Open Access Article
Open Access ArticleMild thermal reduction of graphene oxide as a lubrication additive for friction and wear reduction
Jun Zhao a,
Yingru Lib,
Yongfu Wangc,
Junyuan Maoa,
Yongyong He*a and
Jianbin Luo*a
a,
Yingru Lib,
Yongfu Wangc,
Junyuan Maoa,
Yongyong He*a and
Jianbin Luo*a
aState Key Laboratory of Tribology, Tsinghua University, Beijing, 100084, China. E-mail: heyy@mail.tsinghua.edu.cn; luojb@tsinghua.edu.cn
bInstitute of Materials, China Academy of Engineering Physics, Sichuan 621908, China
cState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China
First published on 9th January 2017
Abstract
Recently, studies on graphene-based lubrication additives have been widely researched, but few refer to their preparation by thermal reduction which shows potential in not only significantly lowering the mass-production cost, but also the simple, nonchemical process. In this study, mild thermal reduction of graphene oxide (MRGO) has been achieved by high temperature (700 °C) treatment and the product used as a lubrication additive. It shows a relatively ordered lamellar structure and a certain level of oxygen by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) analysis, and exhibits excellent tribological properties as a lubrication additive. The friction coefficient can be reduced by as much as 30% and the rubbing surfaces display few scratches at a lower additive concentration (0.5 wt%) compared with that of base oil (Poly Alpha Olefins Type 6: PAO 6) without MRGO additive under the same friction conditions. Based on the advantages of green, low-cost and simple synthesis operation, the MRGO offers significant potential application as a lubrication additive.
Retaining lower friction energy consumption and longer wear durability has been the development trend of the machine industry. In general, severe wear occurs in the start–stop state and low-speed operation of a machine, in which the rubbing surfaces are mainly under boundary regime.1 In that context, using lubrication additives has been an efficient way to improve tribological properties, for they can exhibit great anti-wear characteristics by forming a protective film on the friction interfaces and preventing the metal-to-metal contact.2–5 Nowadays, many additives containing polar components such as sulphur/phosphorus containing groups can lead to reducing wear, nevertheless, simultaneously are prone to make corrosion and pollution.1,6 Thus, to develop more efficient and environment-friendly lubrication additives is an urgent task for academia and industry. Graphene, is nowadays worthy to be used according to its high chemical inertia, extreme mechanical strength and easy to sliding between layers.7–10 However, the properties of graphene for applications varies with its production processes directly. The surface structure characteristics and elastic/plastic properties influencing the friction and wear properties, are strongly dependent on the synthesis methods such as mechanical exfoliation method, chemical vapor deposition (CVD) and reduction of graphene oxide, etc.11,12 Generally, mechanically exfoliated graphene exhibits better lubrication properties due to perfect graphitic structure and lower defects.12 Liquid phase and thermal exfoliation of graphene oxide (GO) has been expected in the marketplace because of convenient and low-cost features13,14 compared with the mechanical method. Lately, our previous works have achieved the scale-up, low-cost and eco-friendly synthesis of GO techniques by an improved Hummers method15–17 which eliminates nitrogen oxygen impurities and simplifies the synthesis process. The above GO have been widely explored for many practical applications,15,17 but study about using it for synthesis of graphene additive in lubrication oil haven't been investigated. In addition, lots of studies have been published on preparation of graphene-based materials and their detailed lubrication properties,7,18–21 but there are few researches referring to the graphene additives from the reduced graphene oxide (RGO) by thermal reduction process which shows potential in not only significantly lowering the cost of mass-produced graphene but also the simple, nonchemical and thermal conversion of GO to RGO,22 while reduction by chemical process is much tedious and unsafe due to the residual hazardous reductants.23,24
Therefore, we investigated the tribological properties of the as-prepared graphene using the GO from above works by thermal reduction in this study. This mildly thermal reduction of graphene oxide (MRGO) displays several integrated layered structure and mild oxygen content, and exhibits excellent tribological properties. Moreover, the rubbing surface shows fewer scratches and smaller wear depth at a lower MRGO concentration (0.5 wt%). This work not only offers an efficient synthesis method of graphene additive for lubrication application due to the simple, low-cost and green operation, but conforms the further application value of the previous works of GO synthesis techniques.
The thermal temperature directly influences the structure and properties of reduced graphene. At a lower thermal temperature, the graphene can be weakly reduced which may result in lower C/O atomic ratio and higher wrinkled and folded structure.25 When reduction under much higher temperature even over 1000 °C, graphitic lattice or re-graphitization would occurs and the reduced sample approaches to bulk graphite.22,26 Therefore, for reaching not only relative high C/O ratio and integrated lamellar microstructure but also nano-thickness of graphene layers, the MRGO was prepared by the mildly thermal reduction of above GO at a temperature of 700 °C for 5 hours in a quartz tube, and then dry ball milling process was operated to make the additive size smaller and more uniform. The structure characteristic of MRGO was investigated by the combination use of scanning electron microscopy (SEM, FEI, Netherlands), transmission electron microscopy (TEM, JEM-2010, Japan), and X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, USA) operating with Al-Kα radiation and Raman spectroscopy (Raman, Horba JobinYvon, France) with 512 nm laser excitation. Friction experiments were carried out by a reciprocating sliding tester (UMT-3 CETR, USA) with a load of 2 N (the max initial Hertz contact pressure is approximate 1 Gpa). The reciprocating frequency and the sliding stroke are 0.4 Hz and 3 mm, respectively. The friction flat disk and the sliding ball (Ø4 mm) were made of bearing steel (GGr15), of which the surface roughness was about 20 nm measured by a white light interfering profilometer (MICROXAM-3D, America). The additives suspension in the PAO6 were mixed using a magnetic stirrer for 2 hours and then ultra-sonication for 0.5 hours to thorough dispersion.
The morphological characteristic of the MRGO is well revealed by SEM and TEM. By the ball milling process, the two dimension size of the MRGO is uniformly distributed within the range of about 1–2 μm (Fig. 1(a)). The surface of the MRGO (inset of Fig. 1(a)) has slightly wrinkled because of some defects, surface/edge dangling bonding and van der Waals interaction inevitably during thermal reduction.22,26 From the TEM image (Fig. 1(b)), the MRGO presents relatively integrated lamellar structure and the layer thickness is about 5 nm. In addition, the chemical structure of the graphene was analyzed by XPS. The C 1s (Fig. 1(c)) is presented into four Gaussian peaks with the binding energies of 284.77 eV (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 286.22 eV (C–O), 287.37 eV (C
C), 286.22 eV (C–O), 287.37 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 289.92 eV (O
O) and 289.92 eV (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH), respectively. Although the oxygen-containing groups are relatively weak compared with that of GO (Fig. 1(c)), the observable content of oxygen (10.31%) remains in the MRGO, suggesting that the MRGO was mildly reduced. Raman spectroscopy is also an effective way to characterize graphene structure, which can reveal the degree of crystalline and order.27 The Raman spectra of the MRGO and GO are shown in Fig. 1(d). It displays prominent Raman peaks of D-band (at 1360 cm−1) and G-band (at 1595 cm−1). The integral intensity ratio (ID/IG) of the MRGO (2.26) is a little larger than that of GO (1.91), which means some local defects residues28 and the creation of numerous new graphite domains (Fig. 1(b))12,29 in the graphene by mild reduction. In a word, under mildly thermal reduction, the MRGO with integrated lamellar structure and mild oxygen content is well prepared.
C–OH), respectively. Although the oxygen-containing groups are relatively weak compared with that of GO (Fig. 1(c)), the observable content of oxygen (10.31%) remains in the MRGO, suggesting that the MRGO was mildly reduced. Raman spectroscopy is also an effective way to characterize graphene structure, which can reveal the degree of crystalline and order.27 The Raman spectra of the MRGO and GO are shown in Fig. 1(d). It displays prominent Raman peaks of D-band (at 1360 cm−1) and G-band (at 1595 cm−1). The integral intensity ratio (ID/IG) of the MRGO (2.26) is a little larger than that of GO (1.91), which means some local defects residues28 and the creation of numerous new graphite domains (Fig. 1(b))12,29 in the graphene by mild reduction. In a word, under mildly thermal reduction, the MRGO with integrated lamellar structure and mild oxygen content is well prepared.
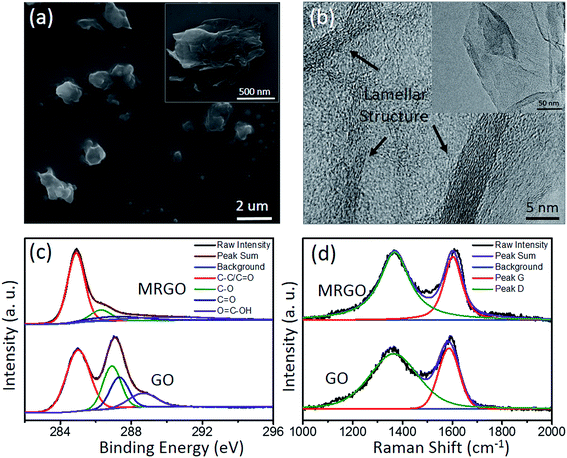 | ||
| Fig. 1 Characteristic of the MRGO and GO. SEM and TEM images for the MRGO (a, b), XPS analysis (c) and Raman spectra (d) for the MRGO and GO, respectively. | ||
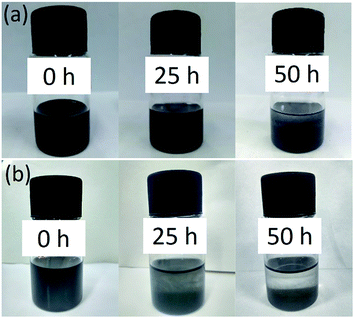
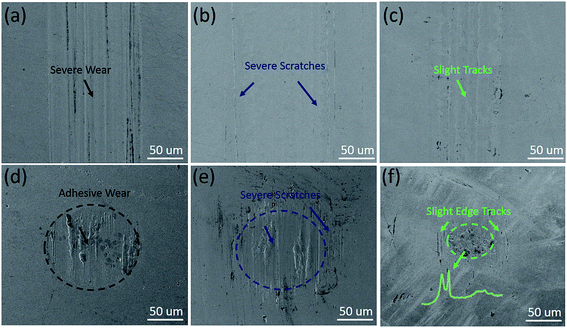
The comparison of dispersion characteristic of GO with MRGO in oil have been tested after the same physical dispersion process above (Fig. 2). The MRGO lubricant showed stable dispersion state for 25 h, but sediment was apparent for the GO lubricant. After 50 h standing, the dispersion of GO displayed directly precipitated which could be due to rick oxygen-containing groups in GO resulting in interaction and agglomeration with each other while only slight agglomeration was observed for the MRGO dispersed oil, which means the MRGO is able to self-disperse in oil to some extent.20 Here, to identify the tribological properties of the MRGO, we also take the GO for comparison. The friction properties of the additives are shown in Fig. 3 after long friction time (5400 s). It can been seen that the MRGO presents excellent self-lubricating characteristic and the friction coefficient reaches a stable value of 0.1 and is decreased by 30% compared with that of the base oil which displays much higher and more tempestuously fluctuant in Fig. 3(a). The optimum concentration for MRGO is about 0.5 wt% for improving the lubrication property of the base oil (Fig. 3(b)). However, the GO lubricant only shows a little smaller friction coefficient than the based oil under the appropriate concentration range from 0.5 wt% to 1.0 wt%, of which the friction coefficient is much larger and more unstable than that of the MRGO lubricant after a short running-in stage, because there exists rick oxygen-containing groups in it, which impedes sliding motion between laminar layers as the result of previous study.12 After the long friction time, the rubbing surface lubricated by MRGO (Fig. 4(a)) only represents slender tracks while that lubricated by base oil or GO lubricant have wide wear tracks and deep furrows (Fig. 4(a) and (b)). According to the relationship between the wear depth and the MRGO or GO concentration (Fig. 4(d)), the wear depth of the rubbing surfaces lubricated by base oil is as large as 1161 nm, while the lowest wear depth for adding the MRGO is as low as 207 nm at the optimum concentration (0.5 wt%), which is decreased by almost 3 times than that of GO effect. However, the wear depths are higher under a larger concentration, which is corresponding to the variation of the friction coefficient, probably because of much higher concentration promoting the additive aggregation and impeding its permeating the rubbing interfaces. The wear behavior is also revealed by the SEM images of the rubbing surfaces in Fig. 5. It is clearly seen that the rubbing surfaces lubricated by the base oil suffer worse wear, because severe scratches and adhesion occurred on the friction disc and ball (Fig. 5(a) and (d)) under boundary lubrication. The rubbing disc and ball lubricated by GO (0.5 wt%) also exhibits severe scratches close to the contact regions (Fig. 5(b) and (e)). However, from the Fig. 5(c) and (f), there is few track on the worn surface lubricated by MRGO, and some slight wear tracks on the contact edge of the friction ball which is probably due to some graphene or wear debris stacking there and fretting the edge. Moreover, the worn diameter of the ball lubricated by MRGO is much smaller than that of both base oil and GO.
From above, the MRGO additive exhibits excellent lubrication and anti-wear ability. The uniform 2D-dimensional size of the as-prepared MRGO and the several nanometer thick of the layers (Fig. 1) promote the additive to permeate the friction interfaces and form a protective lubricating film more easily.30 According to the Raman spectra measured from the wear scar after 15 ultrasonic cleaning (inset of Fig. 5(f)), the characteristic peaks of MRGO (D-band at 1342 cm−1 and G-band at 1607 cm−1) are clearly observed, confirming that the MRGO stably adsorbed on the interfaces and a physical deposition film formed on the rubbing surfaces for prevent metal-to-metal contact.10,18 Moreover, the relatively integrated and ordered structure of the MRGO, composed of some laminated graphite planes by mildly reducing from the TEM image (Fig. 1(b)), may also improve the properties of anti-wear based on sliding between laminar layers after its adsorption on the friction interfaces. Additionally, slighter tracks on the contact edge lubricated by MRGO than that of GO in Fig. 5, may means the inferior self-dispersion of GO (Fig. 2) may be more likely to stack or aggregate there and scratch the surfaces. Therefore, the relative stable dispersion of MRGO improves the lubrication performance.20
In summary, MRGO was prepared and used as lubrication additive, which exhibits relatively integrated and ordered structure based on the uniform 2D-dimensional size (1–2 μm) and several laminar layers (5 nm). The friction coefficient can be decreased by as much as 30% and the scratches of the rubbing surfaces shows much slighter by adding the graphene of 0.5 wt%. Furthermore, this study about the thermal reduction of graphene oxide offers significant potential application as lubricant additive because of combination between the excellent lubrication ability and the advantages of green, low-cost and simple synthesis operation.
Acknowledgements
This work is supported by National Key Basic Research Program of China (973 Program) (No. 2014CB046404) and National Natural Science Foundation of China (51321092 and 51275263).Notes and references
- B. Vengudusamy, A. Grafl, F. Novotny-Farkas, T. Schimmel and K. Adam, Tribol. Int., 2013, 67, 199–210 CrossRef CAS.
- Y. Wu, W. Tsui and T. Liu, Wear, 2007, 262, 819–825 CrossRef CAS.
- L. Rapoport, Y. Bilik, Y. Feldman, M. Homyonfer, S. Cohen and R. Tenne, Nature, 1997, 387, 791–793 CrossRef CAS.
- A. H. Battez, R. González, J. Viesca, J. Fernández, J. D. Fernández, A. Machado, R. Chou and J. Riba, Wear, 2008, 265, 422–428 CrossRef.
- L. Liu, Z. Liu and P. Huang, RSC Adv., 2016, 6, 94876–94883 RSC.
- M. D. B. Bouchet, J. Martin, T. Le Mogne, P. Bilas, B. Vacher and Y. Yamada, Wear, 2005, 258, 1643–1650 CrossRef.
- D. Berman, A. Erdemir and A. V. Sumant, Carbon, 2013, 54, 454–459 CrossRef CAS.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- V. Eswaraiah, V. Sankaranarayanan and S. Ramaprabhu, ACS Appl. Mater. Interfaces, 2011, 3, 4221–4227 CAS.
- X. Fan, Y. Xia, L. Wang and W. Li, Tribol. Lett., 2014, 55, 455–464 CrossRef CAS.
- X. Fan and L. Wang, J. Colloid Interface Sci., 2015, 452, 98–108 CrossRef CAS PubMed.
- Y. Peng, Z. Wang and K. Zou, Langmuir, 2015, 31, 7782–7791 CrossRef CAS PubMed.
- K. S. Novoselov, V. Fal, L. Colombo, P. Gellert, M. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- S. Pei and H.-M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- Y. Li, J. Chen, L. Huang, C. Li, J. D. Hong and G. Shi, Adv. Mater., 2014, 26, 4789–4793 CrossRef CAS PubMed.
- J. Chen, Y. Li, L. Huang, C. Li and G. Shi, Carbon, 2015, 81, 826–834 CrossRef CAS.
- Y. Li, K. Sheng, W. Yuan and G. Shi, Chem. Commun., 2013, 49, 291–293 RSC.
- H.-J. Song and N. Li, Appl. Phys. A: Mater. Sci. Process., 2011, 105, 827–832 CrossRef CAS.
- D. Berman, A. Erdemir and A. V. Sumant, Mater. Today, 2014, 17, 31–42 CrossRef CAS.
- X. Dou, A. R. Koltonow, X. He, H. D. Jang, Q. Wang, Y.-W. Chung and J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 1528–1533 CrossRef CAS PubMed.
- V. Zin, S. Barison, F. Agresti, L. Colla, C. Pagura and M. Fabrizio, RSC Adv., 2016, 6, 59477–59486 RSC.
- S. H. Huh, Thermal reduction of graphene oxide, INTECH Open Access Publisher, 2011 Search PubMed.
- W. Chen, L. Yan and P. R. Bangal, Carbon, 2010, 48, 1146–1152 CrossRef CAS.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217–224 CrossRef CAS PubMed.
- H.-M. Ju, S.-H. Choi and S.-H. Huh, J. Korean Phys. Soc., 2010, 57, 1649–1652 CrossRef CAS.
- C.-M. Chen, Q. Zhang, M.-G. Yang, C.-H. Huang, Y.-G. Yang and M.-Z. Wang, Carbon, 2012, 50, 3572–3584 CrossRef CAS.
- T. Jawhari, A. Roid and J. Casado, Carbon, 1995, 33, 1561–1565 CrossRef CAS.
- J. Xiao, L. Zhang, K. Zhou, J. Li, X. Xie and Z. Li, Carbon, 2013, 65, 53–62 CrossRef CAS.
- C. Xiaohao, L. Shenghua, J. Yuansheng and S. Huaihe, Tribol. Lett., 2003, 14, 53–59 CrossRef.
- J. Lin, L. Wang and G. Chen, Tribol. Lett., 2011, 41, 209–215 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |