Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cu nanoparticles immobilized on montmorillonite by biquaternary ammonium salts: a highly active and stable heterogeneous catalyst for cascade sequence to indole-2-carboxylic esters†
Wencheng Lang,
Qin Yang,
Xueping Song,
Mengyun Yin and
Limei Zhou *
*
Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, China West Normal University, Nanchong 637002, Sichuan, China. E-mail: cwnuzhoulimei@163.com
First published on 28th February 2017
Abstract
Copper nanoparticles immobilized on montmorillonite (MMT) by biquaternary ammonium salts (N1,N6-dibenzyl-N1,N1,N6,N6-tetramethylheptane-1,6-diaminium bromide, Q) were prepared by cation-exchange and impregnation–reduction and designated Cu-Q-MMT. The material was extensively characterized by various characterization techniques such as FTIR, XRD, XPS, SEM, TEM, and N2 adsorption–desorption. The Cu-Q-MMT could be used as a highly active heterogeneous catalyst for cascade sequence to indole-2-carboxylic esters from ortho-bromobenzaldehydes with ethyl acetamidoacetate. Even for inactive chlorobenzaldehydes, a good yield could be obtained. In addition, the catalyst can be reused six times without any significant loss of activity. The high activity and stability of the Cu-Q-MMT catalyst is mainly attributed to the excellent synergistic effects of biquaternary ammonium salts, Cu nanoparticles and the nanospace structure of MMT.
Introduction
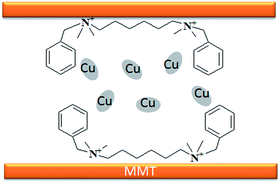
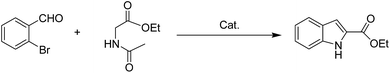
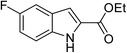
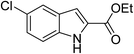
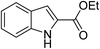
Compared to homogeneous catalysts, heterogeneous catalysts have more advantages such as easy-separation and recyclability.1,2 The application of heterogeneous catalysts in synthetic chemistry is attracting more attention because of their significance from both economic and ecological points of view.3,4 Montmorillonite (MMT), which is a type of naturally available clay, can be structurally defined as layers of negatively charged two-dimensional silicate sheets that are separated by interlayer cationic species with high exchange ability for other cations.5,6 Some quaternary ammoniums such as CTAB, DTAB, are widely used as surfactants to modify montmorillonites which are applied to adsorption, degradation, and polymerization and in retardants.7–11 In addition, the organo-montmorillonites are considered as robust stabilizing materials for metal nanoparticles to form clay catalysts.5,12 For example, Manikandan and coworkers synthesized Pt-CTA-MMT for the hydrogenation of cinnamaldehyde.13 Our group reported a supported Ru catalyst by using CTAB intercalated montmorillonite. The catalyst exhibited high activity and selectivity in the hydrogenation of quinoline. However, it is not negligible that the stability of the catalyst is poor due to the drop of surfactants.7 Inspired by the parallel bar sport that a man with two hands holding on the bar can insist longer than the one with only one hand, we suppose that Gemini quaternary ammonium with two cation positions can anchor on the layer of montmorillonite more stably.The indole motifs are important substructure in numerous synthetic alkaloids.14–18 Their extensive use in the field of biology and pharmaceutics has motivated researchers to promote new synthetic strategies.19–22 Although studies on the synthesis of indole derivatives have achieved a lot, the preparation of some specific substituted patterns remains difficult such as 2-substituted-indole derivatives.23,24 As far as we all know, many indole-2-carboxylic esters and its derivatives are used as powerful precursors for antimicrobial, anticonvulsant and anticancer drugs.25–28 Usually, the synthesis of indole-2-carboxylic esters contains a build of C–N bond. Recently, Cu is reported as a cheap catalyst for the synthesis of indole-2-carboxylic esters. In 2009, Cai group described copper salts catalyzed one-pot synthesis of indole-2-carboxylic esters from ortho-halobenzaldehyde.29 Following, several groups has reported some similar methods with copper catalyzed this kind of reaction.30–32 However, all of the reported methods in the literature to obtain the desired product are homogeneous catalysis systems which have some drawbacks: the separation of the catalyst from the reaction mixture, lack of adequate catalyst recycling methods and the residue of relatively high metal as contamination for the products. The latter problems are intolerable in the context of biological applications. To solve these problems, we immobilized Cu nanoparticles on montmorillonite by Gemini quaternary ammonium salts (Cu-Q-MMT) (Fig. 1) and used it as heterogeneous catalyst for cascade sequence to indole-2-carboxylic esters.
Experimental
Materials
Montmorillonite powder was purchased from Alfa Aesar Company (Tianjin, China). CuCl2·2H2O, acetonitrile, ethanol, ethyl acetate, and NaBH4 were obtained from Kelong Chemical Reagent Co. Ltd. (Chengdu, China). All substrates for the reactions were purchased from Aladdin Industrial Inc. (America) and used without further purification. The deionized (DI) water used throughout all experiments was purified to 18.2 MXm with the Millipore system.Synthesis of Cu-Q-MMT catalyst
N,N-Dimethylbenzylamine (0.1 mol, 15.1 mL), 1,6-dibromohexane (0.025 mol, 4.0 mL) and hydrochloric acid (41.5 mL) were added into a dry round bottom flask and stirred under nitrogen atmosphere. And then acetonitrile (20 mL) was injected as solvent. The reaction mixture was refluxed for 3 h before cooled down to room temperature. After rotary evaporation of the solvent, a waxy product was obtained. The waxy product repeatedly recrystallized from mixture solvent of ethanol and ethyl acetate. After dried in vacuum, the desired product was obtained as a white solid and labelled as Q. The preparation route of Q was shown and the structure of Q was confirmed by 1H NMR, 13C NMR and HRMS (seen in ESI, S2–5†).Q-MMT and Cu-Q-MMT materials were prepared by a method similar to our previous reported method7 (seen in ESI, S6†).
General procedure for indole-2-carboxylic esters
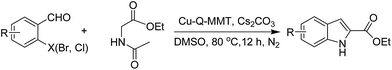
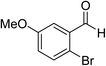
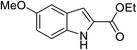
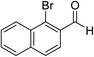
Ethyl acetamidoacetate (156.2 mg, 1.2 mmol), caesium carbonate (652 mg, 2 mmol) and catalyst (52.5 mg) were added to a dried reaction tube. The tube was flushed with nitrogen before DMSO (2 mL) was added. Then 2-bromobenzaldehyde (185 mg, 1 mmol) was added prior to heating the slurry to 80 °C under a nitrogen atmosphere for 12 h with good agitation. After cooled down, the mixture was added DI water (5 mL) and extracted with ethyl acetate (5 mL) for 3 times. The combined organic fraction was washed with DI water (5 mL) and brine (5 mL). After dried over sodium sulfate and filtered, the organic phase was concentrated to give crude product. The crude product was further purified by chromatography, eluting with dichloromethane and petroleum ether.Reuse experiment
After completion of the cascade sequence to indole-2-carboxylic esters catalyzed by Cu-Q-MMT to afford the desired product, the catalyst was recovered by centrifugation, washed with ethyl acetate and water before dried under vacuum at 60 °C for 12 h. The next run reaction was performed with the same amounts of reagents and solvent under the same conditions in the initial reaction.Characterization
The FTIR spectra were recorded on an FTIR system (NICOLET 6700, Thermo Scientific Instrument). XRD measurements were performed on the D/MAX Ultima IV (Rigaku, JAPAN). X-Ray photoelectron spectroscopy (XPS) was performed using an ESCALAB 250 Xi. Transmission electron microscopy (TEM) characterization was carried out using a Tecnai G2 20 field emission electron microscope operated at 200 kV accelerating voltage. SEM was used to determine the morphology of the catalyst system. The experiments were performed on a high-resolution scanning electron microscope (AJEOL JSM-6510LV, JAPAN). TGA was performed on a NETZSCH STA 449F3 (Piscataway, NJ) analyser in a temperature range of 25–500 °C at a heating rate of 5 °C min−1 in N2. The N2 adsorption–desorption were calculated by utilizing a highspeed gas sorption analyzer, Autosorb-IQ (Quantachrome, U.S.A) instrument. The amount of copper in catalysts was recorded by inductively coupled plasma atomic emission spectroscopy (ICP-AES WFX-120). GC (7890 A, Agilent Technologies) with a capillary column (HP-5, 30 m) was used to determine the yields of the reactions. 1H NMR and 13C NMR spectra were recorded on a Bruker DRX-400 instrument. HRMS spectrum was recorded on a Bruker micrOTOF-Q II instrument.Results and discussion
Characterization of Cu-Q-MMT
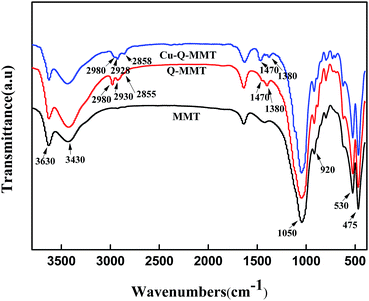
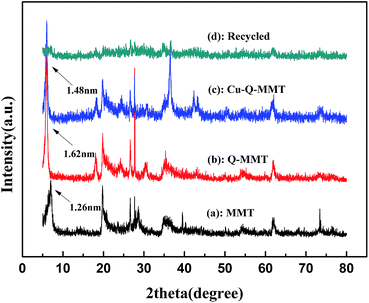
The FTIR spectra of MMT, Q-MMT and Cu-Q-MMT were described in Fig. 2. Compared to MMT, the spectrum of the Q-MMT had characteristic absorptions of native MMT, which suggested that the modification by Q did not destroy the structure of montmorillonite. Besides, the spectrum exhibited some new characterizations. The additional peaks near 2980, 2930 and 2855 cm−1 are attributed to the C–H asymmetric and symmetric stretching vibration.33 And the peaks at 1470 and 1380 cm−1 are assigned to the bending vibration of the C–H bonds of Q.34 The spectrum of Cu-Q-MMT is nearly the same as Q-MMT, which suggested that Q is successfully modified on the two materials.The X-ray diffraction patterns supplied very useful information on the inter layer spacing of the layered material, which provides the information regarding the interlayer distance of montmorillonite. The success of the intercalation was mainly verified by measuring the increase in the basal (001) d-spacing.35 Fig. 3 described the X-ray diffraction patterns of MMT, Q-MMT, Cu-Q-MMT and the recycled catalyst. The curve (a) represents the XRD pattern of MMT. It can be seen that the (001) diffraction peak is at 2θ = 7°. The characteristic basal spacing d is 1.26 nm as calculated from Bragg's law. This reflection shifted to 5.45° in the Q-MMT (curve (b)), meaning an increase in the basal spacing from 1.26 nm to 1.62 nm. The enlarged d-spacing values indicated the intercalation of Q within silicate layers. When the Q-MMT was further modified with copper, this peak shifted to different angles 5.97° with the basal d-spacing decreasing to 1.48 nm (curve (c)). This decrease can be explained by that parts of the unstable Q between the layers of montmorillonite were washed or replaced by Cu2+ in the process of impregnation–reduction. The curve (d) is the XRD pattern of the recycled catalyst. From the spectrum we cannot get its basal d-spacing values which indicated the (001) diffraction peak shift to the left (2θ < 3°). The reason may be that the interlayer structure of MMT has been destroyed under basic reaction conditions.
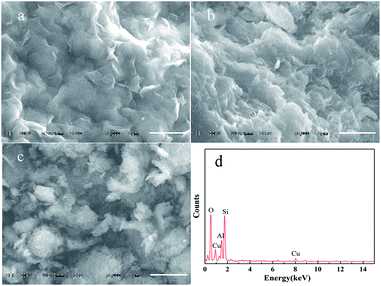
The effective method of study the montmorillonite dispersed state in water is scanning electron microscope (SEM) and the most common method of analysis element composition is energy dispersive X-ray spectrometer (EDS).36 The SEM patterns of MMT, Q-MMT and Cu-Q-MMT are shown in the Fig. 4. The surface morphology of all samples indicated layered structure of montmorillonite. Obviously, the native MMT could disperse well in water (Fig. 4a). It is visible from the Fig. 4b that the modified Q-MMT dispersed worse in water and finally agglomerated. This phenomenon revealed that the surface of silicate platelets changed from hydrophilic one into hydrophobic one, which is caused by the interaction of alkyl chains and benzene rings. After modification of copper nanoparticles, the hydrophobic abate and agglomeration degree of Cu-Q-MMT dropped (Fig. 4c). It is confirmed by the SEM results that biquaternary ammonium salts Q has been successfully modified into the layers of montmorillonite. The EDS analysis also revealed the copper loaded into the montmorillonite (Fig. 4d).
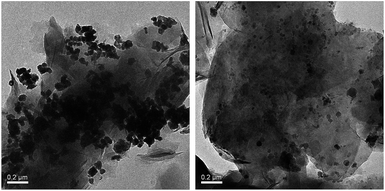
The TEM micrographs of Cu-Q-MMT and the recycled catalyst are displayed in Fig. 5. As shown in Fig. 5, the morphology of copper nanoparticles is close to lamellar structure. After recycled six times, the particle size of copper nanoparticles became smaller. And a part of copper nanoparticles was lost in the recycle experiments. The amount of Cu loaded in virgin Cu-Q-MMT and recycled Cu-Q-MMT were 11.8% and 10.2%, respectively.
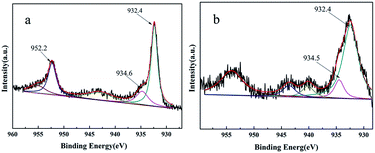
The XPS analysis of virgin catalyst and recycled catalyst were shown in Fig. 6. It was clear that copper was in the Cu2+ and Cu0 components. Cu 2p3/2 peak at 932.4 eV could be assigned to Cu0, whereas the peak at 934.6 eV and 934.5 eV could be assigned to Cu2+ (Fig. 6a and b), which probably results from oxidation of the Cu0 nanoparticles upon exposure to air.37
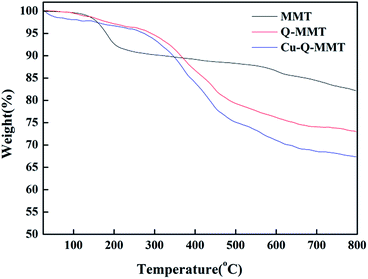
To study the thermal stability of the organoclay and the environments of the inserting molecules, thermogravimetric analysis was performed. The TGA curves of MMT, Q-MMT and Cu-Q-MMT were shown in Fig. 7. It is reported that the decomposition of montmorillonite occurs in two steps: desorption of water from the interlayer space occurs between 25–200 °C and dehydroxylation of the layer crystal lattice structure occurs between 500–800 °C.38 The decomposition of Q-MMT and Cu-Q-MMT has obvious differences from MMT. Studies showed the decomposition of the organoclay often takes place in three steps: desorption of water from the interlayer space, decomposition of organic cation, and dehydroxylation of the layer crystal lattice structure. In this work the decomposition of organic cation steps occurs between 200–500 °C, which also implied that the catalyst Cu-Q-MMT was relatively stable below 200 °C. From all the spectra above, it can be concluded that Cu-Q-MMT has been successfully modified by Q and Cu nanoparticles.
The nitrogen adsorption–desorption isotherms of MMT, Q-MMT, Cu-Q-MMT and the recycle catalyst are given in Fig. 8. The isotherm on montmorillonite is of type IV characteristics according to the classification of Brunauer group, thereby indicating the mesoporous structures of all the samples. Combined with the result of XRD, it can be concluded that Cu-Q-MMT catalyst has nano space structure.
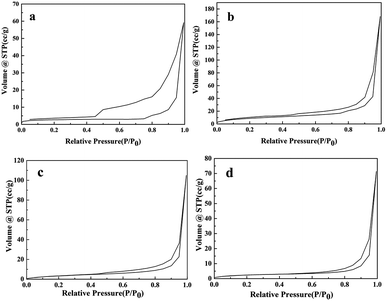 | ||
| Fig. 8 The nitrogen adsorption–desorption isotherms of MMT (a), Q-MMT (b), Cu-Q-MMT (c) and the recycle catalyst (d). | ||
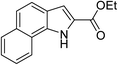
Catalysis of the cascade sequence to indole-2-carboxylic esters
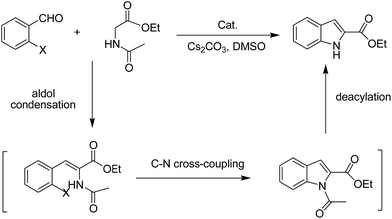
Sequentially, the catalytic performance of Cu-Q-MMT was tested in the cascade sequence to indole-2-carboxylic esters. A presumed three-step sequence that included aldol condensation, C–N cross-coupling (Goldberg reaction), and deacylation was proposed (Scheme 1).30,31The reaction of orthobromobenzaldehyde with ethyl acetamidoacetate was chosen as model for optimizing the reaction conditions (seen in ESI, S8–10†). And the optimal conditions were DMSO as solvent, Cs2CO3 as base, at 80 °C, under N2 atmosphere. With the optimal conditions in hand, the catalytic performances of several catalysts were compared (Table 1).
| Entry | Catalyst | Conditions | Yield |
|---|---|---|---|
| a Condition A: Cs2CO3, DMSO, 12 h, 80 °C, N2; B: 15 mmol% Cu, Cs2CO3, DMSO, 12 h, 80 °C, N2; C: 20 mmol% Cu, Cs2CO3, DMSO, 16 h, 80 °C; D: 20 mmol% Cu, Cs2CO3, 2-Me-THF, 16 h, 80 °C. | |||
| 1 | MMT | A | 18% |
| 2 | Q-MMT | A | 19% |
| 3 | Cu-MMT | B | 75% |
| 4 | Cu-MMT(run 1) | B | 45% |
| 5 | Cu-MMT(run 2) | B | 28% |
| 7 | Cu-Q-MMT | B | 85% |
| 8 | Cu-Q-MMT(run 1) | B | 82% |
| 9 | Cu-Q-MMT(run 2) | B | 82% |
| 10 | Cu-Q-MMT(run 3) | B | 82% |
| 11 | Cu-Q-MMT(run 4) | B | 81% |
| 12 | Cu-Q-MMT(run 5) | B | 83% |
| 13 | Cu-Q-MMT(run 6) | B | 80% |
| 14 | Cu-Q-MMT(run 7) | B | 72% |
| 15 | CuI31 | C | 50% |
| 16 | CuI30 | D | 59% |
Firstly, it can be concluded that Cu is important for the reaction of orthobromobenzaldehyde and ethyl acetamidoacetate to indole-2-carboxylic esters because the yield is up to 75% after the loading of Cu on the MMT (catalyst Cu-MMT). Cu catalysts are often used in the C–N cross-coupling reaction.37 So the C–N cross-coupling is the key step for forming the indole-2-carboxylic esters. And with the amount of Cu and reaction temperature increasing, the yield of indole-2-carboxylic esters increased (seen in ESI, S9†). Although the Cu-MMT gave good yield, the activity declined obviously in the recycle experiments (entry 4 and 5), which also indicated its poor stability. To improve the stability of the catalyst, the MMT was modified by biquaternary ammonium salts Q before the loading of Cu. Encouragingly, the catalyst Cu-Q-MMT could catalyze this reaction with a higher yield (85%) than Cu-MMT (75%). A possible explanation is that biquaternary ammonium salts on the MMT increase the hydrophobicity of MMT surface, benefit the adsorption of product of aldol reaction on the MMT surface, and the coordination of Cu on the MMT surface with product of aldol reaction, thereby enhancing the catalytic activity. Most importantly, the catalyst Cu-Q-MMT could reuse for 6 times without significant loss of activity. The results suggested that biquaternary ammonium salts Q modified on the MMT favor to improve the stability of catalyst as we expected. Besides, the catalyst Cu-Q-MMT showed more significant advantages: lower amount of Cu, shorter reaction time and higher activity, comparing to the reported homogeneous catalysts CuI (entry 15 and 16). The high activity and stability of Cu-Q-MMT catalyst is mainly attributed to the excellent synergistic effects of biquaternary ammonium salts, Cu nanoparticles and the nano space structure of MMT. The nano space structure of catalyst is beneficial to improve the activity, selectivity and stability of catalyst.39–41
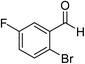
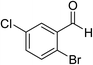
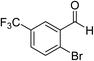
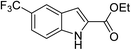
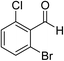
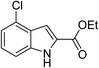
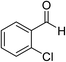
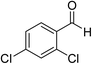
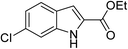
The generality and scope of the reaction catalyzed by Cu-Q-MMT were also explored. The results are summarized in Table 2. And the products were characterized by 1H NMR, 13C NMR and HRMS (seen in ESI, S11–27†). Various benzaldehydes were compatible under the reaction conditions. Electronic effect and steric hindrance had a little impact on the outcome of the reaction. It is clear that the reactivity of the orthohalobenzaldehyde followed the trend of bromobenzaldehyde > chlorobenzaldehyde. So, 4-chloroindole-2-carboxylic ester was obtained as main product when 2-bromo-6-chlorobenzaldehyde was substrate (entry 7). In 2009, Cai group reported the cascade process of chlorobenzaldehyde with ethyl isocyanoacetate to indole-2-carboxylic ester at the yield of 20%.29 Since then, there is little report on the one-pot synthesis of indole-2-carboxylic esters from chlorobenzaldehyde because of its low reactivity. In our experiment, catalyzed by Cu-Q-MMT, 2-chlorobenzaldehydes and 2,4-dichlorobenzaldehyde coupled with ethyl acetamidoacetate to the aimed indole-2-carboxylic esters gave encouraging separation yields of 68% and 75%, respectively (entry 8 and 9).
Conclusion
In conclusion, the MMT supported catalyst Cu-Q-MMT was prepared by using cation-exchanged and impregnation–reduction methods. The obtained catalyst showed high activity for cascade sequence to indole-2-carboxylic esters. Remarkably, this catalyst could catalyze ethyl acetamidoacetate with inactive chlorobenzaldehydes and gave a good yield. In addition, the catalyst could be easily isolated from the reaction mixture by centrifugation and reused up to 6 times. The high activity and stability of Cu-Q-MMT catalyst is mainly ascribed to the excellent synergistic effects of biquaternary ammonium salts, Cu nanoparticles and the nano space structure of MMT. We believe that other transition metals could also be supported on MMT using this method and the resulting materials could be used as efficient and stable nanocatalyst.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (21303139).References
- Z. Dong and Z. Ye, Appl. Catal., A, 2015, 489, 61 CrossRef CAS.
- P. G. Najrul Hussain, P. Khared and M. R. Das, RSC Adv., 2015, 5, 103105 RSC.
- S. Sayyahi, Asian J. Chem., 2013, 25, 6471 CAS.
- P. T. Anastas, M. M. Kirchhoff and T. C. Williamson, Appl. Catal., A, 2001, 221, 3 CrossRef CAS.
- T. Li, J. Zhang, X. Xie, X. Yin and X. An, Fuel, 2015, 143, 55 CrossRef CAS.
- S. Miao, Z. Liu, B. Han, J. Huang, Z. Sun, J. Zhang and T. Jiang, Angew. Chem., 2005, 45, 266 CrossRef PubMed.
- L. Zhou, X. Qi, X. Jiang, Y. Zhou, H. Fu and H. Chen, J. Colloid Interface Sci., 2013, 392, 201 CrossRef CAS PubMed.
- S.-D. Lee, M.-S. Park, D.-W. Kim, I. Kim and D.-W. Park, Catal. Today, 2014, 232, 127 CrossRef CAS.
- H. Fan, L. Zhou, X. Jiang, Q. Huang and W. Lang, Appl. Clay Sci., 2014, 95, 150 CrossRef CAS.
- M. Kozak and L. Domka, J. Phys. Chem. Solids, 2004, 65, 441 CrossRef CAS.
- S. Miao, Z. Liu, B. Han, H. Yang, Z. Miao and Z. Sun, J. Colloid Interface Sci., 2006, 301, 116 CrossRef CAS PubMed.
- Z. Huang, P. Wu, H. Li, W. Li, Y. Zhu and N. Zhu, RSC Adv., 2014, 4, 6500 RSC.
- D. Manikandan, D. Divakar, A. V. Rupa, S. Revathi, M. E. L. Preethi and T. Sivakumar, Appl. Clay Sci., 2007, 37, 193 CrossRef CAS.
- S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, PR215 CrossRef PubMed.
- S. Cacchi and a. G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed.
- M. Inman and C. J. Moody, Chem. Sci., 2013, 4, 29 RSC.
- X. Zhang, R. Guo and X. Zhao, Org. Chem. Front., 2015, 2, 1334 RSC.
- D. F. Taber and P. K. Tirunahari, Tetrahedron, 2011, 67, 7195 CrossRef CAS PubMed.
- A. Ganesan, J. Kothandapani, J. B. Nanubolu and S. S. Ganesan, RSC Adv., 2015, 5, 28597 RSC.
- S. Bahn, S. Imm, K. Mevius, L. Neubert, A. Tillack, J. M. Williams and M. Beller, Chemistry, 2010, 16, 3590 CrossRef PubMed.
- G. Palmisano, A. Penoni, M. Sisti, F. Tibiletti, S. Tollari and K. M. Nicholas, Curr. Org. Chem., 2010, 14, 2409 CrossRef CAS.
- R. G. Vaswani, B. K. Albrecht, J. E. Audia, A. Cote, L. A. Dakin, M. Duplessis, V. S. Gehling, J. C. Harmange, M. C. Hewitt, Y. Leblanc, C. G. Nasveschuk and A. M. Taylor, Org. Lett., 2014, 16, 4114 CrossRef CAS PubMed.
- H. J. A. Sudhakara, K. M. Mahadevan and V. Hulikal, Synth. Commun., 2009, 39, 2506 CrossRef.
- K. K. James McNulty, Eur. J. Org. Chem., 2011, 6902 CrossRef.
- W. Sun, L. Hong and R. Wang, Chemistry, 2011, 17, 6030 CrossRef CAS PubMed.
- R. Mali and V. Yadav, Synthesis, 1984, 1984, 862 CrossRef.
- S. Basceken, S. Kaya and M. Balci, J. Org. Chem., 2015, 80, 12552 CrossRef CAS PubMed.
- N. K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C. H. Kim, A. K. Verma and E. H. Choi, Molecules, 2013, 18, 6620 CrossRef CAS PubMed.
- Q. Cai, Z. Li, J. Wei, C. Ha, D. Pei and K. Ding, Chem. Commun., 2009, 7581 RSC.
- S. G. Koenig, J. W. Dankwardt, Y. Liu, H. Zhao and S. P. Singh, ACS Sustainable Chem. Eng., 2014, 2, 1359 CrossRef CAS.
- S. G. Koenig, J. W. Dankwardt, Y. Liu, H. Zhao and S. P. Singh, Tetrahedron Lett., 2010, 51, 6549 CrossRef CAS.
- Z. Zhu, J. Yuan, Y. Zhou, Y. Qin, J. Xu and Y. Peng, Eur. J. Org. Chem., 2014, 511 CrossRef CAS.
- C. Zhao, Z. Sun, B. Liu and G. Peng, J. Macromol. Sci., Part B: Phys., 2013, 52, 1453 CrossRef CAS.
- T. H. Kim, S. T. Lim, C. H. Lee, H. J. Choi and M. S. Jhon, J. Appl. Polym. Sci., 2003, 87, 2106 CrossRef CAS.
- X. Shi and Z. Gan, Eur. Polym. J., 2007, 43, 4852 CrossRef CAS.
- M. Kozak and L. Domka, J. Phys. Chem. Solids, 2004, 65, 441 CrossRef CAS.
- Q. Huang, L. Zhou, X. Jiang, Y. Zhou, H. Fan and W. Lang, ACS Appl. Mater. Interfaces, 2014, 6, 13502 CAS.
- R. R. Tiwari, K. C. Khilar and U. Natarajan, Appl. Clay Sci., 2008, 38, 203 CrossRef CAS.
- G. Lan, Y. Yao, X. Zhang, M. Guo, H. Tang, Y. Li and Q. Yang, Catal. Sci. Technol., 2016, 6, 2181 CAS.
- M. Zhong, Y. Zhao, Q. Yang and C. Li, J. Catal., 2016, 338, 184 CrossRef CAS.
- Y. Yao, X. Zhang, J. Peng and Q. Yang, Chem. Commun., 2015, 51, 3750 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra25861a |
| This journal is © The Royal Society of Chemistry 2017 |