Open Access Article
Open Access ArticleThe dipeptidyl peptidase-4 inhibitor teneligliptin reduces kidney damage from hypercholesterolemia in apolipoprotein E-deficient mice
Hui Liua,
Nan Lia,
Ying Liua,
Jing Xinga,
Shuai Fengb,
Mengye Lic,
Jinping Liud,
Huiling Gaoe,
Yan Luf and
Hongyang Liu *g
*g
aDepartment of Emergency, The First Affiliated Hospital of Dalian Medical University, 193# Lianhe Road, Dalian, China
bDepartment of Otolaryngology, The First Affiliated Hospital of China Medical University, 155# Nanjing Road, Shenyang, China
cDepartment of Special Medical Unit, The First Affiliated Hospital of Dalian Medical University, 193# Lianhe Road, Dalian, China
dDepartment of Geratology, Dalian Friendship Hospital Affiliated to Dalian Medical University, 8# Sanba Square, People Road, Dalian, China
eCollege of Life and Health Sciences, Northeastern University, Shenyang, China
fDepartment of Cardiology, The First Affiliated Hospital of Dalian Medical University, 193# Lianhe Road, Dalian, China
gDepartment of Heart Intensive Care Unit, The First Affiliated Hospital of Dalian Medical University, 193# Lianhe Road, Dalian, China. E-mail: Yang060188@163.com; Fax: +86-0411-83635963; Tel: +86-0411-83635963
First published on 27th January 2017
Abstract
Hypercholesterolemia is a well-established risk factor for kidney injury that can lead to chronic kidney disease (CKD). Many clinic data show that dipeptidyl peptidase-4 (DPP-4) inhibitor is protective against kidney damage in diabetes patients. The goal of this study was to investigate the possible protective effects of teneligliptin against kidney injury in apolipoprotein E knockout (ApoE−/−) mice. Eight-week-old male ApoE−/− mice were randomly divided into the following 3 groups: a control group fed a normal diet (ND group), a group fed a high cholesterol diet (HD group) or a group fed HD mixed with teneligliptin (HD + Tene group). All groups received different treatments for 6 weeks. The metabolic characteristics of total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-c) and creatinine (Cre) were lower in ApoE−/− HD + Tene mice than ApoE−/− HD mice. Oil-red O staining revealed excessive lipid deposition in the kidneys of ApoE−/− HD mice; however, it was significantly suppressed in the ApoE−/− HD + Tene mice. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) gene and protein expression was lower in the kidney tissue of ApoE−/− HD + Tene mice than ApoE−/− HD mice. These results indicate that teneligliptin may provide a potential therapeutic target for kidney damage from hypercholesterolemia.
Introduction
ApoE−/− mice are considered a well-accepted model of hypercholesterolemia, and they have been used extensively to study the effects of this disease on atherosclerosis and renal injury.1 In ApoE−/− mice, dyslipidaemia-related kidney injury is associated with marked pathological alterations, including lipid deposition in the glomerulus, mesangial expansion, and an increased extracellular matrix (ECM) area.2,3 Increasing evidence has shown that lipid accumulation in the kidney contributes to the progression of chronic kidney disease (CKD).4,5 However, the underlying pathophysiological mechanisms of the relationship between hypercholesterolemia and renal injury are not yet fully understood.Dipeptidyl peptidase-4 (DPP-4), also known as lymphocyte cell surface marker CD26, exists as a smaller soluble form in blood plasma. DPP-4 is widely expressed on T and B cells, subsets of macrophages, haematopoietic stem cells, and haematopoietic progenitor cells as well as on epithelial, endothelial, and acinar cells in a variety of tissues.6,7 The complex biological roles of DPP-4 include cell membrane-associated activation of intracellular signal transduction pathways, cell-to-cell interaction, and enzymatic activity.8 DPP-4 inhibitors have recently emerged as a new class of anti-diabetic drug that have favourable results in improving glycaemic control with a minimal risk of hypoglycaemia and weight gain. Teneligliptin is a novel DPP-4 inhibitor for use in managing type 2 diabetes mellitus.9 Many clinical data suggest that teneligliptin protects against diabetic nephropathy injury.10 Meta-analyses have suggested a potential beneficial effect of DPP-4 inhibitors on cholesterol, which could reduce the cardiovascular risk.11,12 However, the function of teneligliptin in hypercholesterolemia-induced renal injury is unclear.
Materials and methods
Animals and experimental protocols
All animal studies were approved by the Animal Studies Committee of the first affiliated hospital of Dalian Medical University. ApoE−/− mice were purchased from Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China). All mice were housed in a room with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light–dark cycle with a temperature maintained at 24 °C. At 8 weeks old, the male mice were randomly divided into the following three groups: ApoE−/− mice fed a normal diet (n = 7) or a high-cholesterol diet (n = 6) and ApoE−/− mice fed teneligliptin (20 mg kg−1 d−1; Mitsubishi Tanabe Pharma, Osaka, Japan) + a high-cholesterol diet (n = 7). The high-cholesterol diet contained 1.5% cholesterol and 15% fat. The experimental diet was purchased from the Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China). Each group was fed their diet for 6 weeks. Blood samples were obtained from the inferior vena cava and collected in serum tubes; they were then stored at −80 °C until use. Coronal sections of the kidneys were fixed in 10% formalin and then embedded in paraffin for histological evaluation or embedded in OCT compound (Torrance, CA, USA) and stored at −80 °C for oil-red O staining. The remainder of the kidney was snap-frozen in liquid nitrogen for mRNA or immunohistochemical analysis. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals. The study was approved by the ethical committee of the first affiliated hospital of Dalian Medical University.
12 h light–dark cycle with a temperature maintained at 24 °C. At 8 weeks old, the male mice were randomly divided into the following three groups: ApoE−/− mice fed a normal diet (n = 7) or a high-cholesterol diet (n = 6) and ApoE−/− mice fed teneligliptin (20 mg kg−1 d−1; Mitsubishi Tanabe Pharma, Osaka, Japan) + a high-cholesterol diet (n = 7). The high-cholesterol diet contained 1.5% cholesterol and 15% fat. The experimental diet was purchased from the Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China). Each group was fed their diet for 6 weeks. Blood samples were obtained from the inferior vena cava and collected in serum tubes; they were then stored at −80 °C until use. Coronal sections of the kidneys were fixed in 10% formalin and then embedded in paraffin for histological evaluation or embedded in OCT compound (Torrance, CA, USA) and stored at −80 °C for oil-red O staining. The remainder of the kidney was snap-frozen in liquid nitrogen for mRNA or immunohistochemical analysis. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals. The study was approved by the ethical committee of the first affiliated hospital of Dalian Medical University.
Biochemical measurements
TC, LDL-c and Cre were measured using an automatic analyser (Dimension, Wilmington, DE, USA).Morphologic analysis and immunohistochemistry
Kidney samples were collected and either fixed in 4% paraformaldehyde or snap frozen in liquid nitrogen. Samples were embedded in paraffin or OCT and were cut into slices using a microtome (Leica RM 2235 or Leica CM1850UV; Leica, Solms, Germany). Slices were then mounted onto glass slides and histological examinations were performed. Frozen sections were used to evaluate lipid deposition using oil-red O staining (Sigma, Santa Clara, CA, USA).Immunohistochemistry was performed using Histone Simple stain kits (Nichirei, Tokyo, Japan) according to the manufacturer's instructions. Briefly, paraffin-embedded sections were deparaffinised with xylene and then rehydrated in a descending series of ethanol washes. The sections were treated for 15 min with 3% H2O2 in methanol to inactivate endogenous peroxidases and were then incubated at room temperature for 1 hour with primary antibodies for collagen IV (rabbit anti-collagen IV antibody, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; Abcam, England) or LOX-1 (rabbit anti-LOX-1 antibody, 1
500; Abcam, England) or LOX-1 (rabbit anti-LOX-1 antibody, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250; Abcam). All sections were observed under an Olympus B ×40 upright light microscope (Olympus, Tokyo, Japan).
250; Abcam). All sections were observed under an Olympus B ×40 upright light microscope (Olympus, Tokyo, Japan).
RNA isolation and real-time RT-PCR
Total RNA was isolated from renal cortical tissues using the ISOGEN (Nippon gene, Tokyo, Japan) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from total RNA using a first-strand cDNA synthesis kit (SuperScript VILO cDNA Synthesis Kit; Life Technologies Carlsbad, CA, USA) according to the manufacturer's protocol. Gene expression was quantitatively analysed by real-time RT-PCR using fluorescent SYBR Green technology (Light Cycler; Roche Molecular Biochemicals). β-Actin cDNA was amplified and quantitated in each cDNA to normalize the relative amounts of the target genes. Primer sequences are listed in Table 1.| Gene | Primers |
|---|---|
| a Abbreviations: LOX-1, lectin-like oxidized low-density lipoprotein receptor-1; SR–A, scavenger receptor-A; ABCA1, ATP-binding cassette transporter A1. | |
| LOX-1 | F: 5′-CAAAGTCTCCCAACCAACCTGCAA-3′ |
| R: 5′-ACATCCTGTCTTTCATGCGGCAAC-3′ | |
| β-Actin | F: 5′-CGATGCCCTGAGGGTCTTT-3′ |
| R: 5′-TGGATGCCACAGGATTCCAT-3′ | |
| SR–A | F: 5′-GTTAAAGGTGATGGGGGACA-3 |
| R: 5′-TCCCCTTCTCTCCCTTTTGT-3′ | |
| CD36 | F: 5′-CCTTAAAGGAATCCCCGTGT-3′ |
| R: 5′-TGCATTTGCCAATGTCTAGC-3′′ | |
| ABCA1 | F: 5′-AGCCAGAAGGGAGTGTCAGA-3′ |
| R: 5′-CATGCCATCTGGGTAAACCT-3′ | |
Western blot for kidney tissue
Proteins were extracted from renal cortical tissues using radioimmunoprecipitation assay buffer (P0013B; Beyotime, Shanghai, China). Samples were electrophoresed on 10% SDS-PAGE gel and proteins were transferred to polyvinylidene fluoride membrane (Immobilon, Millipore, Billerica, MA, USA). Membranes were blocked in Tris-buffered saline with 0.1% Tween-20 (TBS-T) containing 5% skim milk and then were incubated in primary antibody diluents (P0023A; Beyotime) and gently shaken overnight at 4 °C. Primary antibodies against LOX-1 (rabbit anti-LOX-1 antibody, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250; Abcam), phospho-PKC (rabbit anti-phospho-PKC, 1
250; Abcam), phospho-PKC (rabbit anti-phospho-PKC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; Cell Signaling Technology), and anti-β-actin (1
1000; Cell Signaling Technology), and anti-β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; Cell Signaling Technology). Membranes were then incubated with secondary antibody (anti-rabbit Ig-G, 1
1000; Cell Signaling Technology). Membranes were then incubated with secondary antibody (anti-rabbit Ig-G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; Cell Signaling Technology for 1 hour). This analysis was performed independently three times. Protein levels are expressed as the protein/β-actin ratios to minimize loading differences. The relative signal intensity was quantified using NIH ImageJ software.
1000; Cell Signaling Technology for 1 hour). This analysis was performed independently three times. Protein levels are expressed as the protein/β-actin ratios to minimize loading differences. The relative signal intensity was quantified using NIH ImageJ software.
Statistical analysis
All data were presented as the mean ± SEM. Statistical analysis was performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA). Inter-group variation was measured by one-way ANOVA and subsequent Tukey's test. The minimal level for statistical significance was P < 0.05.Results
Metabolic characteristics
The metabolic characteristics of ApoE−/− mice after 6 weeks of dietary treatment are summarized in Table 2. The HD group showed a marked increased in total cholesterol and low-density lipoprotein levels in ApoE−/− mice, but these were significantly decreased in the HD + Tene group. There was no difference between the HD + Tene and ND groups. Body weights and kidney weights did not differ among the three groups. Creatinine was significantly decreased in the HD + Tene group compared with the HD group.| ApoE−/− ND | ApoE−/− HD | ApoE−/− HD + Tene | |
|---|---|---|---|
| n = 7 | n = 6 | n = 7 | |
| a Abbreviations: TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; CRE, creatinine. Data are means ± SEM; n = 6–7 per group. *P < 0.01 vs. ApoE−/− HD. | |||
| Body weight (g) | 24.29 ± 0.51 | 23.65 ± 0.42 | 24.63 ± 0.86 |
| Kidney weight (mg) | 178.65 ± 7.21 | 158.32 ± 8.16 | 176.1 ± 7.03 |
| T-cholesterol (mg dl−1) | 595.33 ± 72.07* | 2505 ± 386.72 | 830.63 ± 64.29* |
| LDL-c (mg dl−1) | 147.17 ± 36.78* | 677.5 ± 105.16 | 284 ± 13.86* |
| CRE (mg dl−1) | 126.37 ± 17.32* | 352.8 ± 37.26 | 167.15 ± 19.35* |
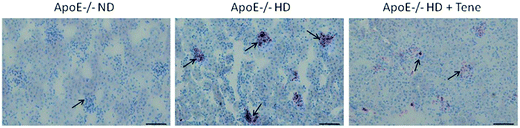
Teneligliptin reduced renal lipid accumulation in ApoE−/− HD group mice
We used oil-red O staining to evaluate renal lipid accumulation. We detected increased lipid retention in the kidneys of ApoE−/− HD group mice. Interestingly, the HD + Tene group mice showed markedly reduced renal lipid deposition compared with ApoE−/− mice despite the consumption of an HD (Fig. 1).Teneligliptin reduced glomerulosclerosis in the ApoE−/− HD group mice
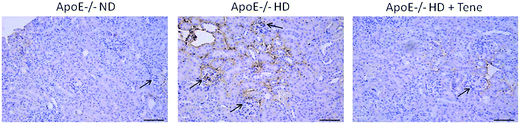
To evaluate glomerulosclerosis, collagen type IV immunostaining was performed (Fig. 2). The HD + Tene group mice showed markedly reduced collagen type IV accumulation in kidney tissue compared with ApoE−/− HD mice. This result indicates that teneligliptin reduced glomerulosclerosis in ApoE−/− HD mice.Teneligliptin reduced LOX-1 gene expression in the kidneys of ApoE−/− mice with HD
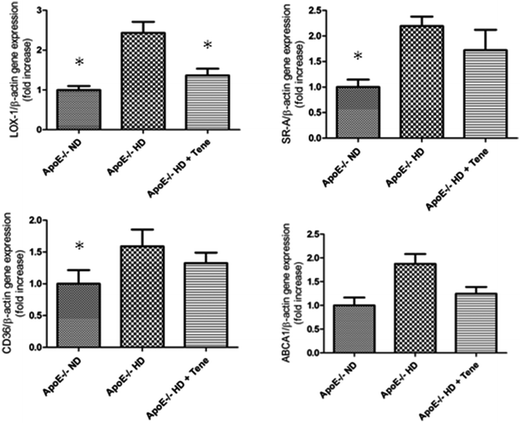
To investigate the mechanism of lipid accumulation in the kidney, kidney tissue gene expression of relevant receptors and the ATP-binding cassette transporter A1 (ABCA1) were examined by RT-PCR. LOX-1 gene expression was significantly increased in the kidney tissue of the ApoE−/− HD group compared with the ND group. The increased expression of LOX-1 was suppressed in the ApoE−/− HD + Tene group. Expression of scavenger receptor-class A (SR-A) and CD36 were increased in the ApoE−/− HD group compared with ND group; however, the levels were similar to those for ApoE−/− HD + Tene mice. Expression of ABCA1 did not differ among the three groups (Fig. 3). These results suggest that LOX-1, SR-A, and CD36 influence lipid accumulation in the kidney tissue of ApoE−/− HD mice. LOX-1, in particular, appears to be a critical factor for mitigating of lipid accumulation in the kidney tissue of ApoE−/− HD + Tene mice compared to ApoE−/− HD mice.Teneligliptin reduced LOX-1 expression in the kidney tissue with immunohistochemistry
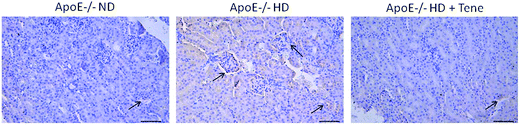
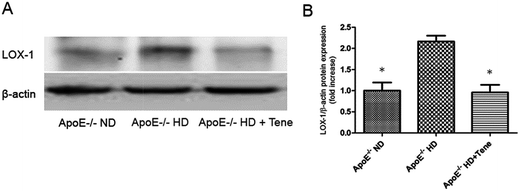
To evaluate LOX-1 expression in the kidney tissue, LOX-1 immunostaining was performed (Fig. 4). The ApoE−/− HD + Tene group had markedly reduced LOX-1 expression in kidney tissue compared to the ApoE−/− HD group. This result indicates that teneligliptin reduced LOX-1 expression in ApoE−/− HD mice.Teneligliptin reduced LOX-1 protein expression in the kidney tissue of ApoE−/− HD mice
To evaluate LOX-1 protein expression in the kidney tissue, LOX-1 protein immunoblotting was performed (Fig. 5A). We found the ApoE−/− HD + Tene group was significantly suppressed compared with the ApoE−/− HD group (Fig. 5B).Teneligliptin reduced the phosphor-PKC expression in the kidney tissue of ApoE−/− HD mice
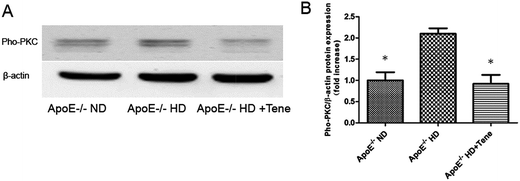
Protein kinases play a role in foam cell formation and lipid deposition, and phosphor-PKC protein immunoblotting was performed (Fig. 6A). We found that phosphor-PKC in the ApoE−/−HD + Tene group was significantly suppressed compared to the ApoE−/− HD group (Fig. 6B).Discussion
This study demonstrates that teneligliptin has a protective effect against progressive lipid deposition and glomerulosclerosis elicited by hypercholesterolemia.According to the metabolic characteristics, we found that TC and LDL-c were increased in the ApoE−/− HD group compared with the ApoE−/− ND group mice. Those results were agreement with reports by Daniel Kolbus.13 Interestingly, TC and LDL-c in the ApoE−/− HD + Tene group were significantly suppressed compared with the ApoE−/− HD group. These results indicated that teneligliptin influences cholesterol metabolism, however, further studies are needed to clarify the mechanisms.
Hypercholesterolemia, is a major independent risk factor for kidney disease.14 Hyperlipidaemia promotes glomerular lipid deposition and glomerulosclerosis.15,16 Cellular lipid homeostasis involves regulation of the influx, synthesis, catabolism, and efflux of lipids. An imbalance in these processes can result in conversion of macrophages, mesangial cells, and vascular smooth muscle cells to foam cells. This process is mediated by several independent pathways, including SR-A, class B (CD36), and LOX-1, and it regulates the expression of its target gene ABCA1.17–19
DPP-4 is a ubiquitous, type II cell surface glycoprotein that is widely expressed in all tissues.20 Treatment with DPP-4 inhibitors, which increase the GLP-1 levels, has been shown to exert numerous renoprotective effects. These effects include a reduction in the blood glucose and lipid levels, inhibition of inflammation and oxidative stress, amelioration of mesangial expansion, and an elevation of the glomerular filtration rate (GFR), among other effects.21 Previous studies have suggested that therapeutic intervention with a DPP-4 inhibitor is effective in postponing the development of neuropathy, cardiovascular disease, and diabetic nephropathy.22–25
In the present study, we analysed the gene expression of scavenger receptors including SR-A, CD36, and LOX-1. We found that LOX-1 gene expression was suppressed in the ApoE−/− HD + Tene group. LOX-1 was originally identified in endothelial cells, and it is a 50 kDa type II membrane glycoprotein that contains a short N-terminal cytoplasmic domain, a single transmembrane domain, a short neck or stalk region, and an ox-LDL-binding C-terminal extracellular C-type lectin-like domain. On the cell surface, LOX-1 consists of 3 homodimers that are bound to ox-LDL, and it plays a leading role in ox-LDL uptake and foam cell formation.26,27 In contrast, deletion of LOX-1 reduced the uptake of oxidized LDL and inhibited atherosclerosis in mice fed a high-cholesterol diet.28 Therefore, suppression of LOX-1 expression in ApoE−/− HD + Tene mice may reduce foam cell formation. Teneligliptin also reduced LOX-1 protein expression in the kidney tissue of ApoE−/− HD mice. Protein kinases play a role in foam cell formation and lipid deposition. As shown earlier, enhanced LOX-1 expression was attenuated by inhibitors of PKC, ERK, and NF-κB inhibitors, indicating that increased production of intracellular ROS and activation of the PKC/MAPK pathways are initial signalling events in LOX-1 gene regulation.29 Our results show that phosphor-PKC expression in the ApoE−/− HD + Tene group was significantly suppressed compared with the ApoE−/− HD group. It is speculated that teneligliptin regulated LOX-1 via the phosphor-PKC pathway.
Hyperlipidaemia has been shown to accelerate the induction and progression of renal injury leading to glomerulosclerosis.30,31 Glomerulosclerosis can be evaluated by measuring the accumulation of collagen IV as detected by immunohistochemistry.32,33 In our study, collagen type IV staining was significantly suppressed in the ApoE−/− HD + Tene group mice compared with the ApoE−/− HD group mice, indicating that teneligliptin contributes to reduced glomerulosclerosis and lipid accumulation.
In conclusion, our data establish that teneligliptin contributes to mitigation of hypercholesteraemic kidney injury as shown by downregulation of LOX-1, as well as suppression of foam cell formation, lipid deposition, and glomerulosclerosis. These findings provide new insights into the role of DPP-4 inhibitor teneligliptin in hypercholesterolemia kidney injury and raise the possibility of a novel therapeutic intervention for treating chronic kidney disease progression.
Author contributions
Hongyang Liu designed this study; Jing Xing, Mengye Li and Ying Liu helped perform the experiments; Hui Liu, Jinping Liu and Nan Li analysed the data and interpreted the experimental results; Hui Liu and Huiling Gao prepared the figures; Hui Liu drafted the manuscript; and Hongyang Liu, Lu Yan and Shuai Feng helped to revise the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was finally supported by the Natural Science Foundation of Liaoning Province, China (No. 2014023033).References
- J. A. Piedrahita, S. H. Zhang, J. R. Hagaman, P. M. Oliver and N. Maeda, Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 4471–4475 CrossRef CAS.
- M. Wen, S. Segerer, M. Dantas, P. A. Brown, K. L. Hudkins, T. Goodpaster, E. Kirk, R. C. LeBoeuf and C. E. Alpers, Renal injury in apolipoprotein E-deficient mice, Lab. Invest., 2002, 82, 999–1006 CrossRef CAS PubMed.
- S. S. Carneiro, R. Z. Carminati, F. P. Freitas, P. L. Podratz, C. M. Balarini, J. B. Graceli, S. S. Meyrelles, E. C. Vasquez and A. L. Gava, Endogenous female sex hormones delay the development of renal dysfunction in apolipoprotein E-deficient mice, Lipids Health Dis., 2014, 13, 176–184 CrossRef PubMed.
- X. Z. Ruan, Z. Varghese and J. F. Moorhead, An update on the lipid nephrotoxicity hypothesis, Nat. Rev. Nephrol., 2009, 5, 713–721 CrossRef CAS PubMed.
- M. Kuwahara, K. Bannai, H. Segawa, K. Miyamoto and H. Yamato, Cardiac remodeling associated with protein increase and lipid accumulation in early-stage chronic kidney disease in rats, Biochim. Biophys. Acta, 2014, 1842, 1433–1443 CrossRef CAS PubMed.
- R. Mentlein, Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides, Regul. Pept., 1999, 85, 9–24 CrossRef CAS PubMed.
- M. D. Gorrell, V. Gysbers and G. W. McCaughan, CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes, Scand. J. Immunol., 2001, 54, 249–264 CrossRef CAS PubMed.
- D. J. Drucker, The biology of incretin hormones, Cell Metab., 2006, 3, 153–165 CrossRef CAS PubMed.
- A. Avignon, A. Radauceanu and L. Monnier, Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes, Diabetes Care, 1997, 20, 1822–1826 CrossRef CAS PubMed.
- A. Halabi, H. Maatouk, K. E. Siegler, N. Faisst, V. Lufft and N. Klause, Pharmacokinetics of Teneligliptin in Subjects With Renal Impairment, Clin. Pharmacol. Drug Dev., 2013, 3, 246–254 CrossRef PubMed.
- R. E. Amori, J. Lau and A. G. Pittas, Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis, J. Am. Med. Assoc., 2007, 298, 194–206 CrossRef CAS PubMed.
- M. Monami, C. Lamanna, C. M. Desideri and E. Mannucci, DPP-4 inhibitors and lipids: systematic review and meta-analysis, Adv. Ther., 2012, 29, 14–25 CrossRef CAS PubMed.
- D. Kolbus, O. H. Ramos, K. E. Berg, J. Persson, M. Wigren, H. Björkbacka, G. N. Fredrikson and J. Nilsson, CD8 + T cell activation predominate early immune responses to hypercholesterolemia in Apoe−/− mice, BMC Immunol., 2010 DOI:10.1186/1471-2172-11-58.
- E. S. Schaeffner, T. Kurth, G. C. Curhan, R. J. Glynn, K. M. Rexrode, C. Baigent, J. E. Buring and J. M. Gaziano, Cholesterol and the risk of renal dysfunction in apparently healthy men, J. Am. Soc. Nephrol., 2013, 14, 2084–2091 Search PubMed.
- H. Bagavant, Y. Scindia, D. Nackiewicz, S. R. Nandula, A. Doran, A. Cutchins, S. Oldham, U. Deshmukh and C. McNamara, Deficiency of a transcriptional regulator, inhibitor of differentiation 3, induces glomerulonephritis in apolipoprotein E-deficient mice: a model linking hyperlipidemia and renal disease, Am. J. Pathol., 2011, 179, 651–660 CrossRef CAS PubMed.
- J. A. Joles, U. Kunter, U. Janssen, W. Kriz, T. J. Rabelink, H. A. Koomans and J. Floege, Early mechanisms of renal injury in Hypercholesterolemic or hypertriglyceridemic rats, J. Am. Soc. Nephrol., 2000, 11, 669–683 CAS.
- C. K. Abrass, Cellular lipid metabolism and the role of lipids in progressive renal disease, Am. J. Nephrol., 2004, 24, 46–53 CrossRef CAS PubMed.
- C. K. Glass and J. L. Witztum, Atherosclerosis: the road ahead, Cell, 2001, 104, 503–516 CrossRef CAS PubMed.
- A. M. Vaughan, C. Tang and J. F. Oram, ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation, J. Lipid Res., 2009, 50, 285–292 CrossRef CAS PubMed.
- C. F. Deacon and J. J. Holst, Dipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspective, Biochem. Biophys. Res. Commun., 2002, 294, 1–4 CrossRef CAS PubMed.
- P. D. Walker, G. P. Kaushal, S. V. Shah and A. Meprin, The major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo, Kidney Int., 1998, 53, 1673–1680 CrossRef CAS PubMed.
- W. J. Liu, S. H. Xie, Y. N. Liu, W. Kim, H. Y. Jin, S. K. Park, Y. M. Shao and T. S. Park, Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats, J. Pharmacol. Exp. Ther., 2012, 340, 248–255 CrossRef CAS PubMed.
- J. Matsubara, S. Sugiyama, K. Sugamura, T. Nakamura, Y. Fujiwara, E. Akiyama, H. Kurokawa, T. Nozaki, K. Ohba and M. Konishi, A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice, J. Am. Coll. Cardiol., 2012, 59, 265–276 CrossRef CAS PubMed.
- H. Y. Jin, W. J. Liu, J. H. Park, H. S. Baek and T. S. Park, Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats, Arch. Med. Res., 2009, 40, 536–544 CrossRef CAS PubMed.
- E. M. D. Abd and S. M. Elshazly, Renoprotective effect of sitagliptin against hypertensive nephropathy induced by chronic administration of L-NAME in rats: role of GLP-1 and GLP-1 receptor, Eur. J. Pharmacol., 2013, 720, 158–165 CrossRef PubMed.
- T. Sawamura, An endothelial receptor for oxidized low-density lipoprotein, Nature, 1997, 386, 73–77 CrossRef CAS PubMed.
- S. Gao and Y. J. Geng, LOX-1: a male hormone-regulated scavenger receptor for atherosclerosis, Vasc. Pharmacol., 2013, 59, 138–143 CrossRef CAS PubMed.
- C. Hu, LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet, Cardiovasc. Res., 2008, 79, 287–293 CrossRef CAS PubMed.
- L. Li, T. Sawamura and G. Renier, Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation, Circ. Res., 2004, 94, 892–901 CrossRef CAS PubMed.
- J. A. Joles, U. Kunter, U. Janssen, W. Kriz, T. J. Rabelink, H. A. Koomans and J. Floege, Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats, J. Am. Soc. Nephrol., 2000, 11, 669–683 CAS.
- B. Rodríguez-Iturbe, T. Sato, Y. Quiroz and N. D. Vaziri, AT-1 receptor blockade prevents proteinuria, renal failure, hyperlipidemia, and glomerulosclerosis in the Imai rat, Kidney Int., 2004, 66, 668–675 CrossRef PubMed.
- Y. Kaneko, S. Shiozawa, K. Hora and K. Nakazawa, Glomerulosclerosis develops in Thy-1 nephritis under persistent accumulation of macrophages, Pathol. Int., 2003, 53, 507–517 CrossRef PubMed.
- R. Korstanje, C. R. Caputo, R. A. Doty, S. A. Cook, R. T. Bronson, M. T. Davisson and J. H. Miner, A mouse Col4a4 mutation causing Alport glomerulosclerosis with abnormal collagen α3α4α5(IV) trimers, Kidney Int., 2014, 85, 1461–1468 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |