Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
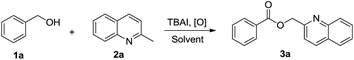
Synthesis of benzyl esters from the commercially available alcohols catalyzed by TBAI via C(sp3)–H bond functionalization†
Dao-Qing Dong,
Hui Zhang and
Zu-Li Wang *
*
College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao, 266109, P. R. China. E-mail: wangzulichem@163.com
First published on 13th January 2017
Abstract
A direct esterification of alcohols catalyzed by TBAI via C(sp3)–H bond functionalization was achieved under mild and clean conditions. A series of benzyl esters were obtained in good to excellent yields via the C(sp3)–H bond functionalization of alkyl-substituted azaarenes.
Alkyl-substituted azaarenes, as key structural skeletal frameworks of many natural products and pharmaceuticals, have attracted considerable interest because of their important biologically active properties.1 One of the most efficient methods for preparing alkyl-substituted azaarenes is direct C(sp3)–H functionalization of the alkyl-substituted azaarenes. For example, the direct addition of C(sp3)–H bond of the alkyl-substituted azaarenes to the unsaturated chemical bonds was first independently realized in 2010 by Huang and co-workers.2f At present, esterification catalyzed by transition metals,2 Bronsted acids,3 and microwave4 has been explored. Even in some cases, these reactions can also be realized without any catalyst.4d,e However, versatile and practical methods for the synthesis of alkyl-substituted azaarenes are still desirable.
On the other hand, Bu4NI has received extensive attention in organic synthesis because it can overcome the drawbacks of the expensive, poisonous, and air-sensitive properties of metals or organometallics.5 In the presence of an oxidant (H2O2, tBuOOH, etc.), Bu4NI has been explored as an efficient catalyst for the formation of C–O,6 C–N,7 and C–C8 bonds. For example, the synthesis of α-ketoamides promoted by TBHP/I2 was described by the Wang group.9 In 2014, Wan and his co-workers reported the synthesis of substituted pyrazoles using TBAI as a catalyst and TBHP as an oxidant.10 Tetrabutylammonium iodide-catalyzed phosphorylation of benzyl C–H bonds via a cross-dehydrogenative coupling (CDC) reaction was also realized by the Tang group.11 In line with our interest in green chemistry,12 herein, we report a TBAI/TBHP-promoted synthesis of benzyl esters from the commercially available alcohols via C(sp3)–H bond functionalization under mild reaction conditions.
At the beginning of our investigation, optimization of the reaction conditions was focused on a variety of reaction parameters using the model reaction of phenylmethanol (1a) with 2-methylquinoline (2a). As shown in Table 1, when H2O2 was chosen as the oxidant for the model reaction, only a 20% yield of the desired product was achieved (Table 1, entry 1). Although O2 was also used as the oxidant for this reaction, the yield of the product was very low (Table 1, entry 2). However, the model reaction smoothly proceeded in the presence of tert-butyl hydroperoxide (TBHP) and the corresponding product was isolated in 86% yield (Table 1, entry 3). With respect to the catalyst, a very low yield of ester 3a was formed when TBAI was replaced by KI, I2O5, or I2 (Table 1, entry 4–6). The effect of the solvent on the model reaction was also investigated and it was found that water was the most effective solvent. Moreover, a lower yield was observed when the reaction was conducted in methanol (Table 1, entry 7) or acetonitrile (Table 1, entry 8). The optimized reaction conditions for the model reaction were TBAI (20 mol%) and TBHP (5.0 equiv.) in water at 90 °C for 12 h.
| Entry | Catalyst | Oxidant | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2a (0.5 mmol), catalyst (0.2 mmol), oxidant (4.0 mmol), water (2 mL), 90 °C and 12 h; isolated yield.b CH3CN was used as the solvent.c CH3OH was used as the solvent. | |||
| 1 | TBAI | H2O2 | 20 |
| 2 | TBAI | O2 | Trace |
| 3 | TBAI | TBHP | 86 |
| 4 | KI | TBHP | 16 |
| 5 | I2 | TBHP | Trace |
| 6 | I2O5 | TBHP | Trace |
| 7b | TBAI | TBHP | 30 |
| 8c | TBAI | TBHP | 7 |
With the optimized reaction conditions in hand, the scope of the substituted alkyl azaarenes and alcohols was investigated. The results are shown in Table 2. A variety of benzyl alcohols bearing substituents on the benzene ring were examined for the synthesis of benzyl esters via the C(sp3)–H bond functionalization of 2-alkyl-substituted azaarenes.
| a Reaction conditions: alcohol (1.0 mmol), 2-alkyl azaarene (0.5 mmol), catalyst (0.2 mmol), oxidant (4.0 mmol), water (2 mL), 90 °C and 12 h; isolated yield. |
|---|
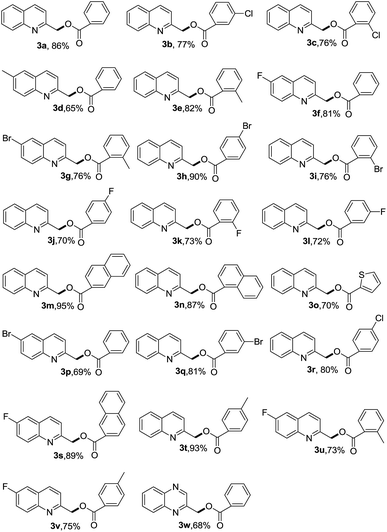 |
The results indicated that a variety of functional groups, including electron-donating (Table 2, 3g, 3e, 3t, and 3v) and electron-withdrawing (Table 2, 3b, 3c, 3h, 3i, 3j, 3k, and 3l) groups were tolerated and the reactions produced the corresponding products in good to excellent yields (Table 2, 3a–w). Note that 2-naphthalene methanol, 1-naphthalene methanol, and 2-thiophenemethanol also smoothly reacted to generate their corresponding products in 70–95% yield (Table 2, 3m, 3n, 3o, and 3s). The present reaction conditions were also suitable for the reaction of benzyl alcohols with other alkyl-substituted azaarenes. Various functional groups, such as fluoro, chloro, bromo, and methyl (Table 2, 3d, 3g, 3r, and 3u) were well tolerated under the reaction conditions. Finally, the reaction of 2-methylquinoxaline with phenylmethanol generated the desired product in 68% yield (Table 2, 3w).
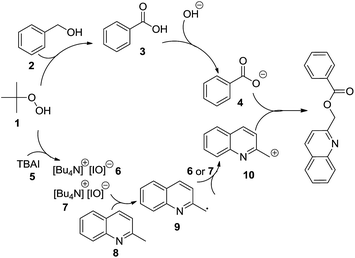
According to a report,5 a plausible mechanism was proposed for this reaction (Scheme 1). The first step involves the reaction between TBHP and TBAI to generate the intermediate ammonium hypoiodite 6 or iodite 7. Then, hypoiodite 6 or iodite 7 induces the production of benzyl radical 9 via the homolysis of a benzyl C–H bond. The benzyl radical could be further oxidized to the benzyl cation 10. Moreover, the oxidation of phenylmethanol 2 by TBHP occurred to form benzoic acid 3; after deprotonation, the benzoate anion 4 reacts with the benzyl cation 10 to provide the final product.
In summary, a direct synthetic method for the preparation of benzyl esters via TBAI-catalyzed C(sp3)–H bond functionalization has been reported. The reactions generate the corresponding products in good yields in most of the cases under simple and mild reaction conditions. This organic TBAI-catalyzed one-step reaction provides a new synthetic protocol for the synthesis of 2-alkyl azaarene derivatives. Further investigations on the detailed reaction mechanism and application of this methodology are in progress.
Acknowledgements
Financial support from the National Natural Science Foundation of China (21402103), the China Postdoctoral Science Foundation (150030), the Scientific Research Foundation of Shandong Province Outstanding Young Scientist Award (BS2013YY024), and the Research Fund of Qingdao Agricultural University's High-level Person (631303) are gratefully acknowledged.Notes and references
- (a) M. C. Bagley, C. Glover and E. A. Merritt, Synlett, 2007, 2459 CrossRef CAS; (b) G. D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef CAS; (c) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC; (d) P. N. W. Baxter, J. M. Lehn, J. Fischerand and M. T. Youinou, Angew. Chem., Int. Ed. Engl., 1994, 33, 2284 CrossRef; (e) J. M. Lehn, Science, 2002, 295, 2400 CrossRef CAS PubMed; (f) D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef; (g) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC; (h) M. C. Bagley, C. Gloverand and E. A. Merritt, Synlett, 2007, 2459 CrossRef CAS.
- (a) J.-J. Jin, H.-Y. Niu, G.-R. Qu, H. M. Guo and J. S. Fossey, RSC Adv., 2012, 2, 5968 RSC; (b) H. Komai, T. Yishino, S. Matsunaga and M. Kanai, Org. Lett., 2011, 13, 1706 CrossRef CAS PubMed; (c) B. Qian, S. Guo, C. Xia and H. Huang, Adv. Synth. Catal., 2010, 352, 3195 CrossRef CAS; (d) B. Qian, P. Xie, Y. Xie and H. Huang, Org. Lett., 2011, 13, 2580 CrossRef CAS PubMed; (e) V. B. Graves and A. Shaikh, Tetrahedron Lett., 2013, 54, 695 CrossRef CAS; (f) B. Qian, S. Guo, J. Shao, Q. Zhu, L. Yang, C. Xia and H. J. Huang, J. Am. Chem. Soc., 2010, 132, 3650 CrossRef CAS PubMed; (g) B. Qian, D. J. Shi, L. Yang and H. Huang, Adv. Synth. Catal., 2012, 354, 2146 CrossRef CAS; (h) B. Qian, L. Yang and H. Huang, Tetrahedron Lett., 2013, 54, 711 CrossRef CAS; (i) J. Y. Liu, H. Y. Niu, S. Wu, G. R. Qu and H. M. Guo, Chem. Commun., 2012, 48, 9723 RSC; (j) Y. Yan, K. Xu, Y. Fang and Z. J. Wang, J. Org. Chem., 2011, 76, 6849 CrossRef CAS PubMed; (k) M. Rueping and N. Tolstoluzhsky, Org. Lett., 2011, 13, 1095 CrossRef CAS PubMed; (l) A. Kumar, P. L. Gupta and M. Kumar, RSC Adv., 2013, 3, 18771 RSC.
- (a) F. F. Wang, C.-P. Luo, Y. Wang, G. Deng and L. Yang, Org. Biomol. Chem., 2012, 10, 8605 RSC; (b) R. Niu, J. Xiao, T. Liang and X. Li, Org. Lett., 2012, 14, 676 CrossRef CAS PubMed; (c) S. V. N. Vuppalapati and Y. R. Lee, Tetrahedron, 2012, 68, 8286 CrossRef CAS; (d) X. Gao, F. Zhang, G. Deng and L. Yang, Org. Lett., 2014, 16, 3664 CrossRef CAS PubMed; (e) S. A. R. Mulla, M. Y. Pathan and S. S. Chavan, RSC Adv., 2013, 3, 20281 RSC; (f) M. Raghua, M. Rajasekhar, B. C. O. Reddy, C. S. Reddy and B. V. S. Reddy, Tetrahedron Lett., 2013, 54, 3503 CrossRef.
- (a) N. N. Rao and H. M. Meshram, Tetrahedron Lett., 2013, 54, 5087 CrossRef; (b) N. N. Rao and H. M. Meshram, Tetrahedron Lett., 2013, 54, 1315 CrossRef; (c) H. M. Meshram, N. N. Rao, L. C. Rao and N. S. Kumar, Tetrahedron Lett., 2012, 53, 3963 CrossRef CAS; (d) H. Y. Li, L. J. Xing, T. Xu, P. Wang, R. H. Liu and B. Wang, Tetrahedron Lett., 2013, 54, 858 CrossRef CAS; (e) Y. Yan, K. Xu, Y. Fang and Z. J. Wang, J. Org. Chem., 2011, 76, 6849 CrossRef CAS PubMed.
- For selected examples, see: (a) R. D. Richardson and T. Wirth, Angew. Chem., Int. Ed., 2006, 45, 4402 (Angew. Chem., 2006, 11, 4510) CrossRef CAS PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (c) M. Uyanik and K. Ishihara, ChemCatChem., 2012, 4, 177 CrossRef CAS; (d) A. Parra and S. Reboredo, Chem.–Eur. J., 2013, 19, 17244 CrossRef CAS PubMed; (e) R. Samanta, K. Matcha and A. P. Antonchick, Eur. J. Org. Chem., 2013, 5769 CrossRef CAS; (f) D.-Q. Dong, S.-H. Hao, Z.-L. Wang and C. Chen, Org. Biomol. Chem., 2014, 12, 4278 RSC; (g) X.-F. Wu, J.-L. Gong and X. Qi, Org. Biomol. Chem., 2014, 12, 5807 RSC; (h) M. S. Kharasch and G. J. Sosnovsky, J. Am. Chem. Soc., 1958, 80, 756 CrossRef CAS; (i) D. J. Rawlinson and G. Sosnovsky, Synthesis, 1972, 1 CrossRef CAS; (j) J. Eames and M. Watkinson, Angew. Chem., Int. Ed., 2001, 40, 3567 CrossRef CAS; (k) M. B. Andrus and J. C. Lashley, Tetrahedron, 2002, 58, 845 CrossRef CAS; (l) L. V. Desai, H. A. Malik and M. S. Sanford, Org. Lett., 2006, 8, 1141 CrossRef CAS PubMed; (m) B. Lucke, K. V. Narayana, A. Martin and K. Jähnisch, Adv. Synth. Catal., 2004, 346, 1407 CrossRef; (n) J. Zhang, E. Khaskin, N. P. Anderson, P. Y. Zavalij and A. N. Vedernikov, Chem. Commun., 2008, 3625 RSC; (o) J. Huang, L.-T. Li, H.-Y. Li, E. Husan, P. Wang and B. Wang, Chem. Commun., 2012, 48, 10204 RSC; (p) L. Liu, L. Yun, Z. Wang, X. Fu and C. Yan, Tetrahedron Lett., 2013, 54, 5383 CrossRef CAS; (q) Q. Wang, J. Feng, W. Chai, H. Geng, M. Xu, K. Wang, C. Xu, R. Fu and R. Yuan, Tetrahedron Lett., 2014, 55, 4785 CrossRef CAS; (r) J. Feng, S. Liang, S. Chen, J. Zhang, S. Fu and X. Yu, Adv. Synth. Catal., 2012, 354, 1287 CrossRef CAS.
- (a) L. Chen, E. Shi, Z. Liu, S. Chen, W. Wei, H. Li, K. Xu and X. Wan, Chem.–Eur. J., 2011, 17, 4085 CrossRef CAS PubMed; (b) W. Wei, C. Zhang, Y. Xu and X. Wan, Chem. Commun., 2011, 10827 RSC; (c) B. Tan, N. Toda and C. F. Barbas III, Angew. Chem., Int. Ed., 2012, 51, 12538 CrossRef CAS PubMed.
- (a) C. Zhu and Y. Wei, ChemSusChem, 2011, 4, 1082 CrossRef CAS PubMed; (b) T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner and B. J. Nachtsheim, Org. Lett., 2011, 13, 3754 CrossRef CAS PubMed; (c) L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 11333 RSC; (d) U. Kloeckner, N. M. Weckenmann and B. J. Nachtsheim, Synlett, 2012, 23, 97 CAS; (e) S. Chen, Y. Xu and X. Wan, Org. Lett., 2011, 13, 6152 CrossRef CAS PubMed; (f) J. Xie, H. Jiang, Y. Cheng and C. Zhu, Chem. Commun., 2012, 979 RSC; (g) Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu and X. Wan, Angew. Chem., Int. Ed., 2012, 51, 3231 CrossRef CAS PubMed; (h) W. Mai, H. Wang, Z. Li, J. Yuan, Y. Xiao, L. Yang, P. Mao and L. Qu, Chem. Commun., 2012, 10117 RSC; (i) H. Li, J. Xie, Q. Xue, Y. Cheng and C. Zhu, Tetrahedron Lett., 2012, 53, 6479 CrossRef CAS.
- (a) A. Rodríguez and W. J. Moran, Org. Lett., 2011, 13, 2220 CrossRef PubMed; (b) L. Li, J. Huang, H. Li, L. Wen, P. Wang and B. Wang, Chem. Commun., 2012, 5187 RSC.
- X.-B. Zhang and L. Wang, Green Chem., 2012, 14, 2141 RSC.
- J. Zhang, Y. Shao, H.-X. Wang, Q. Luo, J.-J. Chen, D.-M. Xu and X.-B. Wan, Org. Lett., 2014, 16, 3312 CrossRef CAS PubMed.
- J. Xu, P.-B. Zhang, X.-Q. Li, Y.-Z. Gao, J. Wu, G. Tang and Y.-F. Zhao, Adv. Synth. Catal., 2014, 356, 3331 CrossRef CAS.
- (a) Z.-L. Wang, RSC Adv., 2015, 5, 5563 RSC; (b) S.-H. Hao, X.-Y. Zhang, D.-Q. Dong and Z.-L. Wang, Chin. Chem. Lett., 2015, 26, 599 CrossRef CAS; (c) X.-Y. Zhang, D.-Q. Dong, T. Yue, S.-H. Hao and Z.-L. Wang, Tetrahedron Lett., 2014, 55, 5462 CrossRef CAS; (d) Z.-L. Wang, L. Wang and J.-C. Yan, Synthesis, 2009, 3744 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26387a |
| This journal is © The Royal Society of Chemistry 2017 |