Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilization and transformation of Pt nanocrystals supported on ZnAl2O4 spinel†
Wei-Zhen Li‡
a,
Libor Kovarika,
Yingwen Chenga,
Lei Niea,
Mark E. Bowdena,
Jun Liu*a and
Yong Wang*ab
aInstitute for Integrated Catalysis, Pacific Northwest National Laboratory, P. O. Box 999, Richland, WA 99352, USA. E-mail: jun.liu@pnnl.gov
bThe Gene & Linda Voiland School of Chemical Engineering and Bioengineering, Washington State University, Pullman, WA 99164, USA. E-mail: yong.wang@pnnl.gov
First published on 13th January 2017
Abstract
The role of interfacial interaction on the stabilization and transformation of Pt nanocrystals supported on zinc aluminate (ZnAl2O4) was systematically studied in this work. In addition to the remarkable stability of Pt nanocrystals supported on the {111} ZnAl2O4 facet under a harsh oxidizing atmosphere, we also identified the phase segregation of ZnAl2O4 to ZnO and Al2O3 and the transformation of Pt to PtZn bimetallic alloy for Pt supported on other facets. High resolution scanning transmission electron microscopy and X-ray diffraction analyses reveal the phase segregation is associated with a cation migration mechanism associated with replacement of Zn2+ by Al3+ in the spinel structure. This work confirms the strong interfacial interactions between precious metal nanocrystals and that the spinel support is a general mechanism for stabilizing precious metal catalysts, with important implications for the rational design of stable integrated catalysis systems.
Supported precious metal nanocrystals are essential for many industrially important catalytic reactions because of their remarkable activities that are difficult or impossible to achieve with alternative materials.1 However, they usually have poor stability under the harsh conditions associated with practical applications especially with high temperatures and oxidizing atmospheres, and readily transform to less reactive particles with much larger sizes.1,2 Therefore, the development of effective approaches to stabilize nanocrystals is of critical importance and has been under intensive research in recent years. The stability of supported nanocrystals depends on many factors, including the phase, exposed facts and interfacial molecular structures of the nanocrystal and the support material, and the instability usually involves several mechanisms. For example, supported Pt nanocrystals migrate and aggregate and form large particles with supports such as Al2O3, SiO2, TiO2 and ZrO2,3,4 on the other hand, they can also react with the support and form solid solutions with supports such as MgO and CeO2.5,6 Overall, these pathways lead to loss in Pt dispersion and substantial reduction in catalytic activity. The stability of Pt nanocrystals can be enhanced by applying novel supports and/or manipulating the surface chemistry of the supports.7–14 However, the results are still unsatisfactory and it is still desired for developing effective and general strategies for synthesizing and processing stable precious metal catalysts systems.
We recently reported the remarkable capability of spinel MgAl2O4 supports on stabilizing Pt nanocrystals with its {111} facet during severe aging at 800 °C in air.15 This prior successful demonstration has motivated us to examine a wider range of spinel-precious metal combinations, with the goal of developing rational approaches for ultra-stable precious metal nanocrystals. In fact, we found that in addition to Pt nanocrystals, Rh and Ir nanocrystals can also be stabilized as small (1–3 nm) particles using MgAl2O4 during severe aging (800 °C in air for 1 week). The underlying mechanism is identified as epitaxial lattice matching between the spinel {111} facet and the metal nanocrystals.16 In this work, we desire to examine the use of alternative supports with similar lattice characteristics but different surface chemistry, i.e., zinc aluminate (ZnAl2O4) support, so that the potential roles of surface chemistry can be examined. ZnAl2O4 was selected because it has similar lattice characteristics with MgAl2O4, (lattice constant of ZnAl2O4 is 8.0848 Å vs. 8.0831 Å for MgAl2O4) but different surface composition and structure. The synthesis of ZnAl2O4 spinel and its use as a catalyst support has been studied previously but limited work has been done specifically aimed at examining the support–catalyst interactions and stabilizations.17–21
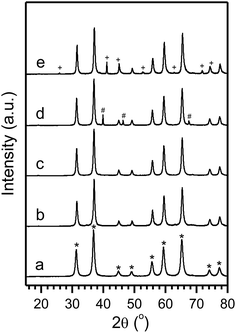
Herein, we report the synthesis of phase-pure ZnAl2O4 spinel and the stabilization and transformation of Pt nanoparticles on this support under oxidizing and reducing atmosphere at high temperatures. Through systematic analysis of aged and reduced samples with high resolution scanning transmission electron microscopy (HR-STEM) and X-ray diffraction (XRD) analysis, we demonstrate the remarkable stability of small Pt nanocrystals attached on the ZnAl2O4 spinel {111} facet, and the partial phase segregation of ZnO as well as the formation of PtZn alloys. In practice, phase-pure ZnAl2O4 spinel was synthesized via an ethanol-mediated controlled hydrolysis method similar to the one reported in our recent papers.15,16 Typically, stoichiometric amount of aluminum isopropoxide (0.1 M) and zinc nitrate hexahydrate (0.05 M) were added to 300 ml ethanol with stirring and the mixed solution was heated at 150 °C (hotplate surface temperature) for 12 hours. The mixture was then dried and the powders obtained were calcined at 800 °C for 12 hours. The XRD pattern confirms formation of pure ZnAl2O4 after calcination without any detectable impurity phases (Fig. 2a). The as-synthesized ZnAl2O4 crystals have the characteristics of well-defined cuboctahedral shape with dominant {111} facet, small grain sizes (∼12 nm, Fig. S1†) and high surface areas (110.3 m2 g−1) (Table S1†). Supported Pt nanocrystals (with a typical loading of 1 wt%) were prepared by the incipient wetness impregnation method using desired amount of PtCl4 with water as the solvent (see ESI† for detailed experiment procedure). The sample was dried and then calcined at 500 °C for 5 hours in air, and was subsequently reduced in 5% H2/He at 800 °C for 2 hours to obtain the 1Pt/ZnAl2O4. The Pt nanocrystals prepared with this method were well dispersed on the surface of ZnAl2O4 and had a dispersion as high as 40.2% as determined via H2 chemisorption. In addition, the Pt nanocrystals had diameter mostly less than 3 nm as revealed with STEM analysis (Fig. 1a–c). The high dispersion was further supported with XRD as no obvious diffraction peaks originated from Pt were detected (Fig. 2b and c).
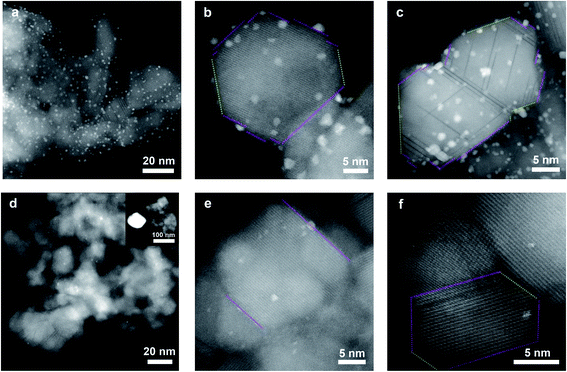 | ||
| Fig. 1 STEM images of 1Pt/ZnAl2O4-FR (a–c), and 1Pt/ZnAl2O4-1WR (d–f). The {111} and {100} facets are marked in magenta and yellow dash lines, respectively. | ||
The stability of Pt nanocrystals under oxidizing and reducing atmospheres was studied via high temperature aging and reduction since severe sintering of nanocrystals usually occurs under elevated temperatures. A typical procedure is aging in air at 800 °C for 1 week and reducing in 5% H2/He at 800 °C for 2 hours. The aging temperatures and the Pt loadings were systematically examined as will be discussed in detail below. The labeling and discussions of samples were organized as follows since a large number of samples were involved: the calcinated samples were labeled with a suffix “O” whereas the reduced samples were labeled with “R”. In addition, the fresh prepared samples had a suffix of “F” and the 1 week aged samples had a suffix of “1W”.
The Pt dispersion decreased to 10.4% with the ZnAl2O4 support after aging in air at 800 °C for 1 week (Table S1,† 1Pt/ZnAl2O4-1WR). This decrease in dispersion is similar to the results observed with the MgAl2O4 support but is much better than other conventional supports such as Al2O3.22 The aged sample was then analyzed with STEM and XRD (Fig. 1 and 2). The diameter of Pt particles after aging and reduction was found to center on the two ranges (therefore a bimodal distribution) of few nanometers similar as prior to aging (1Pt/ZnAl2O4-FR, Fig. 1a–c) and much larger sizes of ∼80 nm (Fig. 1d and S3†). In addition, we observed formation of the PtZn alloy phase (tetragonal, P4/mmm (123)) by XRD (Fig. 2e). Interestingly, the XRD patterns of the 1Pt/ZnAl2O4-1WO sample (after aging but prior to reduction) only exhibited pure metallic Pt phase. This suggests that the formation of PtZn alloy occurred during the H2 reduction.19,20 By using a quantitative multi-component Rietveld analysis, we estimated that ∼70% of Pt forms large Pt particles in 1Pt/ZnAl2O4-1WO sample, which is in line with the loss in Pt dispersion (from 40.2% to 10.4%) determined via H2 chemisorption as discussed above.
The detailed structures and interactions between Pt and ZnAl2O4 both for the fresh and aged samples were further studied with HR-STEM. Fig. 1b shows a typical image for the 1Pt/ZnAl2O4-FR sample. Based on the orientation of the spinel crystal, we can determine the exposed facet ({111} vs. {100}, marked with different color in Fig. 1) and therefore the Pt nanoparticle distribution with regard to facet of the support. The loaded Pt particles had particle size of few nanometers and exhibited random distribution on the ZnAl2O4 spinel facets, suggesting the loading of Pt had no preference on spinel facets during catalyst loading process. After the 1 week aging treatment and the reduction (1Pt/ZnAl2O4-1WR), small Pt nanocrystals were still observed in the STEM images. Their interaction with the support, however, showed clear preferences and most of the particles were selectively attached on spinel {111} facet and had epitaxial relationship with the support. Interestingly, we also noted that the size of Pt particles retained in the aged samples appear smaller than those in the fresh ones, presumably because larger Pt particles have weaker interaction with the support and are more readily to undergo migration and coalesce during aging. The random occupation of Pt in fresh sample and selective occupation in aged one are also evident in additional HR-STEM images (Fig. S2 and S3†), suggesting that Pt nanocrystals attached on the {111} facet has remarkable stability under the conditions examined in this work. These results suggest ZnAl2O4 spinel also have superb capabilities to stabilize Pt nanoparticles in small sizes even during extremely severe aging. Therefore, taking together the results from H2 chemisorption, STEM and XRD, it was concluded that ZnAl2O4 spinel had the capability to stabilize Pt nanocrystals similar as MgAl2O4 spinel and Pt nanoparticles on spinel {111} were stable whereas those on other facets were not. Since these two types of spinel have very similar lattice structure, the origin of the remarkable ability of ZnAl2O4 spinel {111} facet could be rationally understood as the strong interactions between the oxygen termination and platinum {111} facet as have been discussed extensively in our previous works with MgAl2O4.15,16 Overall, these suggest that the strong epitaxial interaction at the metal–support interfaces is a general stabilization mechanism.
The phase transformation of Pt to PtZn alloy is an interesting phenomenon and was therefore further studied. This transformation is different from observations with MgAl2O4 support (where only pure Pt phase was observed) under the same conditions. The STEM images reveal fine structures of dark lines in parallel with the spinel (111) plane in the projection of ZnAl2O4 spinel, which overall appears fault planes in the 1Pt/ZnAl2O4-FR sample (Fig. 1c and S2†). The formation of such dark lines was presumably due to missing of atoms or replacement of heavy atoms by light ones, such as replacement of heavier Zn2+ by lighter Al3+ since the formation energy of antisites of Zn2+ and Al3+ was reported to be small.23,24 Considering the fact that γ-Al2O3 has “spinel structure” (cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (227), lattice constant: 7.9 Å) with Al3+ filling both tetrahedral and octahedral sites and the fact that these dark lines didn't generate significant particle fragmentations, the fault planes can be presumably assigned to the replacement of Zn2+ by Al3+ on spinel (111) planes and phase segregation to ZnO and Al2O3. These features were also observed for particles after aging experiments, suggesting such structure is relatively stable or the phase segregation had relatively slow kinetics. The replaced Zn2+ cannot be detected as zinc oxide by XRD for all the samples processed with 1 wt% of Pt and aged at 800 °C (Fig. 2), implying that the zinc oxide are very small or highly dispersed in the host spinel.
m (227), lattice constant: 7.9 Å) with Al3+ filling both tetrahedral and octahedral sites and the fact that these dark lines didn't generate significant particle fragmentations, the fault planes can be presumably assigned to the replacement of Zn2+ by Al3+ on spinel (111) planes and phase segregation to ZnO and Al2O3. These features were also observed for particles after aging experiments, suggesting such structure is relatively stable or the phase segregation had relatively slow kinetics. The replaced Zn2+ cannot be detected as zinc oxide by XRD for all the samples processed with 1 wt% of Pt and aged at 800 °C (Fig. 2), implying that the zinc oxide are very small or highly dispersed in the host spinel.
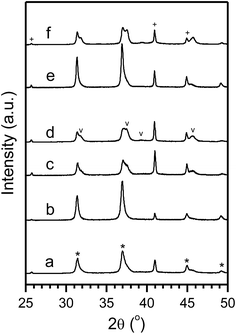
The formation of fault planes and segregation of ZnO and γ-Al2O3 from ZnAl2O4 could decrease the thermal stability of support. Therefore, the thermal stability of the support was subsequently studied systematically and samples were processed with different calcination temperatures and then were analyzed. The spinel was relatively stable at 800 °C since samples processed at this temperature did not show detectable ZnO phases even after calcining for 1 week (Fig. 2). However, the formation of ZnO phase was evident with calcination temperature higher than 900 °C (Fig. 3) and the grain size of ZnO increased markedly from the 32 nm at 900 °C to 54 nm to 1200 °C (Table S2†). Despite the increase in grain size, the weight percentage of ZnO as estimated from XRD results remained small (few wt%, Table S2†) and didn't increase obviously with increased calcination temperature. In addition, the corresponding Al2O3 phase was not detected, which might be due to the overlap of ZnAl2O4 and Al2O3 diffraction peak positions. These results suggest that the phase segregation should be a thermodynamically slow process and might be promoted by the presence of crystals defects (such as dislocations and vacancies) that are introduced during material synthesis. Further improvements in spinel synthesis with controlled defect densities might improve the thermal stability substantially.
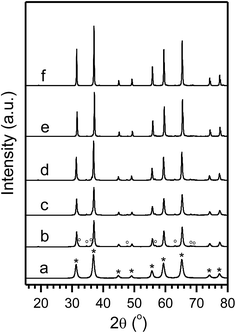
The observation and discussions presented above suggest clearly that the formation of PtZn alloy under reducing atmosphere and the phase segregation of ZnO and γ-Al2O3 from ZnAl2O4 spinel. In order to better understanding the process, we performed additional experiments with varied Pt loadings with supports calcinated at different temperature. Fig. 4a shows the XRD of a freshly prepared 5Pt/ZnAl2O4-800-FR sample (5× Pt loading than samples discussed above). The increases in Pt loading resulted in substantially stronger diffractions of the PtZn phase than the 1Pt/ZnAl2O4-800-FR. In addition, Fig. 4b and c show results obtained with the ZnAl2O4-900 support that has ∼2.4 wt% ZnO and varied Pt loadings of 3, 5 and 8 wt%. Noticeably, all of these samples exhibited evident diffraction responses from PtZn and γ-Al2O3 and their fractions increased from 0.9, 3.7 to 5.3 wt% and from 22, 61 to 54 wt% for loadings of 3, 5 and 8 wt%, respectively (Table S3†). Very similar results were found with the ZnAl2O4-1050 support with Pt loadings of 5 and 8 wt% (Fig. 4e and f and Table S3†). Interestingly, the estimated lattice constant of the “γ-Al2O3” phase generated during phase segregation of ZnAl2O4 was c = 7.93 Å, which is slightly higher than the standard (c = 7.90 Å). Furthermore, small Pt nanocrystals with sizes less than 3 nm and very high particle densities were also evident in the STEM images for the freshly prepared samples with higher Pt loadings (5Pt/ZnAl2O4-FR, Fig. S4; 3Pt/ZnAl2O4-900-FR, Fig. S5; 5Pt/ZnAl2O4-1050-FR, Fig. S6†). Moreover, almost all of the large particles observed with these samples were PtZn alloys. These observations agree well with Pt stabilization and phase transformations discussed above.
In summary, we demonstrated the superior capability of ZnAl2O4 spinel {111} facet on stabilizing Pt nanocrystals especially under harsh oxidizing and reducing conditions. This discovery together with our previous studies on MgAl2O4 spinel support solidifies the stabilization mechanism of strong epitaxial interaction of metal–support interfaces, that overall provided a general approach for rational processing of support materials and metal nanocrystals to meet the rigid requirements for specific catalytic reactions. Furthermore, the observations of phase segregation and transformation might have broader implications for alloy type catalyst particles synthesis and stabilization.
Acknowledgements
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences (DE-AC05-RL01830, FWP-47319). J. Liu and Y. Cheng acknowledge the support from the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Division of Materials Sciences and Engineering, under Award KC020105-FWP12152, for the synthesis of the catalysts and supports. L. Nie acknowledges the support by MS3 Initiative at the Pacific Northwest National Laboratory (PNNL). It was conducted under the Laboratory Directed Research and Development Program (LDRD) at PNNL. The S/TEM and XRD characterizations were performed in the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE Office of Biological and Environmental Research, and located at PNNL. PNNL is a multi-program national laboratory operated by Battelle for DOE by Battelle.References
- V. Ponec and G. C. Bond, Catalysis by Metals and Alloys, Elsevier Science, 1995 Search PubMed.
- S. E. Wanke and P. C. Flynn, The Sintering of Supported Metal Catalysts, Catal. Rev., 1975, 12, 93–135 CAS.
- C. H. Bartholomew, Mechanisms of catalyst deactivation, Appl. Catal., A, 2001, 212, 17–60 CrossRef CAS.
- W.-Z. Li, K.-Q. Sun, Z. Hu and B.-Q. Xu, Characteristics of low platinum Pt–BaO catalysts for NOx storage and reduction, Catal. Today, 2010, 153, 103–110 CrossRef CAS.
- H. Shinjoh, M. Hatanaka, Y. Nagai, T. Tanabe, N. Takahashi, T. Yoshida and Y. Miyake, Suppression of Noble Metal Sintering Based on the Support Anchoring Effect and its Application in Automotive Three-Way Catalysis, Top. Catal., 2009, 52, 1967–1971 CrossRef CAS.
- T. Tanabe, Y. Nagai, K. Dohmae, H. Sobukawa and H. Shinjoh, Sintering and redispersion behavior of Pt on Pt/MgO, J. Catal., 2008, 257, 117–124 CrossRef CAS.
- P. M. Arnal, M. Comotti and F. Schüth, High-Temperature-Stable Catalysts by Hollow Sphere Encapsulation, Angew. Chem., Int. Ed., 2006, 45, 8224–8227 CrossRef CAS PubMed.
- A. Cao, R. Lu and G. Veser, Stabilizing metal nanoparticles for heterogeneous catalysis, Phys. Chem. Chem. Phys., 2010, 12, 13499–13510 RSC.
- S. H. Joo, J. Y. Park, C.-K. Tsung, Y. Yamada, P. Yang and G. A. Somorjai, Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions, Nat. Mater., 2009, 8, 126–131 CrossRef CAS PubMed.
- I. Lee, Q. Zhang, J. Ge, Y. Yin and F. Zaera, Encapsulation of supported Pt nanoparticles with mesoporous silica for increased catalyst stability, Nano Res., 2010, 4, 115–123 CrossRef.
- Y. H. Ng, S. Ikeda, T. Harada, T. Sakata, H. Mori, A. Takaoka and M. Matsumura, High Sintering Resistance of Platinum Nanoparticles Embedded in a Microporous Hollow Carbon Shell Fabricated Through a Photocatalytic Reaction, Langmuir, 2008, 24, 6307–6312 CrossRef CAS PubMed.
- J. M. Schwartz and L. D. Schmidt, Microstructures of Pt–Ce and Rh–Ce particles on alumina and silica, J. Catal., 1992, 138, 283–293 CrossRef CAS.
- J. C. Summers and S. A. Ausen, Interaction of cerium oxide with noble metals, J. Catal., 1979, 58, 131–143 CrossRef CAS.
- Y. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect, Science, 2004, 304, 711–714 CrossRef CAS PubMed.
- W.-Z. Li, L. Kovarik, D. Mei, J. Liu, Y. Wang and C. H. F. Peden, Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres, Nat. Commun., 2013, 4, 2481 Search PubMed.
- W.-Z. Li, L. Kovarik, D. Mei, M. H. Engelhard, F. Gao, J. Liu, Y. Wang and C. H. F. Peden, A General Mechanism for Stabilizing the Small Sizes of Precious Metal Nanoparticles on Oxide Supports, Chem. Mater., 2014, 26, 5475–5481 CrossRef CAS.
- X. Duan, D. Yuan, X. Wang and H. Xu, Synthesis and Characterization of Nanocrystalline Zinc Aluminum Spinel by a New Sol–Gel Method, J. Sol-Gel Sci. Technol., 2015, 35, 221–224 CrossRef.
- X. Y. Chen, C. Ma, Z. J. Zhang and B. N. Wang, Ultrafine gahnite (ZnAl2O4) nanocrystals: hydrothermal synthesis and photoluminescent properties, Mater. Sci. Eng., B, 2008, 151, 224–230 CrossRef CAS.
- N. A. Pakhomov, R. A. Buyanov, E. M. Moroz, G. R. Kotelnikov and V. A. Patanov, Medium effect in thermal pretreatment on the state and catalytic properties of platinum supported on zinc–aluminium spinel, React. Kinet. Catal. Lett., 1978, 9, 257–263 CrossRef CAS.
- A. D. Ballarini, S. A. Bocanegra, A. A. Castro, S. R. Miguel and O. A. Scelza, Characterization of ZnAl2O4 Obtained by Different Methods and Used as Catalytic Support of Pt, Catal. Lett., 2009, 129, 293–302 CrossRef CAS.
- W. Walerczyk, M. Zawadzki and J. Okal, Characterization of the metallic phase in nanocrystalline ZnAl2O4-supported Pt catalysts, Appl. Surf. Sci., 2011, 257, 2394–2400 CrossRef CAS.
- W. Z. Li, L. Kovarik, D. Mei, J. Liu, Y. Wang and C. H. Peden, Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres, Nat. Commun., 2013, 4, 2481 Search PubMed.
- R. Pandey, J. D. Gale, S. K. Sampath and J. M. Recio, Atomistic Simulation Study of Spinel Oxides: Zinc Aluminate and Zinc Gallate, J. Am. Ceram. Soc., 1999, 82, 3337–3341 CrossRef CAS.
- H. Dixit, N. Tandon, S. Cottenier, R. Saniz, D. Lamoen and B. Partoens, First-principles study of possible shallow donors in ZnAl2O4 spinel, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 174101 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26159k |
| ‡ Present address: State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China. E-mail: E-mail: weizhenli@dicp.ac.cn |
| This journal is © The Royal Society of Chemistry 2017 |