Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Integration of Ag/AgCl and Au nanoparticles into isostructural porous coordination polymers of Ni(II), Co(II) and Mn(II): magnetic studies†
Rashmi A. Agarwal *a and
Neeraj K. Guptab
*a and
Neeraj K. Guptab
aDepartment of Chemistry, Indian Institute of Technology Kanpur, Uttar Pradesh 208016, India. E-mail: rashmi.a.agarwal@gmail.com
bDepartment of Mechanical Engineering, Indian Institute of Technology Kanpur, Uttar Pradesh 208016, India
First published on 16th January 2017
Abstract
A systematic investigation of silver/silver chloride (Ag/AgCl) and gold (Au) nanoparticle (NP) formation within non-activated isostructural porous coordination polymers (PCPs) namely Ni-PCP, Co-PCP and Mn-PCP at room temperature is reported without the use of any reducing agent and deterioration of host frameworks. A supercharged environment due to the presence of free anions make these PCPs suitable templates for the growth of nanoparticles (NPs) in high yields. High resolution transmission electron microscopy (HRTEM) images provided insight into the size, shape and distribution of the NPs within the frameworks. Different shapes and sizes of the NPs are attributed to the differences in the levels of supramolecular interactions within these isostructural series and the ionic radii which is related to the charge density of metal nodes. From an electron paramagnetic resonance (EPR) study it is evidenced that synthesis of Au NPs in Ni-PCP is completely through redox reaction via strong charge transfer between coordinated metal and the metal precursor, and the others are via acid formation through anions present in the cavities of PCPs. Au NPs integrated frameworks display different soft ferromagnetic properties when placed in a magnetic field at room temperature.
Introduction
Porous coordination polymers (PCPs) or metal organic frameworks (MOFs) are crystalline materials which have permanent microporosity.1–7 To achieve targeted properties in PCPs various types of multidentate organic ligands based on different geometries have been used with inorganic metal ions having a variety of coordination modes.8 Large internal surface area, easy chemical modification of inner pore surface9 and high thermal stability make these compounds excellent candidates for a variety of applications such as gas storage and separation,10,11 heterogeneous catalysis12 and proton conduction.13 Recently there have been extensive studies to synthesize nanoparticles in PCPs due to their long range order of confined void space which act like reaction vessels. Free NPs tend to aggregate and fuse due to delocalization of free electrons14 which increases their surface energies because of decreased thermodynamic stability. Due to aggregation their inherent properties disappear and storage is also affected from application point of view. That is why to nucleate NPs of uniform size and shape in homogeneous distribution pattern, encapsulation in microporous crystalline solids is an efficient way of preventing agglomeration.15 Embedded NPs in the cavities of PCPs are very much of interest for applications in the field of heterogeneous catalysis,16 gas storage,17 chemical sensing,18 electronics, biomedical assay (anticancer, antibacterial, antifungal and antimicrobial) and devices using quantum confinement effect.19 Recently much attention has been paid to synthesis of plasmonic photocatalysts (Ag/AgCl) which are known to be an efficient and stable catalyst under irradiation of visible light in the field of environmental technology.20 The fabrication of NPs in PCPs/MOFs can be achieved by a variety of methods including solution infiltration method where a solution of metal precursors in the form of inorganic salt are infiltrated into the pores of desolvated PCPs followed by reduction using reducing agents.21,22 Another method involves chemical vapour deposition of volatile organometallic precursors into the inner surface of porous structure followed by thermal decomposition.23 In a third process, double solvent approach where one solvent is hydrophilic (small amount) with metal precursor and the other is hydrophobic (large amount) with adsorbent are utilized with reduction at high temperature.24 Other procedures involve solid grinding followed by hydrogenation25 and photo catalytic reduction of metal precursors.26Some of the above mentioned methods require high temperature while others require reducing agents. In particular redox active coordination polymers have attracted more attention since they react with metal precursors to produce oxidised frameworks and NPs at room temperature.27 Their crystalline nature is maintained even after the redox reaction. Recently Ag and Au NPs have been synthesized by reaction diffusion technique wherein anion alone or cooperatively with the framework reduce the metal precursors.28 In this article we explore the nucleation and growth of NPs through acid formation via anion diffusion.
Herein we report the synthesis of Ag/AgCl and Au NPs from non-activated isostructural series of Ni-PCP, Co-PCP and Mn-PCP which are cationic frameworks wherein anions are free and fix their position via hydrogen bonding within the frameworks. During synthesis of Ag/AgCl NPs, NO3− anions of AgNO3 are exchanged with free Cl− anions in the frameworks followed by oxidation reaction by HCl formation and simultaneously metallic Ag is nucleated through reduction. HCl reacts instantaneously with Ag NPs to form AgCl NPs. While in case of Au NPs synthesis from HAuCl4, Cl− anions are exchanged with already present Cl− anions to produce hydrochloric acid. This acid does not react with Au NPs.
Synthesis of Au NPs in Ni-PCP occurs through redox reaction between coordinated Ni(II) species of the framework and Au(III), as evidenced by EPR spectrum. In this case this PCP acts as a redox active framework. Differential ferromagnetic properties are shown by these Au NPs integrated frameworks at room temperature, which are influenced by size and distribution of the NPs.
Experimental
Materials
All chemicals and solvents used in the synthesis were of reagent grade and were used without further purification.Physical measurements
Fourier transform infrared spectra (FTIR) were obtained (KBr disk, 400–4000 cm−1) using a Perkin-Elmer model 1320 spectrometer. Solid-state diffuse reflectance spectra (DRS) were recorded using UV-vis-NIR spectrophotometer (Varian Model Cary 5000) at room temperature by using BaSO4 as standard. EPR spectra were recorded on a Bruker EPR EMX spectrometer. Thermo-gravimetric analyses were recorded using a Mettler Toledo (heating rate of 10 °C min−1) TGA instrument. Powder X-ray diffraction (PXRD) was performed using a Rigaku Rint 2000 X-ray diffractometer with CuKα radiation. X-ray photoelectron spectra were measured on a Sigma Probe. HRTEM images were obtained on a FEI Titan G2 60-300. The magnetic properties were analyzed on a LDJ9600 vibrating sample magnetometer.Synthesis of isostructural PCPs
Ni-PCP {[Ni2(DBIBA)3]·Cl·18H2O}n, {[Ni2(DBIBA)3]·(ClO4)·3H2O}n Co-PCP {[Co2(DBIBA)3]·Cl·9H2O}n and Mn-PCP {[Mn2(DBIBA)3]·Cl·3H2O}n (DBIBA = 5-di(1H-benzo[d]imidazol-1-yl)benzoate) were synthesized as previous reports.10,11Synthesis of NPs@PCPs
All PCPs were used without activation or without guest removal, in powdered form (to increase their surface area). Solution infiltration methods were applied for NPs synthesis at room temperature.Synthesis of Ag/AgCl@Ni-PCP
Ni-PCP {[Ni2(DBIBA)3]·Cl·18H2O}n (0.04 g, 0.026 mmol) was immersed in a methanol/water (3 mL, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution of AgNO3 (0.013 g, 0.078 mmol) at room temperature for 10 h under stirring. A brown coloured solid was filtered and washed several times with excess of methanol to remove any free AgNO3. The solid material dried under vacuum. The obtained sample has 32 wt% Ag/AgCl@Ni-PCP (the mass ratio of AgCl to Ni-PCP were 32%).
1 v/v) solution of AgNO3 (0.013 g, 0.078 mmol) at room temperature for 10 h under stirring. A brown coloured solid was filtered and washed several times with excess of methanol to remove any free AgNO3. The solid material dried under vacuum. The obtained sample has 32 wt% Ag/AgCl@Ni-PCP (the mass ratio of AgCl to Ni-PCP were 32%).
Synthesis of Ag/AgCl@Co-PCP
Co-PCP (0.04 g, 0.029 mmol) was immersed in a methanol/water (3 mL, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution of AgNO3 (0.015 g, 0.087 mmol) at room temperature for 10 h under stirring followed by same procedure. Here 33 wt% were acquired.
1 v/v) solution of AgNO3 (0.015 g, 0.087 mmol) at room temperature for 10 h under stirring followed by same procedure. Here 33 wt% were acquired.
Synthesis of Ag/AgCl@Mn-PCP
With similar method 25 wt% was prepared by using Mn-PCP (0.04 g, 0.031 mmol) and AgNO3 (0.016 g, 0.093 mmol).Synthesis of Au@Ni-PCP
In this case following {[Ni2(DBIBA)3]·(ClO4)·3H2O}n was used as host framework with different anion. Same procedure was followed to synthesize 10 wt% Au@Ni-PCP with varying amount of Ni-PCP (0.04 g, 0.030 mmol) and HAuCl4 (0.03 g, 0.09 mmol).Synthesis of Au@Co-PCP
Here 6 wt% was obtained by utilizing Co-PCP (0.04 g, 0.029 mmol) and HAuCl4 (0.029 g, 0.087 mmol).Synthesis of Au@Mn-PCP
In this reaction Mn-PCP (0.04 g, 0.031 mmol) and HAuCl4 (0.031 g, 0.093 mmol) were employed to obtain 21 wt% of Au@Mn-PCP.Results and discussion
The isostructural series of PCPs comprising of Ni(II), Co(II) and Mn(II) nodes are thermally and structurally stable with permanent microporosity described through adsorption measurements. Slightly varying pore sizes and varying potential of different types of non-covalent interactions (π–π bonding between two benzene rings, hydrogen bonding by free anions, anion–π interactions and CH–π bonding) exist within their 1D hexagonal cavities as previous reports.10,11 These different types of supramolecular interactions with varying ranges are shown in Fig. 1a–f and Table S1.†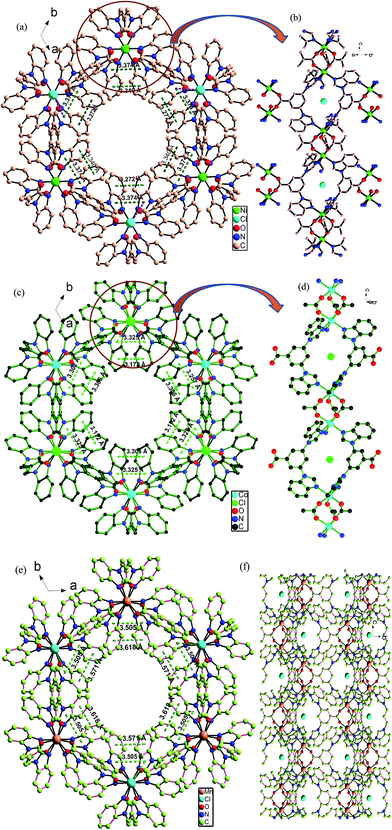 | ||
| Fig. 1 Isostructural host PCPs with varying potential of electrostatic charges (a and b) Ni-PCP, (c and d) Co-PCP and (e and f) Mn-PCP. | ||
Their stable 3D structures and permanent microporosities with internally charged environment due to the presence of lattice anions (Cl−) and varying potential of non-covalent interactions are suitable for nucleation, growth and storage of NPs. That is why these PCPs have been chosen for synthesis of NPs.
All three of the PCPs were immersed separately in methanol/water solvent system for 24 h to examine any structural changes. But PXRD patterns indicate (Fig. 3b) that there are no structural changes because these frameworks have been synthesized in ethanol/water solvent system. To fabricate Ag and Au NPs, these non-activated host PCPs were immersed, in suitable solutions of AgNO3 and HAuCl4 at room temperature for 10 h. Colour of the solutions were changed to brownish in case of Ag/AgCl loading indicating generation of surface plasma by Ag NPs whereas pale yellow colour appears in case of Au NPs integration. To check the deposition of NPs, characterizations have been done by diffuse reflectance spectroscopy (DRS), X-ray photoelectron (XPS) and Fourier transform infrared (FTIR) spectroscopy. High resolution transmission electron microscopy (HRTEM) and energy dispersive spectroscopy (EDEX) give information about morphology, size, distribution and coordination environment of NPs while structural changes of PCPs were analyzed through powder X-ray diffraction (PXRD) patterns after loading NPs.
From UV-visible diffuse reflectance spectra of Ni-PCP as well as synthesized Ag/AgCl@Ni-PCP, Ag/AgCl@Co-PCP and Ag/AgCl@Mn-PCP (Fig. 2), it is observed that broader and stronger absorptions in the visible-light region are due to the surface plasmon resonance of Ag NPs and unique hetero-junction structures of Ag/AgCl NPs.29
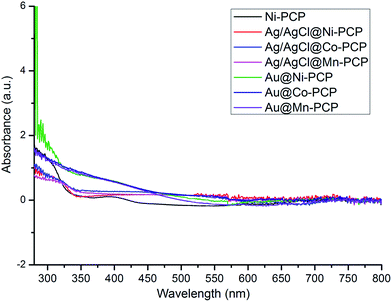 | ||
| Fig. 2 UV-vis DRS spectra of Ni-PCP, Ag/AgCl@Ni-PCP, Ag/AgCl@Co-PCP and Ag/AgCl@Mn-PCP; and Au@Ni-PCP, Au@Co-PCP and Au@Mn-PCP. | ||
To study the possible structural changes of PCPs before and after fabrication of NPs, PXRD patterns of Ag/AgCl@Ni-PCP, Ag/AgCl@Co-PCP and Ag/AgCl@Mn-PCP show that the host frameworks are stretched due to high loading of Ag/AgCl, yet maintaining their structural integrity (Fig. 3a and c). Prominent diffraction peaks at 2θ = 27.8°, 32.3° and 46.3° are representative of face centered cubic structure of AgCl NPs.30 A smaller peak at 38.1° corresponds to Ag NPs synthesis in [111] crystal plane. The low intensity of this peak infers that Ag NPs are of very small size. In comparison, the diffraction patterns of Au@Ni-PCP, Au@Co-PCP and Au@Mn-PCP (Fig. 3d) reveal that the host frameworks are not stretched to any appreciable degree and the 2θ peaks assigned to Au NPs indicate that the size is smaller than Ag/AgCl NPs.31,32
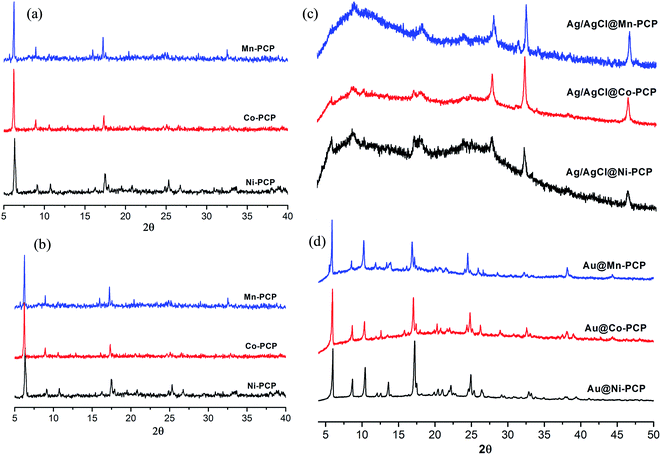 | ||
| Fig. 3 PXRD patterns of (a) host PCPs, (b) PCPs after exposure to methanol/water solvent system for one day, (c) Ag/AgCl NPs integrated PCPs and (d) Au NPs integrated PCPs. | ||
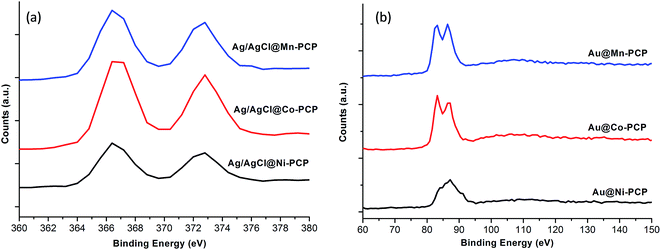
From XPS (Fig. 4) and EDS (ESI, Fig. S1†), it is evident that Ni–Ag, Ni–Au, Co–Ag, Co–Au, Mn–Ag and Mn–Au coexist within the frameworks forming nanocomposites. In XPS, 3d5/2 and 3d3/2 Ag(0) peaks are identified at 367.2 and 372.8 eV respectively, which are comparable to literature values.33 These two peaks are further deconvoluted respectively in to two peaks at 366.4, 367.2 eV and 372, 372.8 eV. The peaks at 366.4 and 372 eV are ascribed to Ag+ of AgCl NPs.34 These results confirm the presence of both Ag/AgCl NPs in the frameworks. The peaks for Au(0), 4f7/2 and 4f5/2 exist at 83 and 86.5 eV comparable to literature values for all loaded PCPs.35
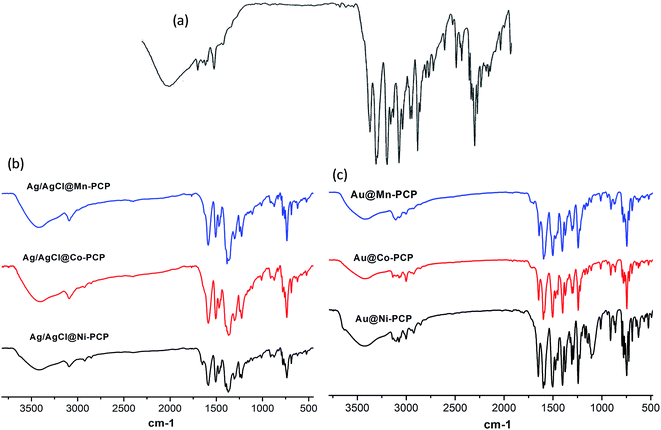
FTIR spectra (Fig. 5) of Ag/AgCl loaded PCPs show the presence of free NO3− at 1383 cm−1 which is due to acid formation (HCl) during anionic exchange. This evidences the synthesis of NPs within the frameworks. The spectral peaks of the host PCPs become broader and slightly shift toward lower wavenumbers inferring that Ag/AgCl NPs interact with the charged environment of the coordinated framework. The spectra of Au@PCPs indicate that there are no structural differences before and after Au NPs fabrication. Spectral peaks do not show any broadening nor any significant shifts. This shows that the NPs do not interact with the charged environment of the framework cavities. All the above spectra also support that the long range crystallinity of the frameworks are essentially intact.
EPR spectroscopy is sensitive to bulk properties of a sample. The strength of signals is indicative of NPs population throughout the bulk of the host frameworks. The spectra (ESI, Fig. S2 and S3†) for Ag/AgCl@PCPs shows g value in the range of 2.030–1.852 which indicates NPs of differing sizes. In comparison the spectra for Au@PCPs show a wider size distribution of Au NPs in the range g = 2.042–1.785. In the case of Ag/AgCl and Au@PCPs, except Au@Ni-PCP, the NPs are formed via acid formation during anion exchange between NO3− and Cl− of AgNO3/HAuCl4 precursors and Cl− of the frameworks. Redox activity of the framework in Au@Ni-PCP is evidenced by gav = 4.135 for Ni(III) and ClO4− FTIR peak at 1090 cm−1. In this redox active reaction Au(III) oxidises the coordinated Ni(II) to Ni(III) and get reduced to Au(0). This is due to strong charge transfer36 between highly anisotropic Ni(II) and Au(III) because of higher ferromagnetic properties than Co-PCP and Mn-PCP.11
In Au@Ni-PCP this strong charge transfer mechanism can be potentially utilized for targeted drug delivery.
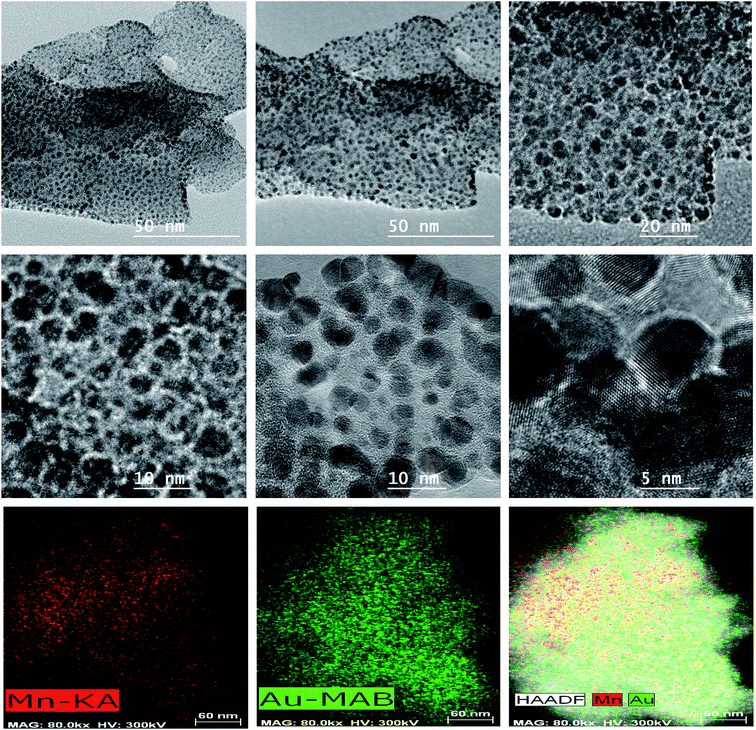
HRTEM images (Fig. 6 and 7, ESI, Fig. S4–S7†) taken at different magnifications show that the Ag/AgCl and AuNPs@PCPs essentially belong to class B wherein the NPs can be observed to be distributed homogeneously throughout the bulk of the crystalline frameworks with lattice fringes clearly visible. The shape of both type of NPs are essentially spheroids. The Ag/AgCl NPs in the isostructural frameworks are present in a broad size distribution with discrete particles 2–12 nm and nanoclusters 20–25 nm whereas in Ag/AgCl@Mn-PCP only discrete NPs exist. In the case of Au NPs, size range of discrete particles and nanoclusters are 1–6 nm and 8–20 nm respectively. However for Au@Mn-PCP, the NPs exist essentially as discrete particles in the range of 1–5 nm and no clusters. The size of fabricated NPs are much larger than the pore dimensions (0.65–0.71 nm) of the frameworks. Though the presence of NPs formed/deposited at the outer surface, which belong to class A is not ruled out, but is not the dominating phenomenon.37 HAADF-STEM images provide chemical information proportional to atomic number of the metals present in the loaded frameworks. It is observed that besides the metal nodes, Ag/AgCl and Au nanoparticles/clusters are clearly visible with their distribution in the porous networks. Despite the difference between pore size and synthesized NPs, it is further evidenced that these NPs exist within the frameworks through the discussion of PXRD patterns wherein the frameworks are stressed by the larger than the pore size NPs.
Thermal and structural stabilities of Ag/AgCl and Au@PCPs were examined by thermo-gravimetry (ESI, Fig. S8†). For Ag/AgCl@PCPs there was almost constant weight loss upto ∼95 °C which was attributed to removal of methanol which substitute water molecules (originally present in the PCPs) during NP synthesis. All these PCPs have significant thermal stability upto ∼220 °C even though stabilities are decreased by ∼150–180 °C in comparison to the host frameworks. This was ascribed to high stresses within the frameworks due to high wt% and catalytic activity of Ag NPs. For Au@PCPs, a constant weight loss upto ∼80–90 °C was observed similar as for Ag/AgCl@PCPs. The Au@PCPs in comparison to Ag@PCPs show higher thermal stability even though they are lower by ∼10–60 °C compared to their hosts.38 It is inferred that these frameworks are less stressed as evidenced by PXRD. Despite the differences in thermal stabilities between the Ag/AgCl and Au@PCPs and host PCPs, the significant thermal stabilities of these systems show that the frameworks can be used as dry storage media for the NPs while preserving their inherent properties. Further the frameworks show potential for being used as templates for the re-fabrication of NPs for applications such as biomedical.
From the above discussions, it is clear that in case of Ag/AgCl@Mn-PCP and Au@Mn-PCP no nanoclusters are formed. The probable reasons for this are; (1) the higher torsion and dihedral angles N2Mn1O1C1 = 64.6(5), N1Mn1O1 = 65.07 for Mn-PCP compared to the other host PCPs (for Ni-PCP; N2Ni1O1C1 = −51.8(6), N1Ni1O1 = 58.22 and for Co-PCP; N2Co1O1C1 = 59.04, N1Co1O1 = 61.86) and (2) weaker electrostatic environment within the structure compared to the other two PCPs wherein the highly charged environment leads to agglomeration of NPs.
Magnetic measurements taken for Au@Ni-PCP, Au@Co-PCP and Au@Mn-PCP at room temperature up to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Oe are shown in Fig. 8. From the data it is seen that the coercive field (Hc) are 75.56, 105.28 and 61.90 Oe and remnant magnetization (Mr) 0.0002, 0.0007 and 0.0005 emu g−1 with saturation magnetization (Ms) values of 0.067, 0.12 and 0.13 emu g−1 respectively for the integrated frameworks. From the curves it is evident that these structures are soft ferromagnets or paramagnets. Further, Au@Mn-PCP and Au@Co-PCP show near comparable mass magnetization while Au@Ni-PCP shows the lowest value. The observed magnetism is attributed to both size and distribution patterns of the NPs within their respective frameworks. Since the above data has been generated on NPs integrated frameworks, we expect that the values would be higher with magnetic characteristics of hard ferromagnets if the NPs were free of their frameworks which act as non-magnetic barriers.
500 Oe are shown in Fig. 8. From the data it is seen that the coercive field (Hc) are 75.56, 105.28 and 61.90 Oe and remnant magnetization (Mr) 0.0002, 0.0007 and 0.0005 emu g−1 with saturation magnetization (Ms) values of 0.067, 0.12 and 0.13 emu g−1 respectively for the integrated frameworks. From the curves it is evident that these structures are soft ferromagnets or paramagnets. Further, Au@Mn-PCP and Au@Co-PCP show near comparable mass magnetization while Au@Ni-PCP shows the lowest value. The observed magnetism is attributed to both size and distribution patterns of the NPs within their respective frameworks. Since the above data has been generated on NPs integrated frameworks, we expect that the values would be higher with magnetic characteristics of hard ferromagnets if the NPs were free of their frameworks which act as non-magnetic barriers.
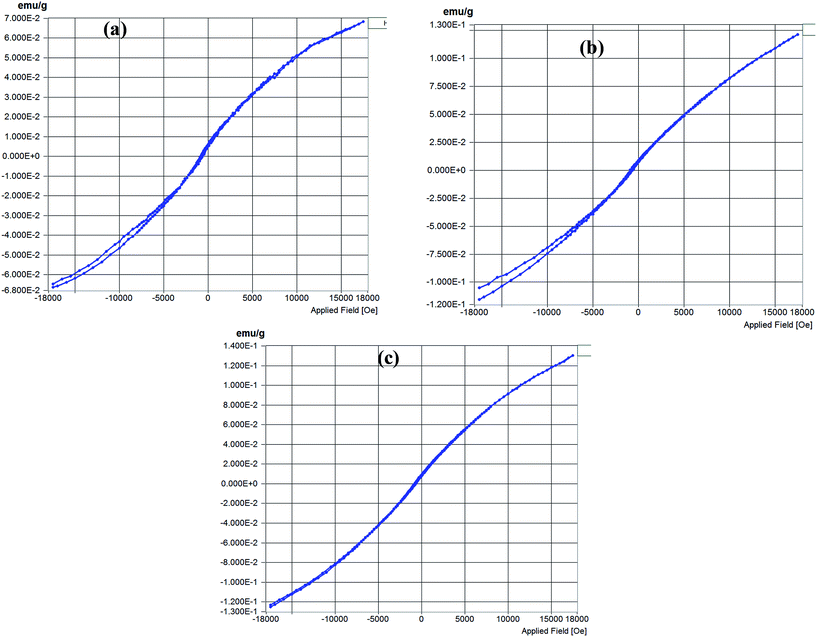 | ||
| Fig. 8 Magnetic properties of Au NPs integrated frameworks (a) Au@Ni-PCP, (b) Au@Co-PCP and (c) Au@Mn-PCP at room temperature. | ||
Conclusions
Conclusively we have demonstrated a very simple method of fabrication with different distribution patterns of Ag/AgCl and magnetic Au NPs in isostructural series of porous coordination polymers of Ni(II), Co(II) and Mn(II) in non-activated state. All NPs are synthesized due to hydrochloric acid formation through anion exchange between NO3− and Cl− of AgNO3/HAuCl4 precursors and Cl− of the frameworks. In Au@Ni-PCP, the framework shows redox activity due to strong charge transfer between coordinated Ni(II) and Au(III). Ag/AgCl and Au(0) fabricated PCPs are thermally stable. Integrated NPs show that their growth and distribution pattern depends on the charge density of the framework metal nodes and electrostatic gradients present. These isostructural PCPs show potential for the nucleation and growth of NPs from a variety of metal precursors. The Au NPs integrated frameworks display soft ferromagnetism at room temperature.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank IIT Kanpur for extending facilities to undertake this study.References
- S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- A. M. Spokoyny, D. Kim, A. Sumrein and C. A. Mirkin, Chem. Soc. Rev., 2009, 38, 1218–1227 RSC.
- S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249–267 CrossRef CAS PubMed.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2011, 112, 869–932 CrossRef PubMed.
- H.-L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351–3370 RSC.
- Y. Sakata, S. Furukawa, M. Kondo, K. Hirai, N. Horike, Y. Takashima, H. Uehara, N. Louvain, M. Meilikhov, T. Tsuruoka, S. Isoda, W. Kosaka, O. Sakata and S. Kitagawa, Science, 2013, 339, 193–196 CrossRef CAS PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed.
- R. A. Agarwal, A. Aijaz, M. Ahmad, E. C. Sañudo, Q. Xu and P. K. Bharadwaj, Cryst. Growth Des., 2012, 12, 2999–3005 CAS.
- R. A. Agarwal, A. Aijaz, E. C. Sañudo, Q. Xu and P. K. Bharadwaj, Cryst. Growth Des., 2013, 13, 1238–1245 CAS.
- S. Hasegawa, S. Horike, R. Matsuda, S. Furukawa, K. Mochizuki, Y. Kinoshita and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 2607–2614 CrossRef CAS PubMed.
- Y. Wang, Z. Tao, X. Yin, J. Shu, L. Chen, D. Sheng, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Inorg. Chem., 2015, 54, 10023–10029 CrossRef CAS PubMed.
- J.-T. Lue, J. Phys. Chem. Solids, 2001, 62, 1599–1612 CrossRef CAS.
- R. J. White, R. Luque, V. L. Budarin, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 481–494 RSC.
- A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed.
- Y. E. Cheon and M. P. Suh, Angew. Chem., Int. Ed., 2009, 48, 2899–2903 CrossRef CAS PubMed.
- L. He, Y. Liu, J. Liu, Y. Xiong, J. Zheng, Y. Liu and Z. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741–3745 CrossRef CAS PubMed.
- F. Cheng, J. W. Betts, S. M. Kelly, J. Schaller and T. Heinze, Green Chem., 2013, 15, 989–998 RSC.
- H. Xu, H. M. Li, J. X. Xia, S. Yin, Z. J. Luo, L. Liu and L. Xu, ACS Appl. Mater. Interfaces, 2011, 3, 22–29 CAS.
- M. S. El-Shall, V. Abdelsayed, A. E. R. S. Khder, H. M. A. Hassan, H. M. El-Kaderi and T. E. Reich, J. Mater. Chem., 2009, 19, 7625–7631 RSC.
- Y. K. Hwang, D.-Y. Hong, J.-S. Chang, S. H. Jhung, Y.-K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Férey, Angew. Chem., Int. Ed., 2008, 47, 4144–4148 CrossRef CAS PubMed.
- P. Serp, P. Kalck and R. Feurer, Chem. Rev., 2002, 102, 3085–3128 CrossRef CAS PubMed.
- A. Aijaz, A. Karkamkar, Y. J. Choi, N. Tsumori, E. Ronnebro, T. Autrey, H. Shioyama and Q. Xu, J. Am. Chem. Soc., 2012, 134, 13926–13929 CrossRef CAS PubMed.
- T. Ishida, M. Nagaoka, T. Akita and M. Haruta, Chem.–Eur. J., 2008, 14, 8456–8460 CrossRef CAS PubMed.
- R. Ameloot, M. B. J. Roeffaers, G. D. Cremer, F. Vermoortele, J. Hofkens, B. F. Sels and D. E. De Vos, Adv. Mater., 2011, 23, 1788–1791 CrossRef CAS PubMed.
- M. P. Suh, H. R. Moon, E. Y. Lee and S. Y. Jang, J. Am. Chem. Soc., 2006, 128, 4710–4718 CrossRef CAS PubMed.
- Y. Wei, S. Han, D. A. Walker, P. E. Fuller and B. A. Grzybowski, Angew. Chem., Int. Ed., 2012, 51, 7435–7439 CrossRef CAS PubMed.
- S.-T. Gao, W.-H. Liu, N.-Z. Shang, C. Feng, Q.-H. Wu, Z. Wang and C. Wang, RSC Adv., 2014, 4, 61736–61742 RSC.
- L. Li and Y.-J. Zhu, J. Colloid Interface Sci., 2006, 303, 415–418 CrossRef CAS PubMed.
- Z. Ban, Y. A. Barnakov, F. Li, V. O. Golub and C. J. O'Connor, J. Mater. Chem., 2005, 15, 4660–4662 RSC.
- H. R. Moon, J. H. Kim and M. P. Suh, Angew. Chem., 2005, 117, 1287–1291 (Angew. Chem., Int. Ed., 2005, 44, 1261–1265) CrossRef.
- P. Wang, B. Huang, Z. Lou, X. Zhang, X. Qin, Y. Dai, Z. Zheng and X. Wang, Chem.–Eur. J., 2010, 16, 538–544 CrossRef CAS PubMed.
- M. Zhu, P. Chen and M. Liu, ACS Nano, 2011, 5(6), 4529–4536 CrossRef CAS PubMed.
- Z. R. Yue, W. Jiang, L. Wang, H. Toghiani, D. Gardner and C. U. Pittman, Carbon, 1999, 37, 1607–1618 CrossRef CAS.
- W. S. Alencar, F. N. Crespilho, M. V. A. Martins, V. Zucolotto, O. N. Oliveira Jr and W. C. Silva, Phys. Chem. Chem. Phys., 2009, 11, 5086–5091 RSC.
- M. Meilikhov, K. Yusenko, D. Esken, S. Turner, G. V. Tendeloo and R. A. Fischer, Eur. J. Inorg. Chem., 2010, 3701–3714 CrossRef CAS.
- B. Gole, U. Sanyal, R. Banerjee and P. S. Mukherjee, Inorg. Chem., 2016, 55, 2345–2354 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDS, EPR, TGA and HRTEM images. See DOI: 10.1039/c6ra26642h |
| This journal is © The Royal Society of Chemistry 2017 |