 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The controllable construction and properties characterization of organic–inorganic hybrid materials based on benzoxazine-bridged polysilsesquioxanes
Huan Liu ab,
Zi-en Fu*c,
Fei Songab,
Qingquan Liuab and
Lijuan Chenab
ab,
Zi-en Fu*c,
Fei Songab,
Qingquan Liuab and
Lijuan Chenab
aCollege of Materials Science and Engineering, Hunan University of Science and Technology, Xiangtan, 411201, People's Republic of China
bHunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers, Hunan University of Science and Technology, Xiangtan, 411201, People's Republic of China
cKey Laboratory of Polymer Material for Electronics, Guangzhou Institute of Chemistry, Chinese Academy of Sciences, P. O. Box 1122, Guangzhou 510650, People's Republic of China. E-mail: fuzien@gic.ac.cn; Fax: +86 20 85232302; Tel: +86 20 85232302
First published on 13th January 2017
Abstract
Using a hydrolysis condensation of triethoxysilane to prepare a synthetic benzoxazine-bridged bis(triethoxysilane) compound (BS), a series of benzoxazine-bridged polysilsesquioxanes precursors were prepared with different degrees of hydrolysis condensation. Ring-opening thermal homopolymerization of the benzoxazine rings in these precursors was employed to obtain several organic–inorganic hybrid materials. The chemical structures of these synthetic compounds were characterized by Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (1H, 13C, 29Si NMR) spectroscopy and elemental analysis (EA). For the morphologies of these hybrid materials, different features of the lamellar structures were explored by transmission electron microscopy (TEM). From the results of energy dispersive X-ray spectroscopy (EDS), these lamellar structures were found to be comprised of carbon-rich (C-rich) and silicon-rich (Si-rich) layers. From a view-point of the structure–property relationships, the optical (photoluminescence (PL)), thermal (glass transition temperature (Tg) and thermogravimetric analysis (TGA)) properties were well characterized and discussed. Based on these results, we proposed that this chemically controllable method has potential as an effective approach used to prepare organic–inorganic hybrid materials with controllable morphology.
1. Introduction
In comparison with disordered nanostructured composites, ordered composites have been created with fantastic physical, chemical and biological properties, which display high potential in biomedicine, catalysis, sensor, energy conversion, and so on.1–5 In order to obtain ordered nanostructured composites, increasing research has been focused on the method of controllable self-organization.6–8 Recently, organic–inorganic hybrid materials constructed using this approach have gained great attention.9–12 Composite nanomaterials with well-defined structures have been extensively explored to realize a combination of the respective properties of each component or achieve cooperatively enhanced performance. Structural control of organic bridged silsesquioxanes has been previously reported concerning self-assembly behaviour.13–16 As a class of versatile functional organic–inorganic hybrid materials, organic bridged polysilsesquioxanes with tunable properties can be prepared via sol–gel processing of the organic bridged silsesquioxanes and have been widely studied.17–20 The organic group acts as a bridge for the silsesquioxane forming groups, is covalently attached to the trifunctional silicon groups through Si–C bonds, and can be varied in length, rigidity, geometry of substitution, and functionality. Due to the presence of the organic bridging group as an integral part of the material, some modulate bulk properties, such as porosity morphology,21 thermal stability,22 dielectric constant,13 optical clarity,23 chemical resistance,24 hydrophobicity,25 and refractive index,26 can be realized by this variability. Based on this enlightenment, by taking advantage of the self-organization ability of the organic bridged polysilsesquioxanes, we speculated whether it was feasible to obtain organic–inorganic hybrid materials with controllable morphology.We studied the methods of preparing organic bridged polysilsesquioxanes materials with long range order on the nanometer length scale; one promising approach is the use of monomers possessing organic bridging groups with intrinsic intermolecular affinity (e.g. hydrogen bonding, π–π interactions and even weak van der Waals forces) to produce materials with uniform nano-sized pores in the final product. Upon carefully controlled polymerization, the repeating units in the polymer are aligned to form an ordered nanostructure, which can be called the self-assembly without templates or “designed”.27–29 For this behavior of self-assembly, the combined effect of both organic bridging groups and the polysilsesquioxane moieties may result in the formation of a nano-scale morphology. In our previous studies,30,31 an investigation on benzoxazine was carried out. From a view-point of the structure–property relationships, the introduction of a heterocyclic benzoxazine moiety that can form strong intermolecular hydrogen bonds may be helpful for the self-assembly of bridged polysilsesquioxanes.31,32 Corriu et al.33 pointed out that polymerization of the bridging groups favored the arrangement of the organic fragments in the bridged polysilsesquioxanes. Hence, it is of interest to incorporate a benzoxazine group into the bridging unit of the bridged silsesquioxane compound for the preparation of organic–inorganic hybrid materials. We expect that thermally activated ring-opening addition polymerization can promote the formation of organic polymer domains; moreover, the appearance of a nano-size phase separation between organic domains and inorganic domains may be generated. Accordingly, we also speculated that some ordered nanostructures could be present in the inorganic–organic hybrid materials.
In this study, benzoxazine-bridged bis(triethoxysilane) (BS) was synthesized and its chemical structure was investigated by Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (1H, 13C and 29Si NMR) spectroscopy and elemental analysis (EA). Using the hydrolysis condensation of triethoxysilane to prepare BS, a series of benzoxazine-bridged polysilsesquioxanes precursors with different degrees of hydrolysis condensation were prepared and monitored using FTIR spectroscopy and EA. Differential scanning calorimetry (DSC) and FTIR spectroscopy were applied to investigate the ring-opening thermal homopolymerization process and the products of the precursors, respectively. The effects of the different degrees of hydrolysis condensation in BS on the morphologies and properties of the hybrid materials have been researched. As expected, the different features of the lamellar structures were explored using TEM of the hybrid materials. The significant results can be explained by a phase separation due to a synergistic effect of the polymerization-induced enrichment of the benzoxazine organic fragments and the self-organization enrichment of the inorganic polysilsesquioxanes. Furthermore, changes in the PL properties and thermal stabilities of the hybrid materials indirectly demonstrate the existence of the lamellar structure. Based on these results, we propose that this research will provide an effective approach to prepare hybrid materials with controllable morphology.
2. Experimental
2.1 Materials
Bisphenol A (98%), (3-aminopropyl)triethoxysilane (95%), paraformaldehyde (96%), calcium hydride (CaH2) (AR), tetramethylammonium hydroxide (TMAH) (AR) and aniline (99%) were purchased from Aladdin Chemical Reagent Co., Ltd. Chloroform and 4-dioxane (99.9%) were obtained from Tianjin Chemical Reagent Co., Ltd and dried with molecular sieves prior to use.2.2 Hybrid materials preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The reaction was carried out at room temperature with different hydrolysis times of 2 h, 4 h and 8 h. At the end of each reaction, the volatile components were removed immediately by the rotating evaporation instrument. The corresponding benzoxazine-bridged polysilsesquioxanes precursors were abbreviated as HBS2, HBS4 and HBS8. The colors of these products were as follows: HBS2 was translucent and yellow, HBS4 was opaque and brown-yellow and HBS8 was also opaque and brown.
2. The reaction was carried out at room temperature with different hydrolysis times of 2 h, 4 h and 8 h. At the end of each reaction, the volatile components were removed immediately by the rotating evaporation instrument. The corresponding benzoxazine-bridged polysilsesquioxanes precursors were abbreviated as HBS2, HBS4 and HBS8. The colors of these products were as follows: HBS2 was translucent and yellow, HBS4 was opaque and brown-yellow and HBS8 was also opaque and brown.2.3 Instrumental analysis
Proton (1H), carbon (13C) and silicon (29Si) nuclear magnetic resonance (NMR) spectra were measured on a Bruker AM-400S (400 MHz) spectrometer using tetramethylsilane (TMS) as an internal standard and deuterated chloroform (at 7.26 ppm) as solvent. Fourier transform infrared spectra (FTIR) were obtained on a Nicolet Avatar 320 FT-IR spectrophotometer. Samples were finely ground with KBr powder and pressed into disks. The quantitative analysis of C, H, N, O and Si were carried out on an F002 Heraeus CHN-O rapid elemental analyzer employing acetanilide as a standard. The dynamic homopolymerization reactions of these benzoxazine compounds were monitored on a Diamond/Pyris DSC (differential scanning calorimeter) under a dry nitrogen atmosphere. All the samples (about 10 mg) were heated from 50 °C to 300 °C at a heating rate of 10 °C min, during the study of the dynamic homopolymerization reactions. The morphologies and chemical components of the hybrid materials were characterized using a JEOL JEM-2010 TEM connected to energy dispersive X-ray spectrometer (EDS) at an acceleration voltage of 3 kV for elemental analysis. The electron beam energy was 50 keV in all cases. The samples were trimmed using an ultra-thin microtome machine, and the sectioned samples (ca. 50 nm in thickness) were placed on 200 mesh copper grids for observations. The thermal stabilities of the thermal homopolymerization samples were investigated using a Perkin-Elmer TGA-6 thermogravimetric analyzer at a heating rate of 10 °C min−1 from 50 °C to 800 °C under nitrogen atmosphere at a flow rate of 90 mL min−1 in all cases. The glass translation temperature (Tg) was determined using DSC under a dry nitrogen atmosphere. All the samples (about 10 mg) followed this thermal treatment process. First, they were quickly heated up to 330 °C for 5 min; second, they were cooled down to ambient temperature at a cooling rate of 20 °C min. After that, they were heated from ambient temperature to 330 °C at a heating rate of 20 °C min−1 again for the detection of the Tg. Photoluminescence spectra were measured on a Hitachi 4500 fluorescence spectrophotometer. The size of samples for the fluorescence test was 10 mm × 40 mm × 1 mm (width × length × thickness).3. Results and discussion
3.1 Preparation and characterization
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–N and O–C
2–N and O–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–N in the benzoxazine group, respectively. The characteristic resonance peaks of BS appear at 3.77 ppm for O–C
2–N in the benzoxazine group, respectively. The characteristic resonance peaks of BS appear at 3.77 ppm for O–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH3, 2.71 ppm for N–C
2–CH3, 2.71 ppm for N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2, 1.64 ppm for CH2–C
2–CH2, 1.64 ppm for CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2, 1.55 ppm for C–C
2–CH2, 1.55 ppm for C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, 1.19 ppm for CH2–C
3, 1.19 ppm for CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3 and 0.64 ppm for Si–C
3 and 0.64 ppm for Si–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2. The aromatic protons display multiple characteristic resonance peaks at 6.92–6.88, 6.79–6.76, and 6.64–6.60 ppm. In the corresponding 13C NMR spectrum shown in Fig. 1b, the resonances appearing at 50.5 ppm and 82.5 ppm were assigned to the methylene carbons in the benzoxazine group. The characteristic resonance peaks appear at 58.3 ppm for O–
2. The aromatic protons display multiple characteristic resonance peaks at 6.92–6.88, 6.79–6.76, and 6.64–6.60 ppm. In the corresponding 13C NMR spectrum shown in Fig. 1b, the resonances appearing at 50.5 ppm and 82.5 ppm were assigned to the methylene carbons in the benzoxazine group. The characteristic resonance peaks appear at 58.3 ppm for O–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–CH3, 54.2 ppm for N–
H2–CH3, 54.2 ppm for N–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–CH2, 21.6 ppm for CH2–
H2–CH2, 21.6 ppm for CH2–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–CH2, 41.5 ppm for CH3–
H2–CH2, 41.5 ppm for CH3–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –CH3, 31.1 ppm for C–
–CH3, 31.1 ppm for C–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3, 18.3 ppm for CH2–
H3, 18.3 ppm for CH2–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3 and 7.64 ppm for Si–CH2. The characteristic resonance peaks at 115.7, 119.5, 125.2, 126.2, 142.7, and 151.7 ppm were assigned to the aromatic carbons. The appearance of the characteristic resonance peaks for the protons and carbons in the benzoxazine group indicate that the structure contains a benzoxazine ring. If the ring is not formed, characteristic resonance peaks at chemical shifts of about 3.0–3.5 ppm are observed. There are no characteristic resonance peaks appearing at about 3.0–3.5 ppm, which also provides evidence for the formation of the benzoxazine ring. Moreover, BS displays only one resonance peak at about −45.1 ppm in the 29Si NMR spectrum (see Fig. 1c), suggesting the high purity of BS and also the absence of the hydrolysis reaction of Si–O–CH2CH3 in the preparation process of BS.
H3 and 7.64 ppm for Si–CH2. The characteristic resonance peaks at 115.7, 119.5, 125.2, 126.2, 142.7, and 151.7 ppm were assigned to the aromatic carbons. The appearance of the characteristic resonance peaks for the protons and carbons in the benzoxazine group indicate that the structure contains a benzoxazine ring. If the ring is not formed, characteristic resonance peaks at chemical shifts of about 3.0–3.5 ppm are observed. There are no characteristic resonance peaks appearing at about 3.0–3.5 ppm, which also provides evidence for the formation of the benzoxazine ring. Moreover, BS displays only one resonance peak at about −45.1 ppm in the 29Si NMR spectrum (see Fig. 1c), suggesting the high purity of BS and also the absence of the hydrolysis reaction of Si–O–CH2CH3 in the preparation process of BS.
 | ||
| Fig. 1 The NMR spectra of BS (a) 1H NMR; (b) 13C NMR and (c) 29Si NMR and the NMR spectrum of HBS8 (d) 29Si NMR. | ||
The FTIR spectrum of BS is shown in Fig. 2. The characteristic absorption at 1171 cm−1 was assigned to the asymmetric stretching of C–N–C. The characteristic absorptions at 1068 and 1238 cm−1 are attributed to the symmetric and asymmetric stretching of C–O–C. The bond at 1367 cm−1 suggests the wagging mode of CH2 groups in the benzoxazine structure. The absorptions at 956 cm−1, 1463 and 1491 cm−1 were attributed to benzene with an attached oxazine ring. The peak located at 1108 cm−1 is the characteristic absorption of Si–O–C. The absorption at 1600 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of benzene ring. The absorptions at 1463 and 1491 cm−1 were attributed to the stretching vibrations of the benzene ring attached to the oxazine ring. In addition, no peaks were observed at 3000–3500 cm−1, which also demonstrates that the structure contains a benzoxazine ring.
C stretching of benzene ring. The absorptions at 1463 and 1491 cm−1 were attributed to the stretching vibrations of the benzene ring attached to the oxazine ring. In addition, no peaks were observed at 3000–3500 cm−1, which also demonstrates that the structure contains a benzoxazine ring.
The elemental analysis results obtained for BS show that the elements' content values (C, 61.63%; H, 8.54%; N, 4.17%; O, 17.92% and Si, 7.62%) are in reasonable agreement with those of the theoretical values (C, 61.8%; H, 8.69%; N, 3.9%; O, 17.8% and Si, 7.81%).
 | ||
| Fig. 3 The non-isothermal DSC curves obtained for the benzoxazine-bridged polysilsesquioxanes precursors (BS, HBS2, HBS4 and HBS8). | ||
| Sample | Tp (°C) | ΔH (J g−1) |
|---|---|---|
| BS | 222 | −246 |
| HBS2 | 237 | −282 |
| HBS4 | 236 | −237 |
| HBS8 | 246 | −243 |
The FTIR spectra of the hybrid polybenzoxazine-bridged polysilsesquioxanes materials are displayed in Fig. 4, which further prove the bulky polysilsesquioxanes cages' steric hindrance influence on the thermal homopolymerization. After post-curing at 250 °C for 2 h, the absorptions for 1,2,3-trisubstituted benzene obtained via the ring-opening thermal homopolymerization were observed at about 1610 cm−1 (1618 cm−1 for PBS, 1620 cm−1 for PHBS2, 1636 cm−1 for PHBS4, and 1642 cm−1 for PHBS8) and 1496 cm−1. The intensities of the characteristic absorptions for the benzoxazine ring at 1232 cm−1 (for Ar–O–C), 939 cm−1 (for oxazine absorption), and 1600 and 1498 cm−1 (for the ortho-substituted benzene absorption) exhibit a distinct weakening. It was noted that these characteristic absorption peaks of the benzoxazine ring in PHBSn did not disappear completely except those in PBS. Hence, we can speculate that a handful of benzoxazine rings were probably wrapped in the polysilsesquioxanes during the hydrolysis condensation process, which could not be involved into the ring-opening thermal homopolymerization reaction during the polymerization stage of the diffusion-controlled process.
 | ||
| Fig. 6 Photoluminescent spectrum of the hybrid materials under excitation at 365 nm (PBS, PHBS2, PHBS4 and PHBS8). | ||
| Sample | Chemical composition content (%) | |||
|---|---|---|---|---|
| Grey domains | Gray domains | |||
| C | Si | C | Si | |
| PHBS2 | 18.7 | 30.2 | 68.6 | 6.9 |
| PHBS4 | 11.3 | 38.6 | 72.5 | 4.7 |
| PHBS8 | 6.5 | 48.3 | 75.8 | 1.8 |
The thermal degradation stabilities of the hybrid materials have been studied using TGA and the weight loss curves obtained for PBS, PHBS2, PHBS4 and PHBS8 are shown in Fig. 8. From the TG and DTG curves, the parameters for all of the samples, two onset degradation temperatures (Tds) evaluated by the temperature with 5% weight loss (T5%) and the temperature with 10% weight loss (T10%) and one maximum weight loss rate (Tmax) temperature are shown in Fig. 8b, and the residual char at 800 °C (Rc800) is listed in Table 3. The values of Tds, Tmax and Rc800, important parameters for evaluating the thermal degradation stability of polymer materials, for PBS are all lower than those obtained for PHBSn. For the three samples of PHBSn, the Tds, Tmax and Rc800 values obtained for PHBS8 are higher than those observed for PHBS2 and PHBS4. Between PHBS2 and PHBS4, there are no evident differences in the T5% values; however, the T10%, Tmax and Rc800 values obtained for PHBS2 are lower than those observed for PHBS4. According to the complete results, it can be proposed that the incorporation of polysilsesquioxanes can promote the enhancement of the thermal stability of these hybrid materials. Ishida et al.50 have acknowledged that the thermal stabilities of polybenzoxazine with secondary cross-linking mechanisms are expected since no new chemical functionalities have been incorporated in this series of compounds. To further significantly increase the char yield and thermal degradation stability, the possibility of forming more thermally stable cyclic structures should be introduced. Polysilsesquioxane possessing excellent thermal stability will play a significant role. One insight into the role played by the polysilsesquioxanes inorganic phase at T10% is its impact on the reticulated structure of the hybrid material.51,52 This structure restricts the movement of the polybenzoxazine chains, and hence retarding the onset of thermal decomposition improves the residual char properties of the hybrid materials. Due to the presence of the char layer with relatively high pyrolysis resistance, further degradation of the materials is retarded.
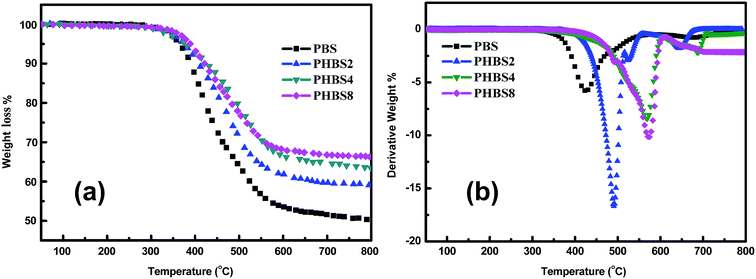 | ||
| Fig. 8 TG (a) and DTG (b) thermograms of the hybrid materials (PBS, PHBS2, PHBS4 and PHBS8) under nitrogen at a heating rate of 10 °C min−1. | ||
| Sample | Specific temperature of weight loss (°C) | Residual char at 800 °C (%) | ||
|---|---|---|---|---|
| T5% | T10% | Tmax | ||
| PBS | 355 | 386 | 421 | 50.1 |
| PHBS2 | 372 | 411 | 499 | 58.5 |
| PHBS4 | 378 | 440 | 568 | 63.7 |
| PHBS8 | 391 | 441 | 571 | 66.2 |
4. Conclusions
A series of inorganic–organic hybrid materials has been prepared via the thermal homopolymerization of benzoxazine-bridged polysilsesquioxanes precursors obtained from the controllable degree of hydrolysis condensation of silsesquioxanes. It was found that because of the application of this chemical controllable preparation method, the degree of the characteristic lamellar structure was increased upon increasing the degree of hydrolysis. Due to the chemical bridging between the polysilsesquioxanes and polybenzoxazine, phase separation is nanodimensional and well controlled. This significant result can be explained by the phase separation due to the synergistic effect of the polymerization-induced enrichment of the benzoxazine organic fragments and the self-organization enrichment of the inorganic polysilsesquioxanes. Upon increasing the degree of hydrolysis, the PL properties of the hybrid materials exhibited a weakening tendency due to the presence of the polysilsesquioxane cage effects. Furthermore, due to the incorporation of polysilsesquioxanes, the thermal stabilities of the hybrid materials were also improved. As expected, the nanodimensional inorganic phase layer was embedded in the organic phase layer and plays a key role in the structure–property relationships, which has been demonstrated by the changes in the optical and thermal properties of the hybrid materials. Hence, in view of the present situation, we propose that this research provides an effective approach to preparing hybrid materials with controllable morphology.Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (21406058).References
- M.-h. Sun, S.-z. Huang, L.-h. Chen, Y. Li, X.-y. Yang, Z.-y. Yuan and B.-l. Su, Chem. Soc. Rev., 2016, 45, 3479–3563 RSC.
- H.-y. Hsueh, C.-t. Yao and R.-m. Ho, Chem. Soc. Rev., 2015, 44, 1974–2018 RSC.
- Y.-h. Deng, Y. Cai, Z.-k. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D.-y. Zhao, J. Am. Chem. Soc., 2010, 132, 8466–8473 CrossRef CAS PubMed.
- Y.-f. Shen, J. Mater. Chem. A, 2015, 3, 13114–13188 CAS.
- Z.-q. Hu and G.-m. Chen, Adv. Mater., 2014, 26, 5950–5956 CrossRef CAS PubMed.
- X. Ji, K.-t. Lee and L.-f. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- T.-h. Kim, J. Hwang, W.-s. Hwang, J. Huh, H.-c. Kim, S.-h. Kim, J.-m. Hong, E.-l. Thomas and C. Park, Adv. Mater., 2008, 20, 522–527 CrossRef CAS.
- X.-m. Zhu, L.-q. Wang, J.-p. Lin and L.-s. Zhang, ACS Nano, 2010, 4, 4979–4988 CrossRef CAS PubMed.
- F. Liu, Y. Zhang, L. Xu and W.-a. Zhang, Chem.–Eur. J., 2015, 21, 5540–5547 CrossRef CAS PubMed.
- W.-a. Zhang and A. H. E. Müller, Prog. Polym. Sci., 2013, 38, 1121–1162 CrossRef CAS.
- C.-y. Gao and G.-m. Chen, Compos. Sci. Technol., 2016, 124, 52–70 CrossRef CAS.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- H.-c. Liu, W.-c. Su and Y.-l. Liu, J. Mater. Chem., 2011, 21, 7182–7187 RSC.
- T. Kishida, N. Fujita, K. Sada and S. Shinkai, J. Am. Chem. Soc., 2005, 127, 7298–7299 CrossRef CAS PubMed.
- A. Shimojima, Z. Liu, T. Ohsuna, O. Terasaki and K. Kuroda, J. Am. Chem. Soc., 2005, 127, 14108–14116 CrossRef CAS PubMed.
- X.-f. Zhou, S. Yang, C.-z. Yu, Z.-h. Li, X.-x. Yan, Y. Cao and D.-y. Zhao, Chemistry, 2006, 12, 8484–8490 CrossRef CAS PubMed.
- K. Yamamoto, Y. Nohara, Y. Domon, Y. Takahashi, Y. Sakata, J. Plévert and T. Tatsumi, Chem. Mater., 2005, 17, 3913–3920 CrossRef CAS.
- S. Yun, H.-j. Luo and Y.-f. Gao, J. Mater. Chem. A, 2015, 3, 3390–3398 CAS.
- L. Xu, V. R. Manda, L. E. McNamara, M. P. Jahan, H. Rathnayake and N. I. Hammer, RSC Adv., 2014, 4, 30172–30179 RSC.
- M. A. Wahab and J. N. Beltramini, RSC Adv., 2015, 5, 79129–79151 RSC.
- D. A. Loy and K. J. Shea, Chem. Rev., 1995, 95, 1431–1442 CrossRef CAS.
- U. Díaz, T. García, A. Velty and A. Corma, Chem.–Eur. J., 2012, 18, 8659–8672 CrossRef PubMed.
- D.-x. Wang, W.-y. Yang, L.-g. Li, X. Zhao, S.-y. Feng and H.-z. Liu, J. Mater. Chem. A, 2013, 1, 13549–13558 CAS.
- S.-m. Wu, T. Hayakawa, R. Kikuchi, S. J. Grunzinger and M.-a. Kakimoto, Macromolecules, 2008, 41, 3481–3487 CrossRef CAS.
- Y. Kaneko, H. Imamura, T. Sugioka and Y. Sumida, Polymer, 2016, 92, 250–255 CrossRef CAS.
- Y.-c. Gan, X.-s. Jiang and J. Yin, J. Mater. Chem. C, 2014, 2, 5533–5539 RSC.
- H. E. Romeo, M. A. Fanovich, R. J. J. Williams, L. Matějka, J. Pleštil and J. Brus, Macromolecules, 2007, 40, 1435–1443 CrossRef CAS.
- J. P. Randall, M. A. B. Meador and S. C. Jana, ASC Appl. Mater. Interfaces, 2011, 3, 613–626 CrossRef CAS PubMed.
- Y. Fujimoto, M. Heishi, A. Shimojima and K. Kuroda, J. Mater. Chem., 2005, 15, 5151–5157 RSC.
- Z.-e. Fu, K. Xu, X. Liu, J. Wu, C.-j. Tan and M.-c. Chen, Macromol. Chem. Phys., 2013, 214, 1122–1130 CrossRef CAS.
- Z.-e. Fu, H. Liu, H.-l. Cai, X. Liu, Y. Guo, K. Xu and M.-c. Chen, Polym. Eng. Sci., 2012, 52, 2473–2481 CAS.
- P. Velez-Herrera, K. Doyama, H. Abe and H. Ishida, Macromolecules, 2008, 41, 9704–9714 CrossRef CAS.
- R. J. P. Corriu, J. J. E. Moreau, P. Thepot and M. W. C. Man, Chem. Mater., 1996, 8, 100–106 CrossRef CAS.
- H. Ishida and Y. L. Hong, J. Appl. Polym. Sci., 1998, 69, 2559–2567 CrossRef CAS.
- X. Ning and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1121–1129 CrossRef CAS.
- B. P. Pichon, M. Wong Chi Man, P. Dieudonné, J.-L. Bantignies, C. Bied, J.-L. Sauvajol and J. J. E. Moreau, Adv. Funct. Mater., 2007, 17, 2349–2355 CrossRef CAS.
- J. Peng, K. Xu, H.-l. Cai, J.-c. Wu, W.-h. Lin, Z.-w. Yu and M.-c. Chen, RSC Adv., 2014, 4, 7124–7131 RSC.
- Y. Kaneko, M. Shoirikia and T. Mizumob, J. Mater. Chem., 2012, 22, 14475–14478 RSC.
- H. Ghanbari, B. G. Cousins and A. M. Seifalian, Macromol. Rapid Commun., 2011, 32, 1032–1046 CrossRef CAS PubMed.
- C.-h. Chou, S.-L. Hsu, K. Dinakaran, M.-y. Chiu and K.-h. Wei, Macromolecules, 2005, 38, 745–751 CrossRef CAS.
- Y. Gao, C.-l. He, Y.-g. Huang and F.-l. Qing, Polymer, 2010, 51, 5997–6004 CrossRef CAS.
- L.-g. Li, R.-d. Liang, Y.-j. Li, H.-z. Liu and S.-y. Feng, J. Colloid Interface Sci., 2013, 406, 30–36 CrossRef CAS PubMed.
- Y.-w. Li, K. Guo, H. Su, X.-p. Li, X.-y. Feng, Z. Wang, W. Zhang, S.-s. Zhu, C. Wesdemiotis, S. Z. D. Cheng and W.-b. Zhang, Chem. Sci., 2014, 5, 1046–1053 RSC.
- C.-x. Liu, Z.-q. Li and Y.-g. Wang, J. Sol-Gel Sci. Technol., 2016 DOI:10.1007/s10971-016-4219-5.
- P. Velez-Herrera, K. Doyama, H. Abe and H. Ishida, Macromolecules, 2008, 41, 9704–9714 CrossRef CAS.
- M. L. Gómez, D. P. Fasce, R. J. J. Williams, H. A. Montejano and C. M. Previtali, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 289–296 CrossRef.
- M. A. Zaitoun, T. Kim and C. T. Lin, J. Phys. Chem. B, 1998, 102, 1122–1125 CrossRef CAS.
- X. Yang, L. Liu, C. He, W.-s. Chin and T. Lin, J. Mater. Chem., 2006, 16, 829–836 RSC.
- P. M. And and S. L. Wunder, Chem. Mater., 2002, 14, 4494–4497 CrossRef.
- H. Ishida and D. P. Sanders, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 3289–3301 CrossRef CAS.
- H. W. P. Carvalho, A. F. Suzana, C. V. Santilli and S. H. Pulcinelli, Polym. Degrad. Stab., 2014, 104, 112–119 CrossRef CAS.
- N. Katsikis, F. Zahradnik, A. Helmschrott, H. Munstedt and A. Vital, Polym. Degrad. Stab., 2007, 92, 1966–1976 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |





