Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microbial xanthan gum production from alkali-pretreated rice straw
M. H. Jazini *a,
E. Fereydounia and
K. Karimi
*a,
E. Fereydounia and
K. Karimi ab
ab
aDepartment of Chemical Engineering, Isfahan University of Technology, Isfahan 84156-83111, Iran. E-mail: m.h.jazini@cc.iut.ac.ir
bIndustrial Biotechnology Group, Research Institute for Biotechnology and Bioengineering, Isfahan University of Technology, Isfahan 84156-83111, Iran
First published on 13th January 2017
Abstract
Xanthan is the first polysaccharide commercially produced by abacterial fermentation using simple sugars. In this study, rice straw was introduced as an inexpensive and widely available carbon source for microbial xanthan gum production. The production yield was improved by pretreatment with NaOH and NaHCO3. The pretreatment with 2 M sodium hydroxide was performed at 0 and 100 °C, and the pretreatment with 0.25, 0.5, and 1 M NaHCO3 was conducted at 120, 150 and 180 °C. The results showed that xanthan yield was increased up to five times in the case of pretreatment with NaOH at 100 °C as well as 1 M NaHCO3 at 180 °C. Although the temperature considerably influenced xanthan yield in the pretreatment with NaOH, the yield was not affected by temperature and concentration in the case of NaHCO3. The quality of the produced xanthan was compared with its commercial equivalent using thermogravimetric thermal analysis, differential thermal analysis, and Fourier transform infrared spectroscopy. The comparison showed that the quality of the produced xanthan was close to the commercial one. It was concluded that rice straw can be a promising substrate for xanthan gum production after the pretreatments.
1. Introduction
Environmentally friendly polysaccharides have versatile applications in many industries because of their biocompatibility and biodegradability. They serve as additives in foods, textiles and cosmetics to improve rheological properties of products. Plant resources of polysaccharides are costly. However, they can be produced more conveniently by microorganisms.1–3Xanthan is the first polysaccharide produced by microorganisms on an industrial scale.4 Additionally, it has the greatest production rate in the world among all natural gums.5,6 Xanthomonas campestris is able to synthesize xanthan gum efficiently. The effects of operating conditions of cultivation of this strain, such as temperature, agitation rate and pH were investigated by Psomas et al.7 and Mirik et al.8 The nutritional requirements of this bacterium should be optimized to attain high yield of gum production. Umashankar et al.9 studied the effects of several N and C mineral sources to maximize xanthan yield. Souw et al.10 studied the nutritional requirements of X. campestris in a defined medium. It can be cultivated solely on glucose or glucose enriched with organic acids.10 Nevertheless, many efforts were undertaken to introduce other carbon sources. Production of microbial xanthan gum using molasses,6 cheese whey,11 waste sugar beet pulp,12 palm date (Phoenix dactylifera L.) juice byproducts13 and starch14 were previously reported. Bioconversion of agro-waste to xanthan gum was reported by Gunasekar et al. they investigated the effects of different concentration of sulphuric acid on hydrolysate obtained from tapioca pulp. They found a strong relationship between the concentration of sulphuric acid with the quality of xanthan gum produced.15
Application of lignocelluloses for the production of polysaccharides, e.g., dextran and curdlan, was reported recently. This research emphasised that pretreatment is necessary to enhance polysaccharide yield from lignocelluloses.16
The production of xanthan from glucose–xylose mixtures were performed by Zhang et al. They aimed to compare the quality of the xanthan produced from glucose–xylose mixture with the one produced from glucose. They found that the quality of the produced xanthan was improved by the consumption of xylose.17
Among introduced carbon sources, those that are renewable and widely available are of great importance. Nowadays, lignocelluloses attract researchers' attention as novel renewable substrates to produce chemicals. Rice straw is one of the abundant and mainly unused lignocellulosic materials worldwide.18 However, because of its recalcitrant structure, it cannot be used directly as a carbon source for the cultivation of microorganisms. For efficient conversion of cellulose, the main constitutional building block of lignocelluloses, they must be pretreated prior to enzymatic hydrolysis.19
Different pretreatment methods for the improvement of cellulose hydrolysis from lignocelluloses have been investigated. The purpose of pretreatment is to disrupt the recalcitrance structure of lignocellulose to make cellulose accessible to enzymes converting cellulose to glucose.20 The three-dimensional polyaromatic matrix of lignocelluloses can be fractured by several methods, including acid, base, steam explosion, and solvents.21 Chemical pretreatment processes, such as alkali pretreatment, are the most effective methods.22
Pretreatment with sodium hydroxide is a low cost, energy consuming process for improving lignocelluloses' hydrolysis. This process can be conducted at either low concentration and high temperature (e.g., 1% NaOH at 180 °C) or high concentration and low temperature (6–20% NaOH at 0–100 °C).23 In the latter conditions, cellulose is dissolved, regenerated and prepared for hydrolysis. This process has several advantages, e.g., possibility to recycle and reuse the alkali solution, thorough disruption of the compact structure of lignocelluloses, and fewer environmental impacts. Alkali pretreatment at high concentration, e.g. 2 M NaOH, has shown promising results for improvement of ethanol and biogas production from different lignocelluloses.24,25 In this work, for the first time, alkali-pretreated lignocelluloses was reported as a potential substrate for production of xanthan. To our knowledge, there is no work reporting the use of rice straw as a substrate for xanthan production. This may pave the way for use of renewable substrates for microbial production of xanthan.
The goal of the current study was to introduce lignocelluloses as renewable substrates for xanthan production. In this work, rice straw was used as a raw material and pretreatment with NaOH and NaHCO3 were used to improve the production yields. Effects of the pretreatment on the composition and structure of the straw was also investigated. Moreover, the quality of the produced xanthan gum was compared with a commercial one.
2. Experimental
2.1. Pretreatment
Rice straw was obtained from the Nowshahr region (36° 38′ 56′′ N, 51° 29′ 46′′ E, Mazandaran, Iran). It was then crushed and sieved using 20 and 80 mesh to achieve particle size of less than 0.8 mm. The pretreatment was performed using two alkalis, namely sodium hydroxide (NaOH) and sodium bicarbonate (NaHCO3). In the pretreatment with sodium hydroxide, 20 g of prepared rice straw was mixed thoroughly with 380 g of 2 M NaOH solution in a bottle. Then it was placed in a bath at a fixed temperature for a specified period. Afterwards, the remaining rice straw was separated via filter and subsequently washed with distilled water. Then it put in an oven at 105 °C for 48 hours. The dried materials were then weighed. In order to compare the capability of the pretreatment methods to destroy the structure of rice straw, the rice straw recovery factor (RSRF) was defined as below:| RSRF= (initial weight of the sample (g) × 100)/(dry weight obtained after pretreatment (g)) | (1) |
Pretreatment with sodium bicarbonate was conducted in a similar way. An amount of 20 g of untreated rice straw was mixed with 380 g of sodium carbonate solution. The completely intermingled substance was then transferred into an oil bath at a desired temperature for 3 hours. Then, the treated straw was separated and washed with distilled water. The separated solid was dried in the same manner as mentioned above and subsequently, RSRF was calculated.
The pretreatment with sodium hydroxide was conducted at 0 and 100 °C for different pretreatment periods (30, 60, 90, 120, and 150 min). The pretreatment with sodium bicarbonate was conducted at three different concentrations of 0.25, 0.5 and 1 M. Each pretreatment performed at three different temperatures (120, 150, and 180 °C). In a previous study, Salehi et al.,25 showed that the treatment with 0.25–1 M Na2CO3 at temperature lower than 90 °C is not effective. Hence, the experiments were arranged to be conducted at 120 °C and higher. Table 1 shows the list of experiments performed.
| (A) Pretreatment with 8% NaOH | |||||
|---|---|---|---|---|---|
| Exp. Nr.a | Temp.b | Pret. Per.c | Exp. Nr. | Temp. | Pret. Per. |
| a Exp. Nr. = experiment number.b Temp. = temperature (°C).c Pret. Per = pretreatment period (minutes).d SBi. conc. = sodium bicarbonate concentration (molar). | |||||
| 1 | 0 | 30 | 6 | 100 | 30 |
| 2 | 0 | 60 | 7 | 100 | 60 |
| 3 | 0 | 90 | 8 | 100 | 90 |
| 4 | 0 | 120 | 9 | 100 | 120 |
| 5 | 0 | 150 | 10 | 100 | 150 |
| (B) Pretreatment with NaHCO3 solution | ||||||||
|---|---|---|---|---|---|---|---|---|
| Exp. Nr. | Temp. | SBi conc.d | Exp. Nr. | Temp. | SBi conc. | Exp. Nr. | Temp. | SBi conc. |
| 11 | 120 | 0.25 | 14 | 150 | 0.25 | 17 | 180 | 0.25 |
| 12 | 120 | 0.5 | 15 | 150 | 0.5 | 18 | 180 | 0.5 |
| 13 | 120 | 1 | 16 | 150 | 1 | 19 | 180 | 1 |
2.2. Materials and microorganisms
The cellulase (Celluclast 1.5 L, Novozymes, Bagsværd, Copenhagen, Denmark) and β-glucosidase (Novozyme 188, Novozymes, Bagsværd, Copenhagen, Denmark) were used for hydrolysis. The activity of cellulase was 30 FPU ml−1 and measured according to the standard method given by NREL (National Renewable Energy Laboratory). The β-glucosidase activity was 147 IU ml−1, measured by the method described by Ximenes et al. (Ximenes et al., 1996).262.3. Enzymatic hydrolysis
Pretreated rice straw (5 g) was mixed with 30 ml of citrate buffer in a 118 ml glass bottle (717561, Pajuhesh Setayesh Sepahan, Isfahan, Iran). The bottle then was autoclaved at 121 °C for 20 min. After the mixture cooled, 20 FPU of cellulase and 30 IU of β-glucosidase per gram of pretreated rice straw were added. The bottle then was incubated at 45 °C and 100 rpm for 72 hours. In order to evaluate the effectiveness of pretreatment, glucose yield was calculated according to the following equation:| Glucose yield= (produced glucose (g l−1) × 100)/(1.111 × glucan in sample (g l−1)) | (2) |
2.4. Xanthan gum fermentation
X. campestris (PTCC 1473, Persian Type Collection Culture, Tehran, Iran) was used for microbial production of xanthan. The cultivation procedure was according to the method reported elsewhere.14The medium used for preparation of the inoculum was: 20.0 g l−1 sucrose, 0.86 g l−1 NH4NO3, 3.0 g l−1 yeast extract, 2.5 g l−1 Na2HPO4 and 2.5 g l−1 KH2PO4. The experiments were conducted in shake flasks at 150 rpm and 28 °C. The average biomass concentration of inoculum was 0.3 g l−1.
The medium used for xanthan production was as follows: 27.0 g l−1 sucrose, 0.8 g l−1 NH4NO3, 2.0 g l−1 yeast extract, 2.5 g l−1 Na2HPO4, 2.5 g l−1 KH2PO4. The carbon source of the medium was replaced by the hydrolysate obtained after pretreatment of rice straw.
In all experiments the pH was adjusted to 7.5 before inoculation.
Commercial xanthan gum from X. campestris, purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, United States, CAS Number: 11138-66-2), was used for comparison.
2.5. Analytical methods
The carbohydrate and lignin content of treated and untreated samples was determined using a standard method proposed by the National Renewable Energy Laboratory (NREL, Denver, United States). Scanning electron microscopy (SEM, Zeiss, Jena, Germany) was used to study the morphology of treated and untreated rice straw. Structure of rice straw before and after treatment was compared based on the results obtained from Fourier transform infrared spectroscopy (FTIR, Bruker Tensor 27 FT-IR, Billerica, MA, USA). In addition, the structure of the produced xanthan was investigated using FTIR. Thermogravimetric analysis (TGA, NETZSCH, Selb, Germany) was performed to investigate the change in properties of the xanthan as a function of temperature. The xanthan samples were heated under nitrogen flow at a heating rate of 20 °C per min. The temperature was raised from room temperature to about 600 °C. Both TGA and DTA (differential thermal analysis) curves were obtained. A high-performance liquid chromatograph equipped with ultraviolet-visible (UV/VIS) spectroscopy and refractive index (RI) detectors was used (Jasco International Co., Tokyo, Japan) to analyse sugar content of the hydrolysate after pretreatment. An Aminex HPX-87H column (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was implemented to measure glucose.Produced xanthan was purified and measured according to the method reported by Niknezhad et al.14 1.5 ml of the fermentation broth was centrifuged for 25 min at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Then the cell free supernatant was mixed with 3 ml of 0.1% calcium chloride. This resulted in precipitation of xanthan, which was separated by centrifugation (30 min, 15
000 rpm. Then the cell free supernatant was mixed with 3 ml of 0.1% calcium chloride. This resulted in precipitation of xanthan, which was separated by centrifugation (30 min, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). The separated xanthan was dried at 50 °C for 48 hours.
000 rpm). The separated xanthan was dried at 50 °C for 48 hours.
All experiments were performed in triplicate to provide sufficient data to analysis and comparison.
2.6. Statistical methods
In order to compare the results from different types of pretreatment, the yields were compared according to analysis of variance (ANOVA). Where appropriate, the yields were compared according to proper type of t-test. These analyses was performed using Excel 2013.3. Results and discussion
3.1. Effects of pretreatment on composition and structure of rice straw
The composition of rice straw before and after pretreatment was measured as presented in Table 2. In this table, the weight percentage of ash, lignin, glucan and xylan as well as rice straw recovery factor (RSRF) in the pretreatments are reported.| (A) Pretreatment with 8% NaOH | ||||||
|---|---|---|---|---|---|---|
| Pretreatment conditions | Xylan (%) | Glucan (%) | Lignin (%) | Ash (%) | RSRF | |
| Temperature (°C) | Time (min) | |||||
| 0 | 30 | 23.2 ± 0.4 | 48.5 ± 0.1 | 13.85 ± 0.3 | 6.75 ± 0.2 | 75 |
| 0 | 60 | 21.8 ± 0.4 | 49 ± 0.2 | 12.49 ± 0.2 | 6.6 ± 0.2 | 71 |
| 0 | 90 | 21.7 ± 0.2 | 50 ± 0.4 | 12.69 ± 0.4 | 6 ± 0.3 | 69 |
| 0 | 120 | 20.6 ± 0.4 | 50.64 ± 0.3 | 12.47 ± 0.5 | 5.2 ± 0.4 | 65 |
| 0 | 150 | 18.77 ± 0.2 | 52.6 ± 0.1 | 12.22 ± 0.1 | 5.13 ± 0.2 | 62 |
| 100 | 30 | 17.2 ± 0.1 | 53.1 ± 0.1 | 11.7 ± 0.3 | 5.7 ± 0.2 | 55 |
| 100 | 60 | 15.7 ± 0.2 | 59.4 ± 0.3 | 11.2 ± 0.2 | 5.3 ± 0.4 | 50 |
| 100 | 90 | 14.9 ± 0.3 | 64 ± 0.2 | 10.5 ± 0.3 | 5.1 ± 0.4 | 49 |
| 100 | 120 | 14.1 ± 0.1 | 67.6 ± 0.2 | 10.3 ± 0.1 | 4.9 ± 0.1 | 48 |
| 100 | 150 | 10 ± 0.1 | 69.1 ± 0.4 | 10.1 ± 0.1 | 4.7 ± 0.2 | 47 |
| Untreated rice straw | 24.5 ± 0.4 | 45.5 ± 0.4 | 16 ± 0.1 | 7.1 ± 0.1 | — | |
| (B) Pretreatment with NaHCO3 | ||||||
|---|---|---|---|---|---|---|
| Pretreatment conditions | Xylan (%) | Glucan (%) | Lignin (%) | Ash (%) | RSRF | |
| Temperature (°C) | Molarity | |||||
| 120 | 0.25 | 17.4 ± 0.2 | 58.7 ± 0.3 | 15.3 ± 0.3 | 3.3 ± 0.3 | 58 |
| 150 | 0.25 | 15.7 ± 0.4 | 60.5 ± 0.3 | 13 ± 0.4 | 3.2 ± 0.3 | 49 |
| 180 | 0.25 | 13.4 ± 0.6 | 62.3 ± 0.7 | 12.4 ± 0.6 | 3 ± 0.1 | 48 |
| 120 | 0.5 | 15.5 ± 0.9 | 61.6 ± 0.5 | 13.1 ± 0.7 | 3 ± 0.2 | 54 |
| 150 | 0.5 | 13.4 ± 0.6 | 62.5 ± 0.1 | 11.6 ± 0.3 | 2.8 ± 0.2 | 46 |
| 180 | 0.5 | 13 ± 0.7 | 65.3 ± 0.3 | 10.6 ± 0.5 | 2.5 ± 0.7 | 43 |
| 120 | 1 | 14 ± 0.5 | 63.5 ± 0.3 | 12.8 ± 0.4 | 2.8 ± 0.2 | 52 |
| 150 | 1 | 11.8 ± 0.3 | 65.7 ± 0.2 | 11.4 ± 0.2 | 2.6 ± 0.2 | 45 |
| 180 | 1 | 11 ± 0.4 | 67.1 ± 0.2 | 10.2 ± 0.1 | 2.4 ± 0.3 | 41 |
| Untreated rice straw | 24.5 ± 0.4 | 45.5 ± 0.4 | 16 ± 0.1 | 7.1 ± 0.1 | 99.5 | |
As it can be seen from Table 2-A, the composition of rice straw has been changed by pretreatment. In the samples pretreated with 2 M sodium hydroxide, at 0 °C, the lignin content was reduced from 16% to 12.22%. In contrast, at 100 °C, it was reduced from 16% to 10.1%.
Pretreatment affected the glucan content as well. The glucan content increased as the pretreatment time increased. The maximum glucan content was observed after 150 min. This increase was 15.6% and 51.8%, compared to the untreated sample in the case, at 0 °C and 100 °C, respectively.
Table 2-A shows that xylan decreased as the pretreatment proceeded. This reduction was 23.6% and 59.2% for the pretreatments at 0 °C and 100 °C, respectively. The ash content decreased as well. The percentage reduction of ash content was 27.7% and 33.8% for the pretreatment at 0° and 100 °C, respectively.
As can be seen in Table 2-B, glucan content, in the pretreated samples with NaHCO3, was greater than control. At constant temperature, the glucan content increased with the increase in the concentration of sodium bicarbonate. At constant concentration of sodium bicarbonate, the glucan content increased when the temperature increased. The glucan content in the pretreatment with 0.5 M sodium bicarbonate at 180 °C was 65.3%. Salehi et al.25 reported that 65.2% content can be achieved in the pretreatment of rice straw with 0.5 M sodium carbonate at 180 °C.
Xylan, lignin and ash contents decreased as the concentration of NaHCO3 increased. At constant concentration, xylan, lignin content decreased when the temperature increased.
The rightmost column of Table 2 shows the RSRF calculated after the different pretreatment methods implemented in this work. RSRF values reveal the fact that the pretreatment at 0 °C resulted in higher RSRF than the pretreatment at 100 °C. For example, the RSRF calculated for treated rice straw at 0 °C after 150 min was 62%, while that of the treated sample at 100 °C was 47% (Table 2-A). The RSRF of the samples pretreated with NaHCO3 was reduced with the increase of temperature at constant concentration. At constant temperature, the RSRF was reduced as the concentration increased. The lowest RSRF, 40%, was observed at 1 M concentration of sodium bicarbonate at 180 °C.
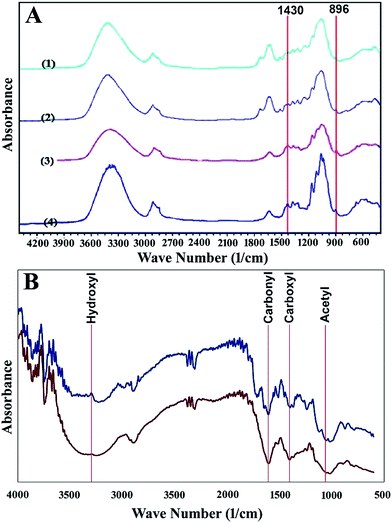
In order to study the crystallinity of the pretreated and untreated rice straw, it was analysed using FTIR. Fig. 1 shows the spectra of four different samples. These samples include (1) untreated rice straw, (2) pretreated one at 0 °C for 150 min with 2 M sodium hydroxide solution, (3) sample pretreated at 180 °C for 180 min with 0.5 M sodium bicarbonate solution and (4) the sample pretreated with 2 M sodium hydroxide at 100 °C for 120 min. The crystallinity index of these samples was calculated as the absorbance ratio of A1430/A896. The crystallinity indexes were determined to be 0.81, 0.78, 0.72 and 0.67 for samples 1 to 4, respectively. This means that the crystallinity index decreased after pretreatment.
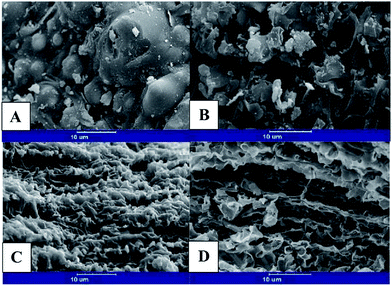
SEM analysis was used to investigate the morphology and surface characteristics of the pretreated and untreated samples (Fig. 2). As can be seen in this figure, pretreatment resulted in destruction of rice straw and an increase in porosity. In other words, pretreated samples show more accessible surfaces (Fig. 2B–D) compared to the untreated sample, which shows a compact structure (Fig. 2A). Comparison of Fig. 2B with 2-D pinpoints that pretreatment at 100 °C led to more porosity than the pretreatment at 0 °C.
3.2. Enzymatic hydrolysis of untreated and pretreated rice straw
All pretreated and untreated samples were subjected to enzymatic hydrolysis. The hydrolysates were analysed for sugars and fermentation inhibitors. The concentration of inhibitory compounds, i.e., furfural, hydroxymethyl furfural, and acetic acid, were less than 0.06 g l−1. In order to compare the performance of hydrolysis, the glucose yield was calculated for each experiment. Fig. 3A shows the glucose yield obtained in pretreatment with 2 M sodium hydroxide at 0 and 100 °C. As it is clear from this figure, the glucose yields obtained from the treated samples were more than that of the untreated sample. The yields were 191% and 286% more than the untreated one in the pretreatments at 0 °C and 100 °C for 150 min, respectively. All yields from the pretreatments were subjected to on-sample t-test in order to compare with the yield obtained from untreated sample. The results of the t-test confirmed the significant higher yields at 100 °C compared to 0 °C (P value = 2.8 × 10−7 while the level of significance was 0.05 for the case of 100 °C. P value = 0.0006 while the level of significance was 0.05 for the case of 0 °C).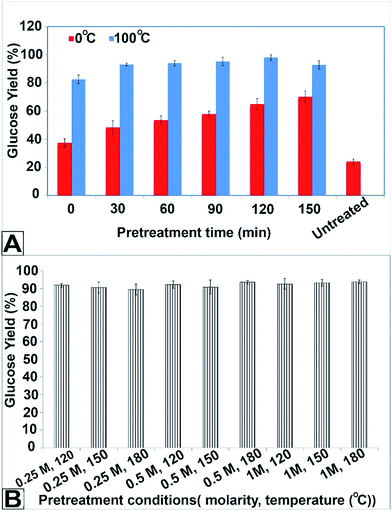 | ||
| Fig. 3 Glucose yield obtained from (A) pretreatment with 2 M NaOH at 0 °C, (B) pretreatment with 2 M NaOH at 100 °C. | ||
The comparison of samples pretreated at 0 and 100 °C showed that the higher the pretreatment temperature, the higher the glucose yield (Fig. 3A). For example, the highest glucose yield obtained at 0 °C was 70.2%, while at 100 °C, it was 92.8%. ANOVA confirmed the significant difference between the yields obtained from pretreatment at 100° C compared to those of 0 °C (the P-value was 8.99 × 10−10 while the level of significant considered as 0.05).
Fig. 3B displays the glucose yield obtained from hydrolysis of samples pretreated with sodium bicarbonate. This figure shows that the glucose yields of pretreated samples are more than four times that of the untreated sample. For instance, the yields were 20.1% and 92.66% for untreated sample and treated sample with 1 M NaHCO3 at 180 °C.
Nevertheless, the glucose yields obtained at different temperatures and concentration of NaHCO3 were not significantly different from each other (Fig. 3B). The average glucose yield was 92.07 ± 1.4.
3.3. Xanthan production
X. campestris was inoculated in the medium containing hydrolysate of enzymatic hydrolysis. This means that the carbon source was provided to the strain by means of glucose produced during enzymatic hydrolysis. Cultivation lasted 72 hours. Then the produced xanthan was measured. The yield of xanthan production (g xanthan per 100 g of raw rice straw) was then calculated accordingly. The results are presented in Fig. 4.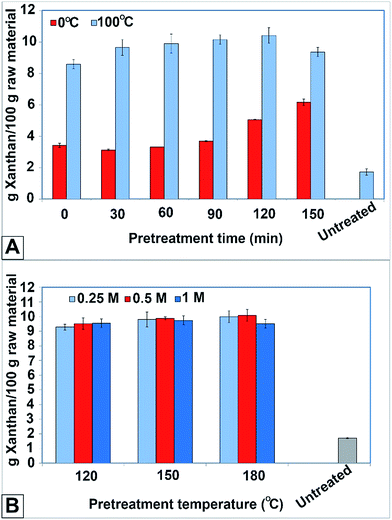 | ||
| Fig. 4 Xanthan yield obtained from (A) pretreatment with 2 M NaOH at 0 °C and 100 °C and (B) pretreatment with NaHCO3 at different concentrations and temperatures. | ||
Fig. 4A presents the xanthan yield obtained by pretreatment with 2 M sodium hydroxide. This figure reveals the fact that the pretreatment temperature has great influence on the xanthan yield. For example, the xanthan yields were 5.1 and 10.41 for the pretreatment at 0 and 100 °C after 120 min, respectively. This was also confirmed by ANOVA. The P-value was 8.44 × 10−7 while the level of significance considered as 0.05. As well, Fig. 4A shows that the change in xanthan yield was notable at 0 °C as pretreatment time increased. It increased from 3.41 to 6.16 g/100 g at 0 °C (96% increase). Nevertheless, this change was not remarkable at 100 °C. The yield changed from 8.6 to 9.36 g/100 g, which corresponds to an 8% increase.
In the pretreatment with sodium bicarbonate, the xanthan yield was nearly the same at different concentrations of sodium bicarbonate and varying temperatures. The average yield obtained was 9.7 with a standard deviation of 0.25. This was also validated by ANOVA since the P-value was 0.6459 at the level of significance of 0.05. (Null hypothesis was not rejected meaning no significant difference among the yields.)
To make a qualitative comparison of the molecular structure of the produced xanthan with that of the commercial product, both were analysed with FTIR. The resulted spectra appear in Fig. 1B. This figure shows similar results for both samples. The comparison of the each peak value, representing a specific functional group in the commercial and in the produced xanthan, revealed that they have almost the same value. For example, the peak value of commercial and produced xanthan at wavelength corresponds to the hydroxyl, carbonyl, carboxyl and acetyl groups, which were (3332, 3346), (1602, 1606), (1404, 1398) and (1037, 1035), respectively.
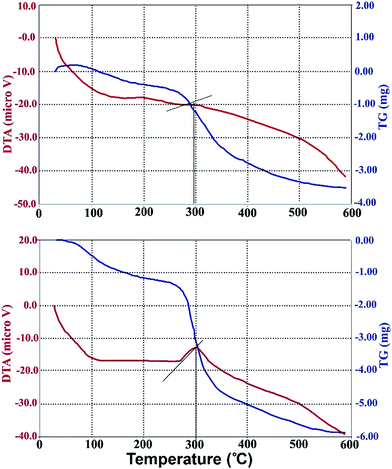
The produced xanthan was also subjected to TGA/DTA analysis. This analysis gives valuable information for a substance subjected to heating. The results of this analysis are presented in Fig. 5A and B for produced xanthan and commercial xanthan, respectively. As can be inferred from Fig. 5, the first mass loss occurred at 35 to 140 °C for produced xanthan and at 33 to 112 °C for the commercial xanthan. These mass losses correspond to 10% and 8% of the total mass reduction for produced and commercial xanthan, respectively. The second weight loss was took place at 250 to 350 °C and 240 to 260 °C, which corresponds to 30% and 53% of total weight loss for produced and commercial xanthan. Fig. 5A and B exhibits that the maximum rate of weight loss occurred at 297 and 300 °C for produced and commercial xanthan, respectively. These points can be identified by the drastic drop in TGA curve and the sudden increase in DTA curve. The exact points were identified by the built-in software of the equipment and are shown in Fig. 5.
Rice straw, as a renewable and inexpensive source of carbon, has great potential as a substrate for microbial xanthan production. The recalcitrant structure of rice straw is the limiting step to prepare it as a carbon source. The results of this work demonstrated that alkali pretreatment enhanced hydrolysis efficiency and consequently increased xanthan yield. The xanthan yield could be boosted to more than ten times the yield obtained from untreated rice straw. The maximum yield obtained in this study was about 10 g xanthan per 100 g of raw rice straw.
The main reason for enhancing yield of enzymatic hydrolysis and, subsequently, xanthan production is the occurrence of several reactions in the presence of Na+ ions. Hydrolysis of glycosidic bonds (depolymerization) and acetyl groups as well as dissolution of cellulose and regeneration of cellulose, resulting in crystallinity reduction and changes in distribution of crystalline and amorphous regions, are among the phenomena resulting in the improvement of hydrolysis. On the other hand, there are several reactions, e.g., formation of alkali-stable end-groups, called peeling reactions of end groups, and decomposition of dissolved polysaccharides, can negatively affect the hydrolysis. Besides, hydroxyl ions at the alkali conditions are responsible for fragmentations and dissolution and of lignin, which are desired in the pretreatment. However, the condensation of lignin and its derivatives on the lignocellulosic surfaces can be happened, resulting in lower cellulose hydrolysis yields.27 Therefore, the alkali pretreatments are very complicated processes that can either improve or reduce the cellulose hydrolysis yield. Generally, the main mechanism in the pretreatment of lignocelluloses with Na+ ions at low temperature (e.g., 0 °C) is dissolution of cellulose, while the main mechanism at high temperature (e.g., 100 °C) is reactive destruction and solubilization of lignin and hemicellulose.24,27
The results of this work confirm that the maximum xanthan yield can be obtained in the pretreatment with 2 M NaOH at 100 °C for 120 min. The maximum xanthan yield at 0 °C was considerably lower than that at 100 °C (9.36 g/100 g compared to 6.16 g/100 g). However, temperature lacks influence on the xanthan yield in the case of pretreatment with NaHCO3. The average xanthan yield obtained at different temperatures and concentrations was 9.7 g/100 g, with a standard deviation of 0.25 (Fig. 4B). The significant influence of temperature occurred upon increase from 0 to 100 °C, which may be due to the increased of rate of reactions that took place during pretreatment. In the case of 0 °C, the reaction requires time to be completed. Hence, the pretreatment needs time to progress. The gradual increase in glucose and xanthan yield (Fig. 4A) is evidence of that. Nevertheless, the temperature of pretreatment with NaHCO3 was high enough that the reactions took place very quickly. This might be the reason for the similar glucose and xanthan yields at different temperatures when rice straw was pretreated with NaHCO3 (Fig. 4B). In other words, the temperature was probably so high that all possible destructive reactions occurred in a short period immediately after the start of pretreatment.
NaHCO3 concentration did not influence the pretreatment notably (Fig. 4B). This could be due to the presence of Na+ ions exceeding the amount required for involvement in the destructive reactions. Therefore, from economic point of view it is recommended to use lower concentration (0.25 M) if sodium bicarbonate is going to be used in pretreatment.
The trend of xanthan yield obtained in both pretreatment methods is in accordance with the trend of glucose yield achieved in the enzymatic hydrolysis. The glucose and xanthan yields in NaOH pretreatment at 100 °C deviate slightly from 92.6% and 9.7 g/100 g respectively. However, the glucose yield obtained at 0 °C was variable from 37.33% to 70.2%, and the xanthan yield increased from 3.1 to 6.1 g/100 g. This means that the bioconversion of hydrolysate to xanthan is not the bottleneck. The main challenge is to make cellulose accessible for enzymatic hydrolysis. The subsequent bioconversion of glucose to xanthan takes place irrespective of the type of alkaline used and pretreatment conditions implemented.
FTIR analysis provides a qualitative study of the chemical composition of the materials being pretreated. The absorption bound at 1430 cm−1 was related to the presence of cellulose type I. This type of cellulose is resistant to hydrolysis, in contrast to cellulose type II, which is more susceptible to be hydrolysed. Absorbance band at 896 cm−1 was related to cellulose type II. Hence, the reduction in crystallinity index is a qualitative measure of the reduction in the presence of cellulose type I. Since the crystallinity decreased after pretreatment (data was presented in section results), the pretreatment investigated in this work was able to reduce cellulose type II and increase the presence of cellulose type I.
As shown in Fig. 1A, absorbency at 3175 and 1335 cm−1 increased after pretreatment. These wave numbers correspond to hydroxyl groups. The absorbency at 1730, which was assigned to acetyl groups, was decreased by pretreatment. Acetyl groups are the key functional groups connecting lignin and hemicellulose. These connections are the main barrier for hydrolysis of hemicellulosic polysaccharides.28 The change in absorbency of the above-mentioned functional groups is evidence for the fact that the pretreatment enhanced accessibility to cellulose. Moreover, SEM analysis confirms the FTIR results. As can be inferred from Fig. 2, pretreatment with 2 M sodium hydroxide was able to swell the recalcitrant structure of rice straw and increase the available surface area. This was also observed in the pretreatment with NaHCO3. As Fig. 2 shows, the pretreatment at 100 °C was more effective than that at 0 °C in terms of making more surface area accessible and disintegrating rice straw structure. As mentioned above, this might be due to the higher temperature, which accelerated the destructive reactions.
Although the pretreatment could enhance xanthan yield, one could question whether the produced xanthan has the same quality as the commercial product. This question was answered qualitatively by TGA and FTIR analyses. The similarity in the spectra of the produced and commercial xanthan reveals that they have similar chemical structure. This was also cross-validated by TGA/DTA analysis (Fig. 5). In TGA analysis, the first mass loss occurred because the samples were dehydrated. Xanthan gum absorbs water because –OH and other polar groups exist in xanthan gum structure.29 The second weight loss is a result of thermal decomposition. Despite a slight difference in the range of temperatures during which the thermal decomposition occurred, the maximum rate of decomposition took place at nearly the same temperature (297 and 300 °C for produced and commercial xanthan, respectively). Hence, one concludes that the quality of the produced xanthan is very close to the commercial product.
4. Conclusions
Rice straw, as a widely available renewable source of carbon, is not suited to be used in its native form for microbial xanthan production, since the production yield is very low. Because of its recalcitrant structure, it must be pretreated to degrade lignin and hemicellulose, to make cellulose accessible for enzymatic hydrolysis. Alkali pretreatment was implemented in this work to increase enzymatic digestibility of rice straw. The pretreatment with 2 M NaOH was greatly influenced by temperature, as temperature increased from 0 to 100 °C. However, the increase in temperature from 120 to 180 °C did not have a notable influence on glucose yield in the pretreatment with NaHCO3. Nevertheless, pretreatments with both alkali, studied in this work, were able to increase xanthan yield up to five times. The quality of produced xanthan gum was similar to the commercial product. Hence, alkali pretreatment has great potential to provide a renewable carbon source for xanthan production.Acknowledgements
The financial support provided for this project by the Research Institute for Biotechnology and Bioengineering, Isfahan University of Technology, is gratefully acknowledged.References
- F. Zia, K. M. Zia, M. Zuber, S. Kamal and N. Aslam, Carbohydr. Polym., 2015, 134, 784–798 CrossRef CAS PubMed.
- K. M. Zia, F. Zia, M. Zuber, S. Rehman and M. N. Ahmad, Int. J. Biol. Macromol., 2015, 79, 377–387 CrossRef CAS PubMed.
- M. Zuber, F. Zia, K. M. Zia, S. Tabasum, M. Salman and N. Sultan, Int. J. Biol. Macromol., 2015, 80, 366–374 CrossRef CAS PubMed.
- S. Rosalam and R. England, Enzyme Microb. Technol., 2006, 39, 197–207 CrossRef CAS.
- F. García-Ochoa, V. E. Santos, J. A. Casas and E. Gómez, Biotechnol. Adv., 2000, 18, 549–579 CrossRef.
- S. Kalogiannis, G. Iakovidou, M. Liakopoulou-Kyriakides, D. A. Kyriakidis and G. N. Skaracis, Process Biochem., 2003, 39, 249–256 CrossRef CAS.
- S. K. Psomas, M. Liakopoulou-Kyriakides and D. A. Kyriakidis, Biochem. Eng. J., 2007, 35, 273–280 CrossRef CAS.
- M. Mirik, A. S. Demirci, T. Gumus and M. Arici, Food Sci. Biotechnol., 2011, 20, 1243–1247 CrossRef CAS.
- H. Umashankar, G. Annadurai, M. Chellapandian and M. R. V. Krishnan, Bioprocess Eng., 1996, 14, 307–309 CrossRef CAS.
- P. Souw and A. L. Demain, Appl. Environ. Microbiol., 1979, 37, 1186–1192 CAS.
- M. F. Silva, R. C. G. Fornari, M. A. Mazutti, D. de Oliveira, F. F. Padilha, A. J. Cichoski, R. L. Cansian, M. Di Luccio and H. Treichel, J. Food Eng., 2009, 90, 119–123 CrossRef CAS.
- S. D. Yoo and S. W. Harcum, Bioresour. Technol., 1999, 70, 105–109 CrossRef CAS.
- R. Ben Salah, K. Chaari, S. Besbes, N. Ktari, C. Blecker, C. Deroanne and H. Attia, Food Chem., 2010, 121, 627–633 CrossRef CAS.
- S. V. Niknezhad, M. A. Asadollahi, A. Zamani and D. Biria, Int. J. Biol. Macromol., 2016, 82, 751–756 CrossRef CAS PubMed.
- V. Gunasekar, K. R. Reshma, G. Treesa, D. Gowdhaman and V. Ponnusami, Carbohydr. Polym., 2014, 102, 669–673 CrossRef CAS PubMed.
- E. T. Öner, in Pretreatment Techniques for Biofuels and Biorefineries, ed. Z. Fang, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 35–56, DOI:10.1007/978-3-642-32735-3_2.
- Z. Zhang and H. Chen, Appl. Biochem. Biotechnol., 2010, 160, 1653–1663 CrossRef CAS PubMed.
- T. Q. Hu, Characterization of Lignocellulosic Materials, Blackwell Publishing Ltd., 2009, pp. 357–370, DOI:10.1002/9781444305425.index.
- K. Karimi and M. J. Taherzadeh, Bioresour. Technol., 2016, 200, 1008–1018 CrossRef PubMed.
- F. M. Gírio, C. Fonseca, F. Carvalheiro, L. C. Duarte, S. Marques and R. Bogel-Łukasik, Bioresour. Technol., 2010, 101, 4775–4800 CrossRef PubMed.
- P. Wang, J. Chang, Q. Yin, E. Wang, Q. Zhu, A. Song and F. Lu, Bioresour. Technol., 2015, 194, 165–171 CrossRef CAS PubMed.
- M. J. Taherzadeh and K. Karimi, Int. J. Mol. Sci., 2008, 9, 1621–1651 CrossRef CAS PubMed.
- M. S. Noori and K. Karimi, Biochem. Eng. J., 2016, 105(A), 197–204 CrossRef CAS.
- K. Mirahmadi, M. M. Kabir, A. Jeihanipour, K. Karimi and M. Taherzadeh, BioResources, 2010, 5, 928–938 CAS.
- S. M. A. Salehi, K. Karimi, T. Behzad and N. Poornejad, Energy Fuels, 2012, 26, 7354–7361 CrossRef CAS.
- A. E. Ximenes, R. C. Felix and J. C. Ulhoa, Curr. Microbiol.Curr. Microbiol., 1996, 32, 119–123 CrossRef.
- K. Karimi, M. Shafiei and R. Kumar, in Biofuel Technologies: Recent Developments, ed. V. K. Gupta and M. G. Tuohy, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 53–96, DOI:10.1007/978-3-642-34519-7_3.
- N. Poornejad, K. Karimi and T. Behzad, Ind. Crops Prod., 2013, 41, 408–413 CrossRef CAS.
- S. Faria, C. L. de Oliveira Petkowicz, S. A. L. de Morais, M. G. H. Terrones, M. M. de Resende, F. P. de França and V. L. Cardoso, Carbohydr. Polym., 2011, 86, 469–476 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |