DOI:
10.1039/C6RA26028D
(Paper)
RSC Adv., 2017,
7, 2650-2657
Co-immobilization of 1-vinyl-3-octadecylimidazolium cations and p-styrenesulphonate anions on silica and their anti-interference performance for the adsorption of naphthols†
Received
30th October 2016
, Accepted 24th November 2016
First published on 12th January 2017
Abstract
Adsorptive removals of naphthols are often interfered by some coexisting anions of aqueous solutions. In this work, 1-vinyl-3-octadecylimidazolium cations and p-styrenesulphonate anions were chemically co-modified on silica (denoted as Sil-PImC18-SS) via the click chemistry reaction and were characterized. This material was created with the aim to explore the feasibility of removal of 1-naphthol (1-NAP) and 2-naphthol (2-NAP) from aqueous solutions. The prepared Sil-PImC18-SS was used as an adsorbent to remove 1-NAP and 2-NAP from aqueous solutions and the effects of various variables for the adsorption of 1-NAP and 2-NAP were systematically studied. Among the coexisting anions tested, 0.2 mol L−1 background NaCl or Na2SO4 aqueous solutions had no significant effect on the adsorption of 1-NAP and 2-NAP. The adsorption behaviors of 1-NAP and 2-NAP were well described by the pseudo-second order kinetic and the Dubinin–Radushkevich models with the maximum adsorption capacities of 84.4 mg g−1 and 71.9 mg g−1 for 1-naphthol and 2-naphthol, respectively. The adsorption was a spontaneous and exothermic process, and an adsorption mechanism was speculated to be the cooperative contributions from the hydrophobic interaction, hydrogen bonding interaction, and π–π stacking. Thus, Sil-PImC18-SS can be potentially used as an effective adsorption material for naphthol pollutant removal from the water environment.
Introduction
1-Naphthol (1-NAP) and 2-naphthol (2-NAP) are position isomers and differ in the location of the hydroxyl group on the carbon skeleton of naphthalene. Since naphthols are widely used as raw materials or intermediates in many chemical industries, such as dyes, pigments, pharmaceuticals, pesticides, plastics, and synthetic rubber, they are present in the waste effluents discharged from these industries.1 Moreover, naphthols are formed in the environment by the biodegradation of phenanthrene and Reactive Red 2.2,3 1-NAP and 2-NAP often coexist in wastewaters, have detrimental effects on the environment and human health, and have been considered as ubiquitous environmental carcinogens.4,5 Naphthols, as persistent organic pollutants, are resistant to environmental degradation through biological, chemical or photolytic processes. Therefore, the removal of naphthols from wastewater will be of great significance.
There are a number of separation methods for naphthol removal including biodegradation, photodegradation, wet air oxidation, ozone degradation, adsorption, and other methods.6–9 Among these, adsorption has gained more and more attention due to its low-cost, high efficiency, and easy operation. As a result, many adsorbents, such as activated carbon,10 carbon black,11 carbon nanotubes (CNTs),7 graphene, graphene oxide and reduced graphene oxide (RGO),1,12 bamboo hydrochars,13 and montmorillonite,14 have been investigated. In recent years, polymeric ionic liquids (PILs) prepared from ionic liquid (IL) monomers combine the unique properties of ILs together with intrinsic polymer characteristics, such as thermal and chemical stability, improved processability, durability, and spatial controllability.15 Based on these advantages, PILs have been immobilized on different supports, such as silicas,16 RGO,17 CNTs,18 metal–organic frameworks (MOFs),19 and polyhedral oligomeric silsesquioxanes (POSSs),20 for various specific applications. The preparation methods for these PILs-grafted materials mainly include the immobilization of only cation monomers, anion monomers, or both on the supports.16 In particular, for the immobilization of only anion or cation monomers of ILs on the supports, ion exchange easily happens between their corresponding counter ions and other ionic species in the explored medium, whereas co-immobilization can avoid this exchange. In other words, these PILs-grafted materials prepared by the co-immobilization method can have the ability to suppress the interferences of ionic species in water environments in terms of adsorbents;21 to date, little research on the use of these co-immobilization materials as adsorbents to remove the pollutants from water environments has been reported.
Considering the inherent properties of 1-NAP and 2-NAP, such as weak acidity, hydrophobicity, and aromaticity (Table 1),22–25 there is a chance of the removal of naphthols from the aqueous solutions using the co-immobilized PILs-modified silica adsorbents due to their tunable selectivity and multiple beneficial physical characteristics towards the target adsorbate, and the interferences coming from the anions in water environments for adsorption can be simultaneously inhibited. Theoretically, 1-vinyl-3-octadecylimidazolium cations should provide most types of interactions, including strong hydrophobic interactions, π–π stacking, hydrogen bonding, and electrostatic interactions, whereas p-styrenesulphonate anions exhibit additional π–π interactions. Thus, Sil-PImC18-SS prepared from 1-vinyl-3-octadecylimidazolium cation and p-styrenesulphonate anion monomers should exhibit good adsorption performance for 1-NAP and 2-NAP. In this study, the main objective was to explore the feasibility of removal of 1-NAP and 2-NAP from the aqueous solutions using Sil-PImC18-SS as the adsorbent and to elucidate the adsorption mechanism. The effects of various parameters, such as pH, initial concentrations of 1-NAP and 2-NAP, temperature, contact time, and ionic strength, were investigated in detail. Kinetics, isotherm models, and thermodynamics of the adsorption were also studied.
Table 1 Selected physicochemical properties of 1-NAP and 2-NAPa22–25
| Adsorbate |
Chemical structure |
S |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW KOW |
pKa |
| S: aqueous solubility, mmol L−1; KOW: n-octanol–water partition coefficient; pKa: acid dissociation constant. |
| 1-NAP |
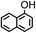 |
6.00 |
2.85 |
9.34 |
| 2-NAP |
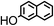 |
6.94 |
2.71 |
9.5 |
Experimental
Materials
1-Vinylimidazole (99%), 1-bromooctadecane (97%), sodium p-styrenesulphonate (90%), 3-mercaptopropyltrimethoxysilane (95%), 1-NAP, and 2-NAP were purchased from Aladdin Chemistry Co. Ltd. Azobisisobutyronitrile (AIBN) was obtained from Guangfu Institute of Fine Chemicals (Tianjin, China) and purified by recrystallization from methanol before use. Silica gel (200–300 mesh), 2-nitrophenol (2-NP), 3-nitrophenol (3-NP), 4-nitrophenol (4-NP) and 2,4-dinitrophenol (2,4-DNP), toluene, chloroform, and acetonitrile were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and the solvents were further dried before use. Dry toluene was obtained by distillation after toluene was dried with calcium chloride and further dried by standing with sodium. Dry chloroform was obtained by distillation after drying over calcium chloride and anhydrous calcium sulfate. Dry acetonitrile was obtained by distillation after drying with anhydrous calcium chloride and phosphoric anhydride.
Preparation of Sil-PImC18-SS
According to the reported studies,16 the synthesis method includes four steps. First, 1-vinyl-3-octadecylimidazolium bromide ([C18VIm]Br) was synthesized. 1-Bromooctadecane (18.33 g) was dissolved in dry acetonitrile, and then 4.71 g 1-vinylimidazole was added dropwise. After this, the mixture was mixed at 60 °C for three days. [C18VIm]Br was obtained after being recrystallized in cold diethyl ether and dried under vacuum for 12 h at 30 °C.
Second, 1-vinyl-3-octadecylimidazolium p-styrenesulphonate ([C18VIm]SS) was synthesized by the exchange of bromide to p-styrenesulphonate. [C18VIm]Br (5 g) and sodium p-styrenesulphonate (2.5 g) were dissolved in 50 mL water. The mixture was mixed at room temperature for two days. After this, white [C18VIm]SS was obtained after being washed with water and dried under vacuum at 30 °C.
Third, mercaptopropyl-modified silica (Sil-MPS) was prepared. Silica gel was first activated by immersing in 6 mol L−1 hydrochloric acid for 24 h, and then washed with water and dried at 100 °C for 12 h. Activated silica gel (6 g) was dispersed in 30 mL dry toluene and 3 g 3-mercaptopropyltrimethoxysilane was added dropwise. The mixture was mixed for 36 h at 125 °C. The obtained Sil-MPS was washed in turn with toluene, methanol, water, methanol and diethyl ether, and then dried for 12 h at 60 °C.
Finally, 1-vinyl-3-octadecylimidazolium p-styrenesulphonate was immobilized onto the silica surface via the click chemistry reaction between the vinyl groups of 1-vinyl-3-octadecylimidazolium p-styrenesulphonate and thiol groups of Sil-MPS. Sil-MPS (2.8 g) and [C18VIm]SS (2 g) were dissolved in 30 mL dry chloroform, and then 0.02 g AIBN was added. After this, the mixture was mixed under nitrogen atmosphere for 30 h at 60 °C. Then, the adsorbent, Sil-PImC18-SS, was obtained after being washed with chloroform, methanol, and diethyl ether. The detailed preparation procedure is described in Fig. S1.†
Characterization
Elemental analyses were carried out using a Vario EL elemental analyzer (Germany). Two parallel analyses were made for each material. The surface morphology was examined using a scanning electron microscope (JEOL, JSM-6390 LV). The Fourier transform infrared (FTIR) spectra were obtained using a Perkin-Elmer-983 IR spectrophotometer in the range of 400–4000 cm−1. Thermogravimetric analysis (STA449C, Netzsch, Germany) was performed with a heating rate of 10 °C min−1 under nitrogen. N2 adsorption–desorption experiments were carried out using a Quantachrome NOVA 2000e sorption analyzer at liquid nitrogen temperature (77 K). The specific surface area (SBET) was estimated by the linear part of the Brunauer–Emmett–Teller (BET) equation, and the pore size distribution was calculated by the Barrett–Joyner–Halenda (BJH) method. The UV-visible spectra of Sil-PImC18-SS before and after the adsorption of 1-NAP and 2-NAP were obtained using a Lambda 950 UV/vis/NIR spectrophotometer (Perkin Elmer).
Adsorption experiments
An aqueous solution of 1-NAP or 2-NAP was freshly prepared by dissolving 1-NAP or 2-NAP in deionized water. Negligible volumes of 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH aqueous solutions were used to adjust the pH value of the aqueous solutions. The adsorption behaviors of 1-NAP and 2-NAP on the adsorbent were investigated using a conventional batch equilibrium technique. A predetermined amount of an adsorbent and 50 mL aqueous solutions with different 1-NAP or 2-NAP concentrations was added to 100 mL conical flasks that were shaken for 5 h to ensure complete adsorption in a THZ-82(A) thermostatic water-bath shaker at 160 rpm (Jintan Scientific Analytical Instrument Co. Ltd., China). After adsorption for a predetermined time, the adsorbent was removed from the mixture by filtration. The concentrations of 1-NAP or 2-NAP in the supernatant were analyzed by a UV/visible spectrophotometer (UV-5100, Shanghai Metash Instruments Co. Ltd., China) at the appropriate optimum UV wavelengths of 293 and 327 nm for 1-NAP and 2-NAP, respectively. The adsorbed amounts of 1-NAP or 2-NAP were calculated by eqn (1).where Qe (mg g−1) is the equilibrium adsorption capacity of 1-NAP or 2-NAP on the adsorbent. C0 and Ce are the initial concentration and the equilibrium concentration of 1-NAP or 2-NAP, respectively. V (L) presents the volume of the solution and W (g) is weight of the adsorbent.
Blank experiments without 1-NAP or 2-NAP were simultaneously carried out to correct the results. In addition, control experiments containing 1-NAP or 2-NAP without the adsorbent were carried out to evaluate the solute losses. The experimental results showed that such losses were negligible.
Results and discussion
Characterization of Sil-PImC18-SS
The elemental compositions of Sil-MPS and Sil-PImC18-SS were C: 5.01%, H: 1.66%; and C: 27.49%, H: 4.39%, N: 1.81%, respectively. Compared to Sil-MPS, the percentages of carbon for Sil-PImC18-SS increased from 5.01% to 27.49%, and this increase in the carbon percentage (22.48%) should be attributed to the successful copolymerization of 1-vinyl-3-octadecylimidazolium p-styrenesulphonate onto the silica substrate. The C/N ratio was 12.42, which was almost equal to the initial C/N ratio (13.28) for 1-vinyl-3-octadecylimidazolium p-styrenesulphonate. This indicated that the anion and cation of 1-vinyl-3-octadecylimidazolium p-styrenesulphonate were simultaneously copolymerized to Sil-MPS with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The elemental analysis data proved that the immobilization on the surface was successful.
1. The elemental analysis data proved that the immobilization on the surface was successful.
The surface morphologies of the activated silica, Sil-MSP, and Sil-PImC18-SS were examined by SEM. As observed in Fig. 1, the surface of the activated silica was relatively smooth; however, Sil-MSP possessed a rough surface. The image of Sil-PImC18-SS showed a granular structure, suggesting that the PILs were successfully grafted onto the surface of activated silica. The FTIR spectra of activated silica, Sil-MSP, and Sil-PImC18-SS are shown in Fig. S2.† The typical peaks of Si–O–Si were observed around 802 and 1100 cm−1. For activated silica, the characteristic peak at 970 cm−1 was attributed to the presence of silanol groups. For Sil-MSP, the peak at 970 cm−1 disappeared, which indicated that the silanol groups had reacted with 3-mercaptopropyltrimethoxysilane. The peak at 689 cm−1 means the presence of C–S. The peak around 2934 cm−1 was assigned to the C–H stretches of tetrahedral carbon. For the Sil-PImC18-SS, the peak at 1008 cm−1 indicated the presence of sulfonate group. The peaks around 2923 and 2853 cm−1 were due to the C–H stretching of the long alkyl chain. The peaks near 1412, 1472, and 1639 cm−1 were attributed to the aromatic benzene, and the peak around 1565 cm−1 indicated the presence of the imidazole ring. From the FTIR spectrum, it can be concluded that the cation and anion of 1-vinyl-3-octadecylimidazolium p-styrenesulphonate were successfully copolymerized onto the silica.
 |
| | Fig. 1 SEM images of activated silica (A), Sil-MSP (B), and Sil-PImC18-SS (C). | |
N2 adsorption–desorption experiments were performed at 77 K (Fig. 2). Compared with activated silica, a significant decrease in the BET surface area and pore volume of Sil-MPS was observed (Table 2). Moreover, compared with Sil-MPS, the BET surface area and pore volume of Sil-PImC18-SS significantly decreased. Thus, compared with activated silica, the average pore diameter of Sil-MPS and Sil-PImC18-SS significantly decreased. The decrease in the porosity was attributed to the extra bulk of 1-vinyl-3-octadecylimidazolium styrenesulfonate.
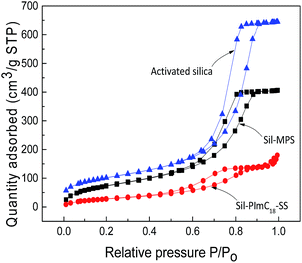 |
| | Fig. 2 N2 adsorption/desorption isotherms of activated silica, Sil-MSP, and Sil-PImC18-SS. | |
Table 2 Textural properties of activated silica, Sil-MSP, and Sil-PImC18-SS
| Materials |
BET surface area (m2 g−1) |
Volume of pore (cm3 g−1) |
Average pore diameter (nm) |
| Activated silica |
357.6 |
0.9995 |
9.6625 |
| Sil-MSP |
278.4 |
0.6126 |
7.2690 |
| Sil-PImC18-SS |
107.8 |
0.2729 |
7.9228 |
The thermogravimetric curves for activated silica, Sil-MPS, and Sil-PImC18-SS are presented in Fig. S3.† For activated silica, the mass loss in the region from 200 to 800 °C was 2.9%, which can be attributed to the condensation of silanol groups. For Sil-MPS, a higher mass loss between 200 and 800 °C was observed (14.5%), which can be attributed to the loss of mercaptopropyl attached to the surfaces of silica. Compared with Sil-MPS, the mass losses between 200 and 800 °C increased from 14.5% to 40.9%; the increase in the mass loss was attributed to the successful immobilization of 1-vinyl-3-octadecylimidazolium p-styrenesulphonate onto the silica surface.
Adsorption properties of activated silica and Sil-PImC18-SS
The adsorption of 1-NAP and 2-NAP onto activated silica and Sil-PImC18-SS is presented in Fig. 3. It can be seen from the figure that the amount of adsorption of Sil-PImC18-SS for 1-NAP and 2-NAP significantly increased as compared to that of activated silica. Fig. 4 further compares the UV-visible spectra of Sil-PImC18-SS before and after the adsorption of 1-NAP and 2-NAP, respectively. It was obvious that the characteristic adsorption peak of 1-NAP (298 nm) and 2-NAP (331 nm) appeared after adsorption; this phenomenon indicated that 1-NAP and 2-NAP could be efficiently adsorbed onto Sil-PImC18-SS.
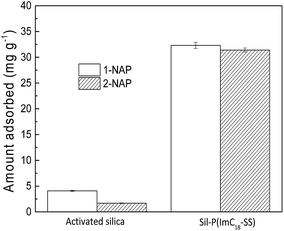 |
| | Fig. 3 Comparison of the adsorption performance of activated silica and Sil-PImC18-SS (experimental conditions: volume of aqueous solution, 50 mL; initial concentration, 50 mg L−1; adsorbent mass, 30 mg; shaking speed, 160 rpm; shaking time, 5 h; and temperature, 30 °C). | |
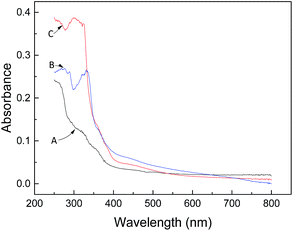 |
| | Fig. 4 UV-visible spectra of Sil-PImC18-SS before (A) and after the adsorption of 1-NAP (C) and 2-NAP (B). | |
Selectivity of Sil-PImC18-SS
To investigate the selectivity of Sil-PImC18-SS, 2-NP, 3-NP, 4-NP, and 2,4-DNP were used as adsorbates. The adsorption experiments were performed in 50 mL of 50 mg L−1 of each nitrophenols aqueous solutions containing 30 mg of Sil-PImC18-SS and shaken at a constant speed of 160 rpm at 30 °C for 5 h. The obtained Qe was 11.3, 10.6, 10.2, and 9.6 mg g−1 for 2-NP, 3-NP, 4-NP, and 2,4-DNP, respectively. Compared with the Qe of Sil-PImC18-SS for 1-NAP and 2-NAP, the four nitrophenols demonstrated lower adsorption amounts. This could be attributed to their different molecular structures. They all have a hydroxyl group on their individual molecular structure. For 1-NAP and 2-NAP, there are two benzene rings. For these nitrophenols, they not only have one benzene ring, but also contain an electron-withdrawing nitro functional group that decreases the electron density of the aromatic ring. The smaller electron density in the aromatic ring supplies less π-electrons to interact with the adsorbent, and accordingly, accounts for the lower amount of adsorption for the four nitrophenols on Sil-PImC18-SS as compared to that of 1-NAP or 2-NAP.
Effect of pH
Adsorptions of 1-NAP and 2-NAP on Sil-PImC18-SS were carried out in the pH range of 2–11. As can be seen in Fig. 5, the adsorption of 1-NAP on Sil-PImC18-SS exhibited the same trend as that of 2-NAP, and the adsorption capacities of 1-NAP and 2-NAP slightly decreased at pH < 8, whereas these obviously decreased at pH > 9.26 For weakly acidic compounds, such as 1-naphthol and 2-naphthol, the occurrence of their nondissociated and dissociated species depends on the pH value of the aqueous solution and their dissociation constants (pKa). The content of nondissociated and dissociated species can be calculated according to eqn (2) and (3), respectively.| | |
fN = 1/(1 + 10pH−pKa)
| (2) |
where fN and f are the content of the nondissociated and dissociated species, respectively. The distributions of the nondissociated and dissociated species for 1-NAP and 2-NAP in the aqueous solution were predicted as a function of solution pH values and are shown in Fig. S4,† respectively. 1-NAP and 2-NAP adsorption on Sil-PImC18-SS exhibited the same trend as that of the dissociation curves of 1-NAP and 2-NAP in the aqueous solution, which displayed that the molecular states of 1-NAP and 2-NAP were suitable for the adsorption, whereas the ionic states were not suitable. Moreover, the adsorption capacity of 1-NAP on Sil-PImC18-SS was larger than that of 2-NAP in the investigated pH range. According to the experimental results, octanol–water partition coefficient, and water solubility of 1-NAP and 2-NAP (shown in Table 1), it can be concluded that the larger KOW and the smaller solubility would benefit the naphthol adsorption onto Sil-PImC18-SS. This adsorption phenomenon was attributed to the possible hydrophobic interactions, hydrogen bonding interactions (N atoms of imidazolium as the hydrogen bonding acceptors and –OH of naphthols as donors), and π–π stacking between Sil-PImC18-SS and 1-NAP and 2-NAP.27 The adsorbent, Sil-PImC18-SS, not only has a long octadecyl chain to increase the hydrophobicity, but also contains imidazolium groups to incorporate the hydrogen bonding interactions and π–π stacking. The naphthols, 1-NAP and 2-NAP, not only have two benzene rings to increase the hydrophobicity, but also contain a –OH group to incorporate the hydrogen bonding interactions. The hydroxyl groups are strong electron donors that make the naphthalene ring of 1-NAP and 2-NAP become π-electron rich, thus facilitating π–π interactions between the naphthalene ring of 1-NAP and 2-NAP and the phenyl and imidazolium rings of Sil-PImC18-SS. At high pH, –OH group of 1-NAP and 2-NAP would be ionized and their polarity would increase. Thus, the hydrophobic interactions between anionic 1-NAP and 2-NAP and the adsorbent would be significantly weakened. At the same time, hydrogen bonding interactions between the –OH group of 1-NAP and 2-NAP (as donors) and the N-containing group of Sil-PImC18-SS (as acceptors) would also be significantly impeded due to the ionization of the –OH group of 1-NAP and 2-NAP. Therefore, the adsorption capacities of 1-NAP and 2-NAP on Sil-PImC18-SS decreased with the increase of pH value.
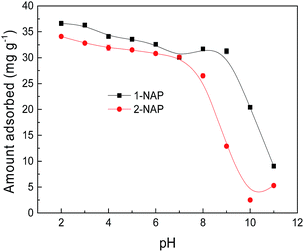 |
| | Fig. 5 Effect of pH on the adsorption of 1-NAP and 2-NAP. | |
Effect of coexisting anions
1-NAP and 2-NAP are always present in waste water with some other ionic species, especially anions, which can affect the adsorption performance of Sil-PImC18-SS.28 Therefore, the effect of inorganic salts, such as NaCl and Na2SO4, on the adsorption ability of Sil-PImC18-SS for 1-NAP and 2-NAP from aqueous solutions was investigated, and the results are depicted in Fig. 6. Fig. 6 shows that the adsorption of 1-NAP and 2-NAP was not affected when the background NaCl and Na2SO4 concentration increased from 0 to 0.2 mol L−1.
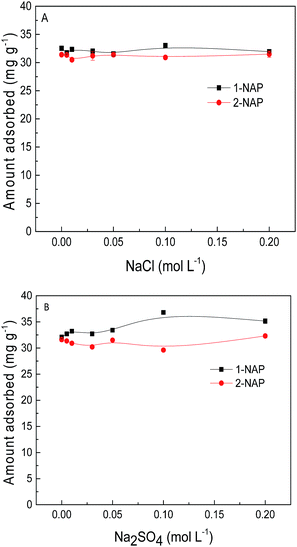 |
| | Fig. 6 Effect of ionic strength ((A) NaCl and (B) Na2SO4) on the adsorption of 1-NAP and 2-NAP. | |
The excellent adsorption performance of Sil-PImC18-SS for 1-NAP and 2-NAP under high ionic strength was attributed to the cooperative contributions of hydrophobic, hydrogen bonding, and π–π interactions.
Adsorption kinetics
The adsorption of 1-NAP and 2-NAP by Sil-PImC18-SS over different contact times was investigated, as presented in Fig. 7. It can be seen that the adsorption equilibrium was reached within 300 min and no significant change was observed from 300 to 540 min. Therefore, a contact time of 300 min was selected for the sure establishment of the adsorption equilibrium in the further adsorption experiments. To evaluate the adsorption kinetics of 1-NAP and 2-NAP onto Sil-PImC18-SS, the kinetic data were further statistically investigated using the pseudo-first order, pseudo-second order, and intra-particle kinetic models, as shown in eqn (4), (5) and (6), respectively.29,30| |
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (4) |
| | |
t/Qt = 1/(Qe2k2) + t/Qe
| (5) |
| |
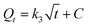 | (6) |
where Qe and Qt are the adsorption capacities at equilibrium and at time t, respectively (mg g−1). k1 (min−1), k2 (g mg−1 min−1), and k3 (mg g−1 min−1/2) are the pseudo-first order rate constant, pseudo-second order rate constant, and the diffusion rate constant, respectively. C (mg g−1) is a constant.
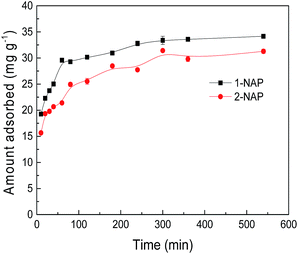 |
| | Fig. 7 Effect of contact time on the adsorption of 1-NAP and 2-NAP. | |
The values of Qe and k2 can be calculated from the slopes and intercepts of the pseudo-second order kinetic plots using eqn (5). When t → 0, the initial adsorption rate, h, can be defined as eqn (7).
Table 3 summarizes the corresponding model parameters. The correlation coefficients suggest that the adsorption process was most suitably fitted by the pseudo-second order equation (R2: 0.9993 for 1-NAP, 0.9931 for 2-NAP) when compared to the pseudo-first order equation (R2: 0.9612 for 1-NAP, 0.8493 for 2-NAP) and the intra-particle kinetic equation (R2: 0.7432 for 1-NAP, 0.9166 for 2-NAP). Moreover, the calculated equilibrium adsorption capacities (Qe,cal: 34.1 mg g−1 for 1-NAP, 34.4 mg g−1 for 2-NAP) were close to the experimental values.
Table 3 Kinetic parameters for the adsorption of 1-NAP and 2-NAPa
| Models |
Parameters |
1-NAP |
2-NAP |
| (Qe,exp: 34.2 mg g−1 for 1-NAP, 34.7 mg g−1 for 2-NAP), which further confirmed the applicability of the pseudo-second order rate equation for the adsorption of 1-NAP and 2-NAP onto Sil-PImC18-SS. |
| Pseudo-first order model |
Qe,exp (mg g−1) |
34.2 |
34.7 |
| k1 (min−1) |
0.0089 |
0.0034 |
| Qe,cal (mg g−1) |
12.4 |
14.9 |
| R2 |
0.9612 |
0.8493 |
| Pseudo-second order model |
k2 (g mg−1 min−1) |
0.0026 |
0.0009 |
| Qe,cal (mg g−1) |
34.1 |
34.4 |
| h (mg g−1 min−1) |
3.0168 |
1.0894 |
| R2 |
0.9993 |
0.9931 |
| Intra-particle diffusion model |
k3 (mg g−1 min−1/2) |
0.5627 |
0.7317 |
| C (mg g−1) |
20.8 |
15.9 |
| R2 |
0.7432 |
0.9166 |
Adsorption isotherms
Fig. 8 shows the adsorption isotherms of 1-NAP and 2-NAP onto Sil-PImC18-SS. The equilibrium adsorption data were analyzed by the most common Langmuir (eqn (8)) and Freundlich (eqn (9)) models.31,32where Ce (mg L−1) is the 1-NAP or 2-NAP concentration at equilibrium, Qe (mg g−1) is the equilibrium adsorption capacity of 1-NAP or 2-NAP by the adsorbent, Qm is the monolayer adsorption capacity (mg g−1), b is the Langmuir constant, KF and 1/n are the constants (indicative of the relative adsorption capacity of the adsorbent and the constant indicative of the heterogeneity factor of the Freundlich adsorption isotherm, respectively).
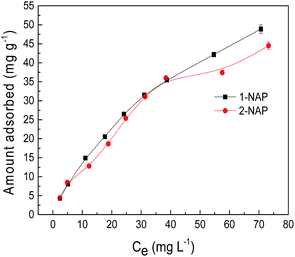 |
| | Fig. 8 Adsorption isotherms of 1-NAP and 2-NAP. | |
The relative parameters calculated from the Langmuir and Freundlich models are presented in Table 4. According to the correlation coefficients, the adsorption isotherms fitted well with those of the Langmuir model. The maximum adsorption capacities (Qm) fitted by the Langmuir model for 1-NAP and 2-NAP were 84.4 and 71.9 mg g−1, respectively. Compared to the adsorption capacities of 1-NAP and 2-NAP on other reported adsorbents (Table 5), Sil-PImC18-SS was a suitable material for the removal of 1-NAP and 2-NAP from the aqueous solutions.
Table 4 Langmuir, Freundlich, and Dubinin–Radushkevich isotherm constants
| Models |
Parameters |
1-NAP |
2-NAP |
| Langmuir |
Qm (mg g−1) |
84.4 |
71.9 |
| b (L mg−1) |
0.0189 |
0.0218 |
| R2 |
0.9987 |
0.9748 |
| Freundlich |
KF (mg g−1)(mg L−1)n |
3.3684 |
3.5332 |
| 1/n |
0.6343 |
0.5979 |
| R2 |
0.9922 |
0.9577 |
| Dubinin–Radushkevich |
Qm,DR (mmol g−1) |
3.407 |
2.6218 |
| KDR (mol2 kJ−2) |
0.0062 |
0.0058 |
| E (kJ mol−1) |
9.0112 |
9.3099 |
| R2 |
0.9989 |
0.9841 |
Table 5 Qm values of adsorbents reported in the literature for the adsorption of 1-NAP and 2-NAP
| Adsorbents |
Qm (mg g−1) |
Reference |
| 1-NAP |
2-NAP |
| Sil-PImC18-SS |
84.4 |
71.9 |
This study |
| 2c-G-MWCNTs |
37.74 |
— |
7 |
| Sludge-based activated carbon |
— |
111.9 |
10 |
| GO/ARG/CRG |
57.7–282 |
— |
12 |
| Anion–cation organopalygorskite |
— |
33.65 |
30 |
| Oxidized MWCNTs |
30.57 |
— |
31 |
| Fe3O4@polyaniline |
28.74 |
9.13 |
33 |
| Biochars of orange peels |
27.07 |
— |
34 |
The Dubinin–Radushkevich (D–R) isotherm model, as shown in eqn (10), was further applied to analyze the adsorption energy for deciding the nature of adsorption process as physical or chemical.35,36
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe,DR = ln Qe,DR = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qm,DR − KDRε2 Qm,DR − KDRε2
| (10) |
where
Qe,DR (mol g
−1) is the equilibrium adsorption capacity,
Qm,DR (mol g
−1) and
KDR (mol
2 kJ
−2) are the D–R isotherm constants related to the capacity and free energy of adsorption, and
ε (kJ mol
−1) is the Polanyi potential, which is equal to
RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
ln(1 + 1/
Ce,DR).
R is the universal gas constant (8.314 J mol
−1 K
−1),
T is the absolute temperature (K), and
Ce,DR (mol L
−1) is the equilibrium concentration.
The free energy of adsorption E (kJ mol−1) was calculated using the following equation:
| |
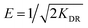 | (11) |
The parameters for the D–R model are shown in Table 4. The correlation coefficients of 1-NAP and 2-NAP were 0.9989 and 0.9841, respectively, which indicated that the adsorption of 1-NAP and 2-NAP onto Sil-PImC18-SS fitted well with the D–R isotherm model. Moreover, the free energy of adsorption was higher than 8 kJ mol−1 for the adsorption of 1-NAP and 2-NAP, revealing that the adsorption process could be considered to be chemical adsorption.37
Thermodynamic analysis
Thermodynamic studies were performed to further elucidate the adsorption mechanism of 1-NAP or 2-NAP onto the adsorbent. As shown in Fig. 9, the adsorption of 1-NAP or 2-NAP continuously decreased as the temperature increased from 20 to 50 °C, which indicated that the adsorption process was an exothermic process and was favored at lower temperature. The thermodynamic parameters, such as Gibbs free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS), were calculated by the following equations:38,39| |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K
| (13) |
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = ΔS/R − ΔH/RT K = ΔS/R − ΔH/RT
| (14) |
where K is the distribution coefficient.
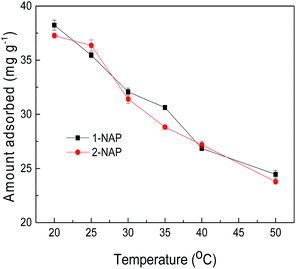 |
| | Fig. 9 Effect of temperature on the adsorption of 1-NAP and 2-NAP. | |
As presented in Table 6, the values of ΔG and ΔH of the process at five temperatures were negative, indicating that the adsorption process was a spontaneous and exothermic process. The negative value of ΔS implied a decrease in the randomness at the solid/liquid interface due to the orderly adsorption of 1-NAP or 2-NAP.
Table 6 Thermodynamic parameters for the adsorption of 1-NAP and 2-NAP
| Adsorbates |
ΔH (kJ mol−1) |
ΔS (kJ mol−1 K−1) |
ΔG (kJ mol−1) |
| 20 °C |
25 °C |
30 °C |
35 °C |
40 °C |
50 °C |
| 1-NAP |
−19.27 |
−0.0056 |
−17.67 |
−17.64 |
−17.51 |
−17.61 |
−17.37 |
−17.56 |
| 2-NAP |
−19.71 |
−0.0072 |
−17.56 |
−17.75 |
−17.42 |
−17.37 |
−17.42 |
−17.46 |
Regeneration and reuse of Sil-PImC18-SS
Desorption and reuse experiments were investigated through three consecutive cycles. After adsorption of 1-NAP and 2-NAP, the adsorbents were collected and fully washed with deionized water. Then, 1-NAP and 2-NAP loaded Sil-PImC18-SS was immersed in ethanol and stirred at 30 °C for 5 h. The Qe of 1-NAP decreased a little from the initial value of 33.4 mg g−1 to 29.1 mg g−1 and the Qe of 2-NAP ranged from 31.4 mg g−1 to 27.9 mg g−1 after three consecutive cycles. These results indicate that Sil-PImC18-SS could be easily regenerated and have the potential for application in the removal of 1-NAP and 2-NAP from the aqueous solutions.
Conclusions
In this work, Sil-PImC18-SS, with a good adsorption capacity for 1-NAP and 2-NAP, was prepared and characterized by elemental analysis, FTIR, SEM, N2 adsorption–desorption experiments, and thermogravimetric analysis. The Sil-PImC18-SS performed well over a wide pH range from 2 to 8. Importantly, Sil-PImC18-SS also had a good adsorption performance for naphthols even in presence of some coexisting anions, such as Cl− and SO42−. The adsorption behavior could be fitted well with the Dubinin–Radushkevich model and pseudo-second order model. The adsorption of 1-NAP and 2-NAP onto the Sil-PImC18-SS was a spontaneous and exothermic process and might be controlled by multiple mechanisms such as hydrophobic, hydrogen bonding, and π–π interactions. These results suggest that Sil-PImC18-SS is a potentially efficient material for the removal of naphthols from water environment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21107022) and the Key Scientific Research Project of Higher Education of Henan Province of China (No. 15A610006).
Notes and references
- M. M. Ali and K. Y. Sandhya, RSC Adv., 2014, 4, 51624–51631 RSC.
- N. V. Balashova, I. A. Kosheleva, N. P. Golovchenko and A. M. Boronin, Process Biochem., 1999, 35, 291–296 CrossRef CAS.
- D. C. Kalyani, A. A. Telke, R. S. Dhanve and J. P. Jadhav, J. Hazard. Mater., 2009, 163, 735–742 CrossRef CAS PubMed.
- Y. Pang, Y. Zhang, W. Li, H. Ding and X. Shen, J. Electroanal. Chem., 2016, 769, 89–96 CrossRef CAS.
- X. Peng, H. Wang, B. Yang, X. Zhan and Y. Wu, Chromatographia, 2016, 79, 327–333 CAS.
- X. Wang, C. Chen, J. Li and X. Wang, Chem. Eng. J., 2015, 262, 1303–1310 CrossRef CAS.
- Z. Wu, H. Yang, F. Jiao, Q. Liu, X. Chen and J. Yu, Colloids Surf., A, 2015, 470, 149–160 CrossRef CAS.
- M. Shiohara, T. Isobe, S. Matsushita and A. Nakajima, Mater. Chem. Phys., 2016, 183, 37–43 CrossRef CAS.
- L. Xu, J. Li and M. Zhang, Ind. Eng. Chem. Res., 2015, 54, 2379–2384 CrossRef CAS.
- L. Gu, Y. Wang, N. Zhu, D. Zhang, S. Huang, H. Yuan, Z. Lou and M. Wang, Bioresour. Technol., 2013, 146, 779–784 CrossRef CAS PubMed.
- L. Zuo, S. Yu, L. Cheng and E. Du, Korean J. Chem. Eng., 2013, 30, 714–723 CrossRef CAS.
- J. Wang and B. Chen, Chem. Eng. J., 2015, 281, 379–388 CrossRef CAS.
- Y. Li, A. Meas, S. Shan, R. Yang and X. Gai, Bioresour. Technol., 2016, 207, 379–386 CrossRef CAS PubMed.
- S. Yang, M. Gao, Z. Luo and Q. Yang, Chem. Eng. J., 2015, 268, 125–134 CrossRef CAS.
- L. Chen and X. Huang, J. Chromatogr. A, 2016, 1466, 42–49 CrossRef CAS PubMed.
- H. Qiu, A. K. Mallik, T. Sawada, M. Takafuji and H. Ihara, Chem. Commun., 2012, 48, 1299–1301 RSC.
- J. Liu, M. Wang, Y. Zhang, L. Han, X. Chen and J. Wang, RSC Adv., 2014, 4, 61936–61943 CAS.
- Q. Wen, Y. Wang, K. Xu, N. Li, H. Zhang and Q. Yang, Anal. Chim. Acta, 2016, 939, 54–63 CrossRef CAS PubMed.
- Z. Li, W. Wang, Y. Chen, C. Xiong, G. He, Y. Cao, H. Wu, M. D. Guiver and Z. Jiang, J. Mater. Chem. A, 2016, 4, 2340–2348 CAS.
- D. Lu, J. Zhao, Y. Leng, P. Jiang and C. Zhang, Catal. Commun., 2016, 83, 27–30 CrossRef CAS.
- J. Feng, M. Sun, L. Xu, S. Wang, X. Liu and S. Jiang, J. Chromatogr. A, 2012, 1268, 16–21 CrossRef CAS PubMed.
- X. Yang, J. Li, T. Wen, X. Ren, Y. Huang and X. Wang, Colloids Surf., A, 2013, 422, 118–125 CrossRef CAS.
- F. Wang, J. J. H. Haftka, T. L. Sinnige, J. L. M. Hermens and W. Chen, Environ. Pollut., 2014, 186, 226–233 CrossRef CAS PubMed.
- X. Sun, W. Huang, Z. Ma, Y. Lu and X. Shen, J. Hazard. Mater., 2013, 252–253, 192–197 CrossRef CAS PubMed.
- S. Yang, M. Gao, Z. Luo and Q. Yang, Chem. Eng. J., 2015, 268, 125–134 CrossRef CAS.
- C. He, J. Huang, C. Yan, J. Liu, L. Deng and K. Huang, J. Hazard. Mater., 2010, 180, 634–639 CrossRef CAS PubMed.
- J. Huang, B. Yuan, X. Wu and S. Deng, J. Colloid Interface Sci., 2012, 380, 166–172 CrossRef CAS PubMed.
- C. Sun, B. Xiong, Y. Pan and H. Cui, J. Colloid Interface Sci., 2017, 487, 175–181 CrossRef CAS PubMed.
- R. Li, C. Lin and X. Liu, RSC Adv., 2016, 6, 19872–19877 RSC.
- Y. Tai, C. Shi and C. Wang, J. Mol. Liq., 2014, 195, 116–124 CrossRef CAS.
- G. D. Sheng, D. D. Shao, X. M. Ren, X. Q. Wang, J. X. Li, Y. X. Chen and X. K. Wang, J. Hazard. Mater., 2010, 178, 505–516 CrossRef CAS PubMed.
- S. Chaudhary, P. Sharma, Renu and R. Kumar, RSC Adv., 2016, 6, 62797–62809 RSC.
- Q. Zhou, Y. Wang, J. Xiao and H. Fan, Synth. Met., 2016, 212, 113–122 CrossRef CAS.
- B. Chen and Z. Chen, Chemosphere, 2009, 76, 127–133 CrossRef CAS PubMed.
- H. R. Nodeh and H. Sereshti, RSC Adv., 2016, 6, 89953–89965 RSC.
- T. Zhao and T. Feng, RSC Adv., 2016, 6, 90878–90886 RSC.
- Y. Seki, S. Seyhan and M. Yurdakoc, J. Hazard. Mater., 2006, 138, 60–66 CrossRef CAS PubMed.
- W. Huang, X. Yu and D. Li, RSC Adv., 2015, 5, 84937–84946 RSC.
- S. Radi, S. Tighadouini, M. Bacquet, S. Degoutin, L. Janus and Y. N. Mabkhot, RSC Adv., 2016, 6, 82505–82514 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26028d |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW
KOW

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The elemental analysis data proved that the immobilization on the surface was successful.
1. The elemental analysis data proved that the immobilization on the surface was successful.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t
Qe − k1t
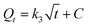
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe,DR = ln
Qe,DR = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qm,DR − KDRε2
Qm,DR − KDRε2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1 + 1/Ce,DR). R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature (K), and Ce,DR (mol L−1) is the equilibrium concentration.
ln(1 + 1/Ce,DR). R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature (K), and Ce,DR (mol L−1) is the equilibrium concentration.
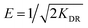
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = ΔS/R − ΔH/RT
K = ΔS/R − ΔH/RT








