.26% of MO is photodegraded, the photocatalytic activity of the flower-like hierarchical Ag/Cu2O composite microspheres almost remain unchanged, indicating that they are photostable during the photodegradation of MO. Furthermore, such micrometer-sized overall structures of the flower-like hierarchical Ag/Cu2O composite microspheres enable them to be recycled and reused easily from solution by natural settlement in a short time.
A possible photocatalysis mechanism for the flower-like hierarchical Ag/Cu2O composite microspheres is illustrated in Fig. 8(b). When the flower-like hierarchical Ag/Cu2O composite microspheres are illuminated by visible light with photon energy higher than the band gap of Cu2O, electrons in the valence band (VB) can be excited to the conduction band (CB), leaving behind the same amount of holes in VB. As the work function of Ag (4.6 eV) is greater than that of Cu2O (4.2 eV),62,63 electrons hence can be transferred from the CB of Cu2O to Ag.1,64 The holes in the VB can be trapped by the OH− (or H2O), resulting in the formation of ˙OH radical species.65 The electrons can be captured by the adsorbed O2 and form the superoxide anion radicals (˙O2− radicals). The ˙O2− radicals can ultimately be reduced to ˙OH radicals.66 It is known that the ˙OH radicals are able to oxidize pollutants due to their high oxidative capacity. Hence, under visible light illumination, MO molecules can be photodegraded by the flower-like hierarchical Ag/Cu2O composite microspheres. When H2O2 is added in the reaction system, the electrons and holes can be also captured by H2O2 molecules, leading to the formation of more ˙OH radicals, as shown in Fig. 8(b), which is in favor of oxidizing MO molecules. Thus, an appropriate dosage of H2O2 can accelerate the photocatalytic activity of the flower-like hierarchical Ag/Cu2O composite microspheres.
4 Conclusion
In summary, we have synthesized the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres via a one-step, environmentally friendly solvothermal method. Influencing factors, such as the molar ratio of Cu(NO3)2·3H2O to AgNO3, play important roles in the contents of Cu in the obtained products. The as-prepared interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres exhibit better photocatalytic properties in photodegradation of MO compared with the commercial Cu2O. Our experimental results for photodegradation of MO also indicate that the existence of Cu in the products does not favor high photocatalytic activity for Ag/Cu2O composites. The photocatalytic activity gradually decreases with increasing Cu content. It may be due to the existence of excess metal in the products which provides the recombination centers for electron–hole pairs. The addition of small amounts of H2O2 can further accelerate the photodegradation rate of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres. After five consecutive cycles, the photocatalytic activity of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres almost remain unchanged, indicating that they are photostable during the photodegradation of MO. Most importantly, such micrometer-sized overall structures of the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres enable them to be recycled and reused easily from solution by natural settlement in a short time. These results suggest that the interlaced nanoflake-assembled flower-like hierarchical Ag/Cu2O composite microspheres had potential applications in visible light photocatalysis for environmental remediation.Acknowledgements
This research was supported by the National Natural Science Foundation of China (51302102, 51402120, 51571166 and 61505167), the Key Natural Science Research Project for Colleges and Universities of Anhui Province (KJ2016A638, KJ2016SD53 and KJ2014B11), the Natural Science Foundation of Anhui Province (1708085ME96), the Huaibei Scientific Talent Development Scheme (20140305 and 20140318), the Huaibei Normal University youth research project (2014xq017). We also thank the support of the Natural Science Research Project of Shaanxi Province (2016JM5001), the Research Fund of the State Key Laboratory of Solidification Processing (NWPU) (Grant No. 147-QZ-2016), The Research Fund of Key Laboratory of Materials Physics, Institute of Solid State Physics, CAS (2016KLMP04), and the Key Scientific and Technological Team from Shaanxi Province (No. 2015KCT-12).References
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- K. Maeda, ACS Catal., 2013, 3, 1486–1503 CrossRef CAS.
- D. J. Martin, G. G. Liu, S. J. A. Moniz, Y. P. Bi, A. M. Beale, J. H. Ye and J. W. Tang, Chem. Soc. Rev., 2015, 44, 7808–7828 RSC.
- X. Li, S. Guo, C. Kan, J. Zhu, T. Tong, W. Choy and B. Wei, Nano Energy, 2016, 30, 549–558 CrossRef CAS.
- Y. Zhang, Y. Ye, X. Zhou, Z. Liu, D. Ma, B. Li, Q. Liu, G. Zhu, S. Chen and X. Li, CrystEngComm, 2016, 18, 7994–8003 RSC.
- X. Li, J. Zhu and B. Wei, Chem. Soc. Rev., 2016, 11, 3145–3187 RSC.
- Y. Zhong, Z. Wang, R. Zhang, F. Bai, H. Wu, R. Haddad and H. Fan, ACS Nano, 2014, 8, 827–833 CrossRef CAS PubMed.
- R. Xie, G. Fan, L. Yang and F. Li, Catal. Sci. Technol., 2015, 5, 540–548 CAS.
- M.-H. Sun, S.-Z. Huang, L.-H. Chen, Y. Li, X.-Y. Yang, Z.-Y. Yuan and B.-L. Su, Chem. Soc. Rev., 2016, 45, 3479–3563 RSC.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
- J.-S. Xu and Y.-J. Zhu, CrystEngComm, 2012, 14, 2702–2710 RSC.
- X. Zheng, Z. Han, S. Yao, H. Xiao, F. Chai, F. Qu and X. Wu, Dalton Trans., 2016, 45, 7094–7103 RSC.
- M. Li, K. Chang, T. Wang, L. Q. Liu, H. B. Zhang, P. Li and J. H. Ye, J. Mater. Chem. A, 2015, 3, 13731–13737 CAS.
- S. Guo, X. Li, J. Zhu, T. Tong and B. Wei, Small, 2016, 12, 5692–5701 CrossRef CAS PubMed.
- C. Lu, L. Qi, J. Yang, X. Wang, D. Zhang, J. Xie and J. Ma, Adv. Mater., 2005, 17, 2562–2567 CrossRef CAS.
- H. Gao, J. Zhang, R. Wang and M. Wang, Appl. Catal., B, 2015, 172, 1–6 CrossRef.
- H.-Y. Jing, T. Wen, C.-M. Fan, G.-Q. Gao, S.-L. Zhong and A.-W. Xu, J. Mater. Chem., 2014, 2, 14563–14570 RSC.
- J. Y. Xiang, J. P. Tu, Y. F. Yuan, X. H. Huang, Y. Zhou and L. Zhang, Electrochem. Commun., 2009, 11, 262–265 CrossRef CAS.
- N. Meir, I. Jenlaplante, K. Flomin, E. Chockler, B. Moshofsky, M. Diab, M. Volokh and T. Mokari, J. Mater. Chem., 2013, 1, 1763–1769 RSC.
- C. Han, L. I. Zhiyu and J. Shen, Asian J. Chem., 2015, 27, 1861–1864 CrossRef CAS.
- B. Lu, A. Liu, H. Wu, Q. Shen, T. Zhao and J. Wang, Langmuir, 2016, 32, 3085–3094 CrossRef CAS PubMed.
- L. Ye, Z. Li, X. Zhang, F. Lei and S. Lin, J. Mater. Chem., 2014, 2, 21010–21019 RSC.
- M. R. Hoffmann, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- X. Z. Li and F. B. Li, Environ. Sci. Technol., 2001, 35, 2381–2387 CrossRef CAS PubMed.
- B. Chen, J. Mater. Chem., 2012, 22, 11651–11657 RSC.
- F. Wenguang, J. Simon, S. Yiyi and M. K. H. Leung, Phys. Chem. Chem. Phys., 2013, 16, 676–680 Search PubMed.
- M. Zhao, H. Xu, S. X. Ouyang, D. W. Li, X. G. Meng and J. H. Ye, Phys. Chem. Chem. Phys., 2016, 18, 3409–3412 RSC.
- Y. Bi and J. Ye, Chem. Commun., 2009, 6551–6553 RSC.
- Y. C. Chen, F. C. Zheng, Y. L. Min, T. Wang, Y. G. Zhang and Y. X. Wang, J. Mater. Sci.: Mater. Electron., 2012, 23, 1592–1598 CrossRef CAS.
- L. Li, X. Chen, Y. Wu, D. Wang, Q. Peng, G. Zhou and Y. Li, Angew. Chem., Int. Ed., 2013, 52, 11049–11053 CrossRef CAS PubMed.
- J. Xiong, Z. Li, J. Chen, S. Zhang, L. Wang and S. Dou, ACS Appl. Mater. Interfaces, 2014, 6, 15716–15725 CAS.
- J. Li, S. K. Cushing, J. Bright, F. Meng, T. R. Senty, P. Zheng, A. D. Bristow and N. Wu, ACS Catal., 2013, 3, 47–51 CrossRef CAS.
- Z. Wang, S. Zhao, S. Zhu, Y. Sun and M. Fang, CrystEngComm, 2011, 13, 2262–2267 RSC.
- W. Wei-Tai, W. Yusong, S. Lei, P. Wenmin, Z. Qingren, X. Guoyong and L. Fei, J. Phys. Chem. B, 2006, 110, 14702–14708 CrossRef PubMed.
- H. Qing, S. Daili, Z. Wenhua, C. Kai, C. Sujie, M. Yunsheng, J. Zhiquan, Y. Jinlong and H. Weixin, Langmuir, 2011, 27, 665–671 CrossRef PubMed.
- Z. Ai, L. Zhang, S. Lee and W. Ho, J. Phys. Chem. C, 2009, 113, 20896–20902 CAS.
- X. Lin, R. Zhou, J. Zhang and S. Fei, Appl. Surf. Sci., 2009, 256, 889–893 CrossRef CAS.
- G. Bacis, M. Catelani, L. Ciani, V. Scarano and R. Singuaroli, Nanotechnology, 2007, 18, 1–4 Search PubMed.
- J. Cao, B. Xu, H. Lin, B. Luo and S. Chen, Dalton Trans., 2012, 41, 11482–11490 RSC.
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang and S. Jiazhong, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef.
- Y. Wang, S. Li, H. Shi and K. Yu, Nanoscale, 2012, 4, 7817–7824 RSC.
- B. Zhou, Z. Liu, H. Wang, Y. Yang and W. Su, Catal. Lett., 2009, 132, 75–80 CrossRef CAS.
- L. Ren, Y. P. Zeng and D. Jiang, Catal. Commun., 2009, 10, 645–649 CrossRef CAS.
- S. Sun, X. Zhang, J. Zhang, L. Wang, X. Song and Z. Yang, CrystEngComm, 2012, 15, 867–877 RSC.
- F. Ji, C. Li, J. Zhang and L. Deng, Desalination, 2011, 269, 284–290 CrossRef CAS.
- S. Sun, X. Zhang, X. Song, S. Liang, L. Wang and Z. Yang, CrystEngComm, 2012, 14, 3545–3553 RSC.
- L. Zhang, B. Yu, P. Ying, L. Wu, S. Chen, J. Wang, X. Gu, R. Zhou and Z. Ni, Superlattices Microstruct., 2015, 84, 181–191 CrossRef CAS.
- J. Yang, Z. Li, C. Zhao, Y. Wang and X. Liu, Mater. Res. Bull., 2014, 60, 530–536 CrossRef CAS.
- L. Xu, F. Zhang, X. Song, Z. Yin and Y. Bu, J. Mater. Chem. A, 2015, 3, 5923–5933 CAS.
- S. Yang, S. Zhang, H. Wang, H. Yu, Y. Fang and F. Peng, Mater. Res. Bull., 2015, 70, 296–302 CrossRef CAS.
- W. Zhang, X. Yang, Z. Qian, K. Wang, J. Lu, C. Min and Z. Yang, Ind. Eng. Chem. Res., 2014, 53, 16316–16323 CrossRef CAS.
- G.-H. Cui, C.-H. He, C.-H. Jiao, J.-C. Geng and V. A. Blatov, CrystEngComm, 2012, 14, 4210–4216 RSC.
- S. Wang, D. Chen, F. Niu, N. Zhang, L. Qin and Y. Huang, RSC Adv., 2016, 6, 34574–34587 RSC.
- C. L. Marchena, L. Lerici, S. Renzini, L. Pierella and L. Pizzio, Appl. Catal., B, 2016, 188, 23–30 CrossRef.
- K. Wang, Z. Chen, M. Huang, Z. Yang, C. Zeng, L. Wang, M. Qiu, Y. Zhang and W. Zhang, RSC Adv., 2016, 6, 103594–103600 RSC.
- D. Nagy, T. Firkala, E. Drotár, A. Szegedi, K. Laszlo and I. M. Szilágyi, RSC Adv., 2016, 6, 95369–95377 RSC.
- G. Granados-Oliveros, E. A. Páez-Mozo, F. M. Ortega, C. Ferronato and J. M. Chovelon, Appl. Catal., B, 2009, 89, 448–454 CrossRef CAS.
- N. Daneshvar, M. A. Behnajady and Y. Z. Asghar, J. Hazard. Mater., 2007, 139, 275–279 CrossRef CAS PubMed.
- W. Luo, L. Zhu, N. Wang, H. Tang, M. Cao and Y. She, Environ. Sci. Technol., 2010, 44, 1786–1791 CrossRef CAS PubMed.
- E. Brillas, I. Sirés and M. A. Oturan, Chem. Rev., 2009, 109, 6570–6631 CrossRef CAS PubMed.
- D. D. Dionysiou, M. T. Suidan, E. Bekou, I. Baudin and J. M. Laîné, Appl. Catal., B, 2000, 26, 153–171 CrossRef CAS.
- Y. Xu, Am. Mineral., 2000, 85, 543–556 CrossRef CAS.
- A. Henglein, Berichte Der Bunsengesellschaft Für Physikalische Chemie, 1997, 101, 1562–1572 CrossRef CAS.
- Y. Pan, S. Deng, L. Polavarapu, N. Gao, P. Yuan, C. H. Sow and Q.-H. Xu, Langmuir, 2012, 28, 12304–12310 CrossRef CAS PubMed.
- Y. Zheng, L. Zheng, Y. Zhan, X. Lin, A. Qi Zheng and K. Wei, Inorg. Chem., 2007, 46, 6980–6986 CrossRef CAS PubMed.
- W. Lu, S. Gao and J. Wang, J. Phys. Chem. C, 2008, 112, 16792–16800 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26870f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |