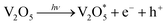Open Access Article
Open Access ArticleVanadium oxide-based photochromic composite film
Hidetoshi Miyazaki*a,
Takahiro Matsuuraa and
Toshitaka Otab
aInterdisciplinary Graduate School of Science and Engineering, Shimane University, 1060, Nishikawatsu, Matsue, Shimane 690-8504, Japan. E-mail: miya@riko.shimane-u.ac.jp
bCeramic Research Laboratory, Nagoya Institute of Technology, 10-6-29, Asahigaoka, Tajimi, Gifu 507-0071, Japan
First published on 4th January 2017
Abstract
V2O5-based composite films were fabricated using peroxo-iso-poly vanadic acid as the filler and transparent urethane resin as the matrix. The size of the V2O5 particles in the films was around 60–80 nm and the fabricated composite films exhibited photochromic properties when irradiated with ultraviolet (UV) light. The composite films displayed multichromism (yellow ↔ green ↔ pale blue) under UV irradiation. The absorption edge of the films shifted to lower wavelengths (Bürstein Moss effect) with the increase of the coloring degree of the films. Almost all the colors of the composite film bleached when placed in a dark room.
1. Introduction
Amorphous and crystalline V2O5 films are amongst the most widely investigated materials for electrochromic devices,1–3 lithium ion batteries,4,5 chemical gas sensing,6,7 and photochromic device applications.8,9 Electrochromism or photochromism of V2O5 is achieved by changing the valency of vanadium in the V2O5 host, using either an electrochemical or a photochemical electron transfer reaction. Thus, V2O5 can be used in display devices or smart windows that are activated photochemically or electrochemically.Previously, we fabricated WO3 (ref. 10 and 11) and MoO3 (ref. 12) based photochromic composite films with rapid coloring and bleaching properties. The photochromism of the films was a direct result of the presence of sub-nanometer sized WO3 and MoO3 particles embedded in the resin matrix. In order to embed the nanoparticles in the matrix, we used tungsten or molybdenum peroxo-isopoly acid as the starting materials because of their solubility in H2O or alcohol and combined these with the transparent resin. It is assumed that by a similar method, a photochromic composite film of vanadium oxide can be created using vanadium oxide nanoparticles. Peroxo-iso-poly vanadic acid (V-IPA), which is readily formed when H2O2 reacts with vanadium metal, is highly soluble in water.13,14 Therefore, we expect to fabricate vanadium oxide-based photochromic composite films, containing sub nanometer size particles, using V-IPA.
In this study, we have fabricated V2O5-based composite films using V-IPA and transparent urethane resin and evaluated their photochromic properties.
2. Experimental procedure
Vanadium powder (1.0 g, Wako, Osaka, Japan) was mixed with 30% aq. H2O2 solution (100 mL) in an ice bath for 15 h. After reaction, the excess H2O2 was removed catalytically using Pt nets. The resulting orange solution was dried using a rotary evaporator with 60 rpm, placed in a water bath maintained at 40 °C for 1 h, to obtain V-IPA powder.V-IPA powder was then dissolved in ion-exchanged water to form a vanadium solution, with a final concentration of 0.2 M. The obtained solution (0.15 mL) was mixed with 3.2 g of liquid urethane resin (M-40; Asahi Kasei Chemicals Corp., Japan). The liquid urethane resin was used as the matrix material in the synthesis of the composite films, and it the resin was cured by irradiation with UV light. The precursor mixture (slurry state) was degassed at 1 kPa for 60 min. The precursor was first placed between two glass slides such that a 150 μm thick film could be formed and irradiated with UV light for 5 min before removing it from the glass. The resulting films were pale blue in color because of the effects of the UV radiation and hence, the composite films were placed in a dark room for three days for clarification.
The structure of the V-IPA powder was characterized by X-ray diffraction (XRD) using a Rigaku Miniflex with CuKα radiation. The microstructure and the crystallographic structure of the particles in the composite film was characterized by transmission electron microscopy (TEM, EM-002B; Topcon Corp., Japan) and selected area electron diffraction (SAED, ibid.). The photochromic properties of the films were evaluated at room temperature with a UV-Vis spectrophotometer (UV-1600; Shimadzu Corp., Japan). Throughout the investigation, a 1 kW high-pressure Hg lamp was used for curing the resin and for color manipulation of the composite films. The coloring properties of the films were evaluated by UV irradiation, while the film bleaching properties were observed by placing the films in a darkened room.
3. Results and discussions
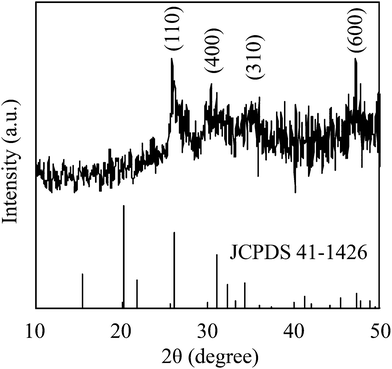
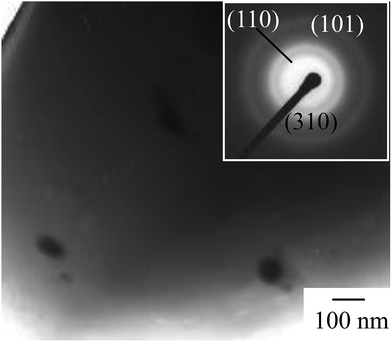
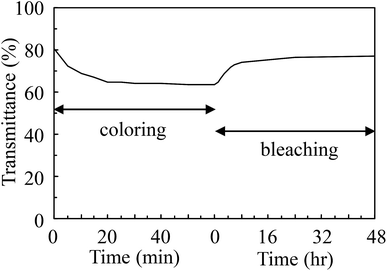
A direct reaction between vanadium metal and hydrogen peroxide was carried out and the resulting precursor solution was dried by evaporation to obtain V2O5 powder. Fig. 1 depicts the XRD pattern of the resulting powder. The resulting powder displayed (110), (410), (310) and (600) planes of the V2O5 peaks, thus exhibiting only the (hk0) planes. The results were in good agreement with previous studies of V-IPA.13 The V-IPA (2D-V2O5) powder, thus synthesized, was further used in our study.Vanadium oxide-based composite films were fabricated using the synthesized V-IPA and the cured urethane resin. Fig. 2 shows the TEM bright field image and the selected area electron diffraction (SAED) image of a composite film. The V2O5 particle size in the composite film was about 60–80 nm and the particles were dispersed homogeneously throughout the film. Using the electron diffraction pattern, the crystallographic structure of the particles in the resulting composite film were confirmed to be V2O5. Photochromic properties of the films were evaluated by UV irradiation. Fig. 3 illustrates the UV-Vis spectra of the composite film before and after UV irradiation and the inset photographs shows the overview of the same. From the overview, it is evident that the initial color of the resultant film was yellow, which color was close to V2O5 film.2,3 However, the color of the film changed gradually from yellow to green to pale blue after exposure to UV light and had a weak, but broad absorption spectrum with peak at around 700 nm. After 60 min of UV irradiation the film displayed a higher transmittance in the wavelength region of 1000–1100 nm than the film exposed to 10 min of UV irradiation. Though the colored film bleached when placed in a dark room, complete bleaching did not occur until 48 h. In the previous investigation of V2O5 electrochromism, it was observed that the V2O5 film color changed from yellow ∼ green ∼ pale blue because of Li ion insertion3 and the previous results of color changing on the V2O5 electrochromism agreed well with the present investigation. Significantly, the composite film in the present study showed a semi-reversible photochromic property, similar to the electrochromic V2O5 film.
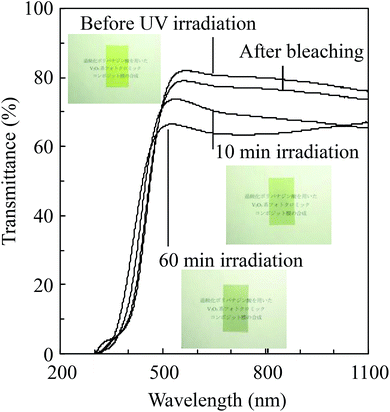 | ||
| Fig. 3 The UV-Vis spectra of the composite films before UV-Vis irradiation, after UV-Vis irradiation and of the re-bleached film. Insets show the overview of the films. | ||
With respect to the electrochromism of V2O5, vanadium valence is electrochemically reduced from “+5” to “+4” and thus the color change is attributed to this change in the valence state. Similar to the photochromism of V2O5, it assumed that change in the valence state of vanadium and the change in color of V2O5 were caused by UV irradiation. The exact photochromic mechanism has been previously reported with regard to MoO3-based composite films.12 Applying the similar theory to V2O5-based composite films; the photochromic mechanism can be explained as follows:
| h+ + H2O (as precursor solvent) → H+ + –OH |
| V25+O5 (colored) + xH+ + xe− (bleached) → HxV2−x5+Vx4+O5 (colored) |
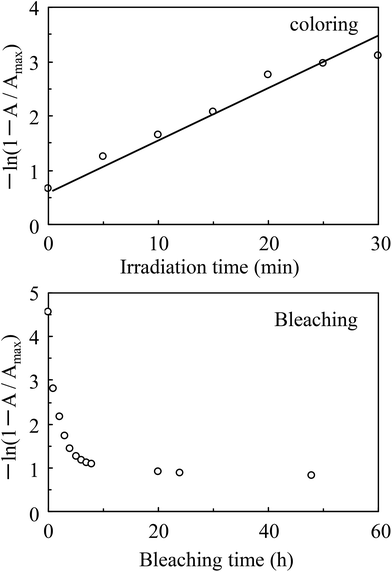
In order to evaluate the coloring and bleaching property of the films, time dependent studies on transmittance of the composite film were performed, as shown in Fig. 5. The colored films showed significant absorption around 700 nm and hence, transmittance at 700 nm was studied. Assuming the reaction to be of first order, the reaction rate constant k can be estimated as follows:
| −ln([A]/[A0]) = kt, |
There have been few reports on the photochromism of V2O5 thin films or bulk ceramics. Nishio et al. reported the photochromism of V2O5 bulk ceramics that were colored by “Ar ion laser irradiation” and bleached by “oxidation at a high temperature of 400 °C”.8 Wan et al. fabricated V2O5 xerogel films by a sol–gel method; the film thickness was less than 10 μm. The V2O5 xerogel films exhibited photochromic property because of hydrogen atoms which were detached under the action of light from organic-molecules adsorbed on the “film surface”.9 However, these reports do not describe the bleaching property. Remarkably, in this study, 130 μm thick V2O5-based composite films with reversible photochromic properties were fabricated by just irradiating the films with UV light at room temperature. In addition, when the particle size in WO3 and MoO3-based composite film was in the order of sub nanometers, reversible photochromic properties were observed at room temperature.10–12 Similarly, the presence of these nanometer size V2O5 is considered to be responsible for the photochromic property of the composite film, in the present study. Furthermore, other nano-composite film fabrication methods have been reported.17,18 We should elucidate the photochromic properties of V2O5 nano-composites fabricated by various methods in future research.
4. Conclusions
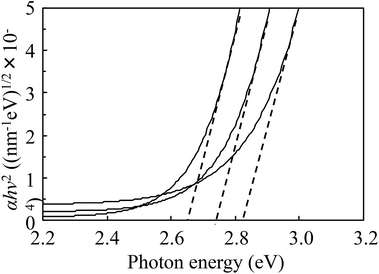
We fabricated vanadium oxide-based composite films with homogeneously dispersed sub-nanometer sized V2O5 particles. The composite films upon irradiation with UV light showed a broad absorption peak at 700 nm. Furthermore, the optical band gap (Eg) of the film increased with increase in UV irradiation time because of the corresponding increase in carrier concentration. This increase in Eg is assumed to be due to the Bürstein Moss effect. Moreover, the colored films bleached when kept in the dark. In conclusion, we successfully fabricated vanadium oxide photochromic composite films using V-IPA and urethane resin.References
- A. Talledo and C. G. Granqvist, J. Appl. Phys., 1995, 77, 4655 CrossRef CAS
.
- S. Krishnakumar and C. S. Menon, Phys Status Solidi, 1996, 153, 439 CrossRef CAS
.
- Y. R. Lu, T. Z. Wu, C. L. Chen, D. H. Wei, J. L. Chen, W. C. Chou and C. L. Dong, Nanoscale Res. Lett., 2015, 10, 387 CrossRef PubMed
.
- G. P. Holland, J. L. Yarger, D. A. Buttry, F. Huguenin and R. M. J. Torresi, J. Electrochem. Soc., 2003, 150, A1718 CrossRef CAS
.
- H. Yamada, K. Tagawa, M. Komatsu, I. Moriguchi and T. Kudo, J. Phys. Chem. C, 2007, 111, 8397 CAS
.
- W. Fergus, Sens. Actuators, 2007, 121, 652 CrossRef
.
- A. D. Raj, T. Pazhanivel, P. S. Kumar, D. Mangalaraj, D. Nataraj and N. Ponpandian, Curr. Appl. Phys., 2010, 10, 531 CrossRef
.
- S. Nishio and M. Kakihana, Chem. Mater., 2002, 14, 3730 CrossRef CAS
.
- Y. Wang, L. Pan, Y. Li and A. I. Gavrilyuk, Appl. Surf. Sci., 2014, 314, 384 CrossRef CAS
.
- H. Miyazaki, Y. Baba, M. Inada, A. Nose, H. Suzuki and T. Ota, Bull. Chem. Soc. Jpn., 2011, 84, 1390 CrossRef CAS
.
- H. Miyazaki, T. Ishigaki, H. Suzuki and T. Ota, Bull. Chem. Soc. Jpn., 2014, 87, 838 CrossRef CAS
.
- H. Miyazaki, H. Ichioka, H. Suzuki and T. Ota, Bull. Chem. Soc. Jpn., 2013, 86, 1323 CrossRef CAS
.
- M. Hibino, M. Ugaji, A. Kishimoto and T. Kudo, Solid State Ionics, 1995, 79, 239 CrossRef CAS
.
- H. Miyazaki, K. Tsunomori, H. Suzuki and T. Ota, J. Ceram. Soc. Jpn., 2016, 124, 34 CrossRef CAS
.
- G. Wu, K. Du, C. Xia, X. Kun, J. Shen, B. Zhou and J. Wang, Thin Solid Films, 2005, 485, 284 CrossRef CAS
.
- M. Benmoussa, A. Outzourhit, R. Jourdani, A. Bennouna and E. L. Ameziane, Act. Passive Electron. Compon., 2003, 26, 245 CrossRef
.
- H. Wei, X. Yan, Y. Li, H. Gu, S. Wu, K. Ding, S. Wei and Z. Guo, J. Phys. Chem. C, 2012, 116, 16286 CAS
.
- N. Asim, S. Radiman and M. A. Yarmo, Mater. Lett., 2008, 62, 1044 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2017 |