Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nano KF/Al2O3 particles as an efficient catalyst for no-glycerol biodiesel production by coupling transesterification
Ying Tang *,
Haomiao Ren,
Feiqin Chang,
Xuefan Gu and
Jie Zhang*
*,
Haomiao Ren,
Feiqin Chang,
Xuefan Gu and
Jie Zhang*
College of Chemistry and Chemical Engineering, Xi'an Shiyou University, Xi'an, Shaanxi 710065, China. E-mail: tangying78@xsyu.edu.cn; zhangjie@xsyu.edu.cn
First published on 17th January 2017
Abstract
In this study, an efficient solid base catalyst, nano KF/Al2O3, for no-glycerol biodiesel production was prepared using nano γ-Al2O3 particles as support, and was used in the tri-component coupling transesterification of canola oil, dimethyl carbonate and methanol. The preparation optimum conditions (blending temperature, blending time, calcination temperature and calcination time) as well as the loading amount of KF were screened in detail. A yield of biodiesel, 98.8%, was obtained under the conditions of KF loading of 10.0 wt%, calcination temperature of 400 °C, 2 h of reaction time at 338 K, 5.0 wt% catalysts and molar ratio of methanol/oil/DMC of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This high conversion of vegetable oil to biodiesel is considered to be associated with the high surface to volume ratio and basicity of the catalyst surface.
1. This high conversion of vegetable oil to biodiesel is considered to be associated with the high surface to volume ratio and basicity of the catalyst surface.
Introduction
Biodiesel is a renewable fuel that can be produced from vegetable oils, animal fats, or recycled restaurant grease for use in diesel vehicles. Its physical properties are similar to those of petroleum diesel, but cleaner when being burnt, thus making biodiesel a good alternative fuel.1,2 The prevailing method of biodiesel production in industry is transesterification of glycerol triglyceride with short-chain alcohols, such as methanol or ethanol, in the presence of soluble inorganic basic catalysts, such as sodium or potassium methoxides.3 Although the reaction rates for this process are high, recovery of catalysts is difficult, and treatment of waste mixtures containing catalysts, water, glycerol and oils presents challenges. Problems can be reduced by using solid catalysts, which can be filtered off or centrifuged out of suspensions. However, heterogeneous catalysis produces glycerol4,5 which needs an additional separation process, thus adding to the overall cost of biodiesel manufacture.6 Glycerin is not very valuable as a by-product which simply adds to the over-supply of the commodity and thus contributes to a price depression.7Generation of glycerol can be avoided through the reaction of vegetable oils and dimethyl carbonate (DMC) to produce glycerol carbonate (GC) as a secondary product, which does not require separation from the fatty acid esters.8 A direct reaction between DMC and triglycerides can occur in the presence of sodium methoxide or potassium hydroxide (KOH) when refluxed at 90 °C for 6 h.9 Ilham,10 Saka11 and Tan et al.12 used DMC to produce biodiesel and GC at supercritical conditions in non-catalytic processes, while other researchers used enzymes to produce biodiesel with DMC as reactant.13–15 In our previous research, an in-direct reaction between DMC and triglycerides has been established by adding methanol as the third reaction component.16 Although the tri-component transesterification improved the reaction rate to a relatively high level, it still didn't reach the theoretical maximum due to low diffusion rate between the three-phase systems made up by the three components. Reaction can only occur on the interfacial region between phases, and the only way to increase yield is to increase contact area.17 Our previous study focused on the use of nanocrystalline calcium oxides catalyst for the transesterification reaction,18 and obtained high biodiesel yield of 99% at room temperature. The high catalytic activity of the nano-based catalyst was deduced from its high surface/volume ratio.
As we know now, γ-Al2O3-supported catalysts have shown high activity in heterogeneous reactions including transesterification for biodiesel production.19 Yield of biodiesel can be greatly enhanced by the enormous increase of surface area of γ-Al2O3-supported nano materials. KF-Impregnated commercial nanoparticles of γ-Al2O3 have been used as heterogeneous catalysts for the transesterification of vegetable oil with methanol for the synthesis of biodiesel. In this paper, we present the results of nano KF/Al2O3 catalyzed coupling transesterification of vegetable oil, dimethyl carbonate and methanol to produce biodiesel at moderate conditions. The catalytic activities and the effect of nano γ-Al2O3 preparation condition on the yield of biodiesel were studied as well.
Experiments
Materials
Refined rapeseed or canola oil was purchased from Xi'an coal Co., Ltd. Dimethyl carbonate (DMC), methanol, analytical reagent grade, calcium carbonate (CC) (99% purity) and calcium oxalate monohydrate (CO) (98% purity) were purchased from Sinopharm Chemical Reagents Co., Ltd. (Beijing, China) and were used without further purification. Methyl heptadecanoate (gas chromatographic standard), nano γ-Al2O3 (95% purity) and surfactant OP-10 (octyl phenol with 10 moles per L of ethoxylate) were purchased from Sigma.Catalyst preparation
Nano KF/γ-Al2O3 catalyst particles were prepared by the sol–gel method. Firstly, 5.0 g γ-Al2O3 was added into 50 mL of ethanol, and then a certain amount of KF·2H2O and 1 mL OP-10 was added in under stirring. The mixture was stirred for 2 h and a clear white gel was obtained. The white gel was kept at room temperature for 2 h. After that, the gel was heated up to 80 °C and kept for 2 h to vaporize water. Then the condensed gel was placed in a desiccator for 12 h to dry completely. The resulting dry gel was milled to white powder and calcinated under high temperature, and cooled to room temperature to complete the preparation procedure of catalyst KF/γ-Al2O3-OP. For comparison, the supported KF/γ-Al2O3 was prepared by impregnating KF over commercial γ-Al2O3 directly under same condition without ethanol.Materials characterizations
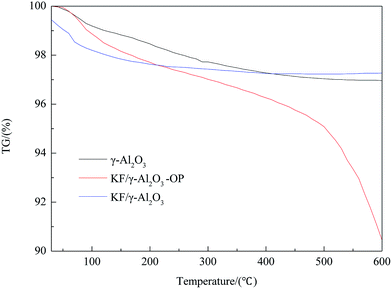
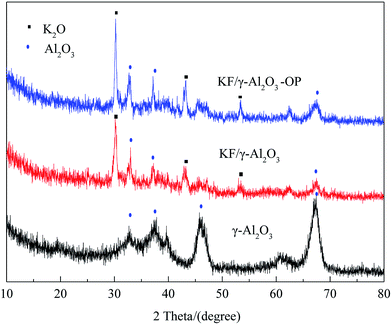
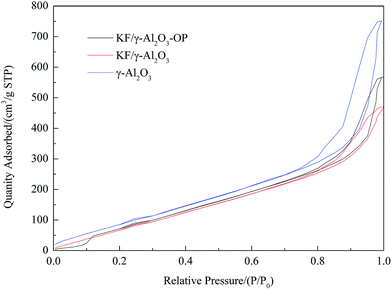
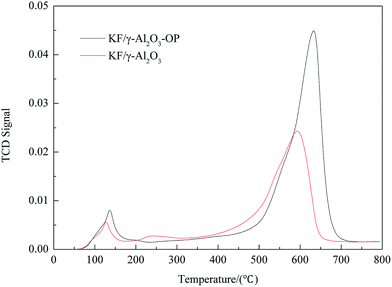
Thermo gravimetric analysis (TGA) of catalyst sample was performed in the static air condition 20–1000 °C with a heating rate of 5 °C min−1, using a thermo-gravimetric analyzer/simultaneous differential thermal analyzer (TGA/SDTA 851e, Mettler-Toledo, Switzerland). Textural characteristics were investigated by means of surface area determined by BET including mean pore diameter and pore volume using desorption isotherms on a Micromeritics ASAP2000. The crystalline structures of the products of calcination were analyzed using an X-ray diffraction device (JDX-3530, JEOL, Japan) with an X-ray tube that has copper Cu as target and released Kα radiation when accelerated at 30 mA and 40 kV. Scanning Electron Microscopy (SEM) was used for the investigation of surface morphology of catalyst samples. Before submitted to SEM characterization, the solid sample was coated with gold in order to achieve sufficient conductivity. Temperature-programmed desorption patterns of carbon dioxide (CO2-TPD) for the products were measured with an automated chemisorption analyzer (Autochem II 2920, Micromeritics, USA) at the temperature range from 40 to 800 °C with a heating rate of 10 °C min−1.Preparation and analysis of biodiesel
Prior to reaction, the oil was treated with sodium hydroxide, at a ratio of 1 mg KOH per g lipids, and with bentonite to, respectively, lower fatty acid and water concentrations below 1 mg g−1 of triglyceride. The reaction was performed in a three necked round bottomed flask equipped with a reflux condenser and a thermometer. A certain amount of KF/γ-Al2O3 and 8.01 g methanol were charged into the flask. Then 20.48 g rapeseed oil and 2.815 g DMC were added. The mixture was heated to and maintained at 65 °C with continuous stirring. Samples were taken from the reaction mixture for each hour, and quenched to room temperature. The catalyst was separated by centrifugation, and the excess methanol was distilled off under vacuum. Samples were quantitatively analyzed for composition on an HP-6890 gas chromatograph equipped with a flame ionization detector and a fused-silica capillary column (HP-5; 0.32 mm × 30 m, 0.1 μm film thickness) using methyl heptadecanoate as standard. The carrier gas was nitrogen with a flow rate of 20 mL min−1. The oven temperature was kept constant at 280 °C. Yield was defined as a ratio of the weight of fatty acid methyl esters in samples, as determined using the GC, to the weight of equivalent fatty acid methyl esters contained in the oil used in the reaction.Results and discussion
Characterizations of various samples
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1 × 10−2) | Pore size (nm) |
|---|---|---|---|
| γ-Al2O3 | 396.34 | 0.9054 | 91.3801 |
| KF/γ-Al2O3-OP | 389.97 | 0.7230 | 75.3185 |
| KF/γ-Al2O3 | 278.46 | 0.4328 | 50.3287 |
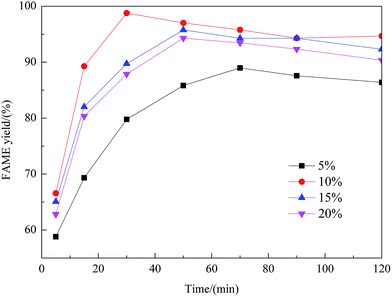
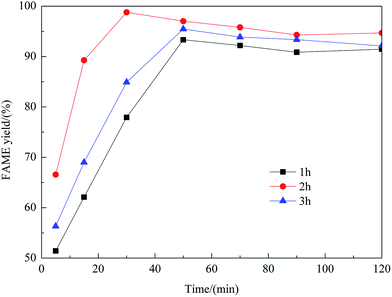
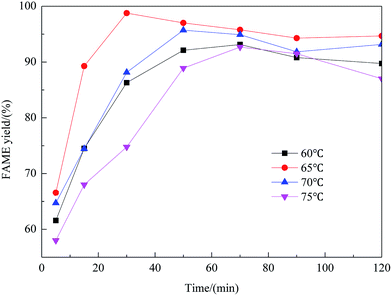
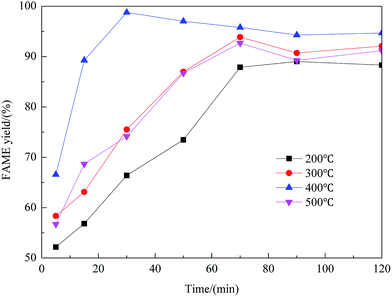
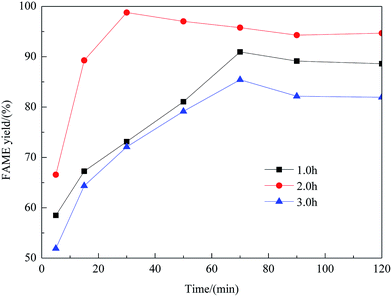
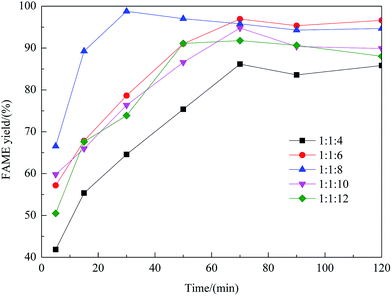
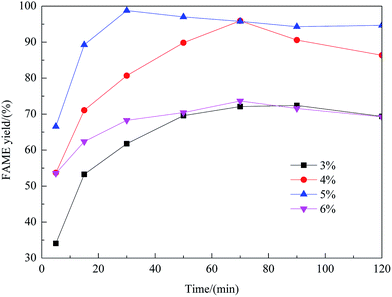
Effect of preparation parameters
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 of oil
8 of oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC
DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol under refluxing. For obtaining maximum reaction rate of the transesterification process, an overall optimization is necessary, and the reaction parameters which affect the yield of FAME have been itemized in detail.
methanol under refluxing. For obtaining maximum reaction rate of the transesterification process, an overall optimization is necessary, and the reaction parameters which affect the yield of FAME have been itemized in detail.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of rapeseed oil to DMC. As shown in this figure, with an increase of the methanol amount, the yield of FAME enhanced considerably. The maximum yield of FAME, 98.8%, was obtained as the molar ratio reached 8
1 molar ratio of rapeseed oil to DMC. As shown in this figure, with an increase of the methanol amount, the yield of FAME enhanced considerably. The maximum yield of FAME, 98.8%, was obtained as the molar ratio reached 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, beyond the molar ratio of 8
1. However, beyond the molar ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, excessive methanol caused the yield to decrease, which was possibly due to the mixing difficulty caused by mass transfer hindrance involving excess reactants, products and solid catalyst. Therefore, the optimum molar ratio of methanol to rapeseed oil was determined as 8
1, excessive methanol caused the yield to decrease, which was possibly due to the mixing difficulty caused by mass transfer hindrance involving excess reactants, products and solid catalyst. Therefore, the optimum molar ratio of methanol to rapeseed oil was determined as 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The comparison study
The catalytic performances of KF–γ-Al2O3-OP for biodiesel production with or without DMC have been investigated, and the properties of the product were studied. As presented in Table 2. Nearly 98.9% yield of FAME was obtained within 30 min using the proposed coupling transesterification under the optimized condition (65 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratio of methanol, oil to DMC), while transesterification between methanol and rapeseed oil resulted in 96.5% yield of FAME within 70 min. The main reason for high FAME yield over KF–γ-Al2O3-OP containing DMC should be contributed to the integration of two transesterifications of methanol/rapeseed oil and glycerol/DMC, which resulted in promoting the transesterification between rapeseed oil and methanol towards the favored direction as suggested in our previous results. As a result, the conversion of rapeseed oil was enhanced by activating and converting glycerol to glycerol carbonate, which shifted the transesterification of methanol and rapeseed oil favorably to give high FAME yield. The comparison study on the catalytic activity of KF/γ-Al2O3-OP and KF/γ-Al2O3 was carried out under the optimum reaction condition obtained above: 65 °C, 1
8 molar ratio of methanol, oil to DMC), while transesterification between methanol and rapeseed oil resulted in 96.5% yield of FAME within 70 min. The main reason for high FAME yield over KF–γ-Al2O3-OP containing DMC should be contributed to the integration of two transesterifications of methanol/rapeseed oil and glycerol/DMC, which resulted in promoting the transesterification between rapeseed oil and methanol towards the favored direction as suggested in our previous results. As a result, the conversion of rapeseed oil was enhanced by activating and converting glycerol to glycerol carbonate, which shifted the transesterification of methanol and rapeseed oil favorably to give high FAME yield. The comparison study on the catalytic activity of KF/γ-Al2O3-OP and KF/γ-Al2O3 was carried out under the optimum reaction condition obtained above: 65 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratios of oil/DMC/methanol, 5 wt% of catalyst. From this study it can be seen that the yield of FAME was enhanced to near to 98.8% in the presence of KF/γ-Al2O3-OP at 30 min, while with KF/γ-Al2O3 it needed 120 min to obtain only 37.3%. In view of the characteristic results of the catalysts, it is obvious that the activity change of the catalysts was well correlated to the change of their basicity. Besides, the surfactant can also improve the dispersion of active species over support as it greatly enhanced the forming of both K2O species and Al–O–K groups in the composite.
8 molar ratios of oil/DMC/methanol, 5 wt% of catalyst. From this study it can be seen that the yield of FAME was enhanced to near to 98.8% in the presence of KF/γ-Al2O3-OP at 30 min, while with KF/γ-Al2O3 it needed 120 min to obtain only 37.3%. In view of the characteristic results of the catalysts, it is obvious that the activity change of the catalysts was well correlated to the change of their basicity. Besides, the surfactant can also improve the dispersion of active species over support as it greatly enhanced the forming of both K2O species and Al–O–K groups in the composite.
| Reaction condition | Unit | KF/γ-Al2O3-OP | KF/γ-Al2O3 | |
|---|---|---|---|---|
| Without DMC | With DMC | With DMC | ||
| Reaction time | min | 70 | 30 | 120 |
| Reaction temperature | °C | 65 | 65 | 65 |
| Molar ratio | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| Amount of catalyst | % | 5 | 5 | 5 |
| Yield of FAME | % | 96.5 | 98.8 | 37.3 |
| Yield of glycerol carbonate | % | — | 98.6 | 37.2 |
The quality of produced oil
For the aim of being commercial applicable, the produced biodiesel must be characterized using specified analytical methods to ensure it meeting the international standards. Therefore, some properties, including viscosity, density, flash point and free glycerol value were investigated and listed in Table 3. The specifications of both biodiesel are close to the European standard, EN14214 and the literature data.25 It should be noted that the free glycerol in biodiesel obtained from the new method is within the range of European standard, thus indicates that the coupling transesterification eliminated glycerol efficiently as we suggested before. The viscosity of obtained biodiesel is lower than the limit of EN14214 due to the presence of glycerol derivates, and this property may improve the spray injection behavior of the fuel.| Fuel property | Unit | Prepared FAME | EU (EN14214) |
|---|---|---|---|
| Density (15 °C) | g mL−1 | 0.88 | 0.86–0.90 |
| Kinematic viscosity (40 °C) | mm2 s−1 | 3.18 | 3.5–5.0 |
| Pour point | °C | −9.4 | — |
| Cold filter plugging point | °C | −10.0 | — |
| Flash point | °C | 144.0 | ≥101 |
| Free glycerol | % (m m−1) | 0.018 | <0.02 |
Conclusion
In this research, nano-KF/γ-Al2O3 was prepared by blending powdered nano-γ-Al2O3 with an aqueous solution of KF followed by calcination at a high temperature in air. The dispersed nano-γ-Al2O3 increased the surfactivity greatly, and the K2O species formed during the thermal decomposition of loaded KF was probably the main reason for the high catalytic activity. Nearly 99.0% yield of FAME was obtained within 30 min using the proposed coupling transesterification under the optimized condition (65 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratio of methanol, oil to DMC). Furthermore, the reaction reduced the free glycerol content in biodiesel product to make it a great advantage of avoiding the risk of plug in fuel filters.
8 molar ratio of methanol, oil to DMC). Furthermore, the reaction reduced the free glycerol content in biodiesel product to make it a great advantage of avoiding the risk of plug in fuel filters.
Acknowledgements
This work was financially supported by grants from Natural Science Research Plan Projects of Shaanxi Science and Technology Department (2016JM2012), National Natural Science Foundation of China (21306149).References
- Q. Liu, R. R. Xin and C. C. Li, J. Environ. Sci., 2013, 25, 823–829 CrossRef CAS.
- A. J. Dassey, S. G. Hall and S. Chandra, Algal Res., 2014, 4, 89–95 CrossRef.
- F. Ma and M. A. Hanna, Bioresour. Technol., 1999, 70, 1–15 CrossRef CAS.
- J. Bone, J. Coata, S. Romain, J. S. Reneaume, A. E. Plesu and V. Plesu, Food Bioprod. Process., 2009, 111, 773–778 Search PubMed.
- G. El Diwani, N. K. Attia and S. I. Hawash, Int. J. Environ. Sci. Technol., 2009, 6, 219–224 CrossRef CAS.
- G. J. Van, Fuel Process. Technol., 2005, 86, 1097–1107 CrossRef.
- D. T. Johnson and K. A. Taconi, Environ. Prog., 2007, 26, 338–348 CrossRef CAS.
- D. Delledonne, F. Rivetti and U. Romano, Appl. Catal., A, 2001, 221, 241–251 CrossRef CAS.
- D. Fabbri, V. Bevoni, M. Notari and F. Rivetti, Fuel, 2007, 86, 690–697 CrossRef CAS.
- Z. Ilham and S. Saka, Bioresour. Technol., 2009, 100, 1793–1796 CrossRef CAS PubMed.
- S. Saka and Y. Isayama, Fuel, 2009, 88, 1307–1313 CrossRef CAS.
- G. T. Ang, K. T. Tan and K. T. Lee, Renewable Sustainable Energy Rev., 2014, 31, 61–70 CrossRef CAS.
- P. J. Seong, B. W. Jeon, M. Lee, D. H. Cho, D. K. Kim, K. S. Jung, S. W. Kim, S. O. Han, Y. H. Kim and C. Park, Enzyme Microb. Technol., 2011, 48, 505–509 CrossRef CAS PubMed.
- L. P. Zhang, S. Z. Sun, Z. Xin, B. Y. Sheng and Q. Liu, Fuel, 2010, 89, 3960–3995 CrossRef CAS.
- Y. M. Ji and E. Y. Lee, Biotechnol. Lett., 2011, 33, 1789–1796 CrossRef PubMed.
- Y. Tang, Q. T. Cheng, H. Cao, L. Zhang, J. Zhang and H. F. Li, C. R. Chim., 2015, 18, 1328–1334 CrossRef CAS.
- G. Wen, Z. F. Yan, M. Smith, P. Zhang and B. Wen, Fuel, 2010, 89, 2163–2165 CrossRef CAS.
- M. L. Granados, Appl. Catal., B, 2006, 3, 317–326 Search PubMed.
- K. Noiroj, P. Intarapong, A. Luengnaruemitchai and S. Jai-In, Renewable Energy, 2009, 34, 1145–1150 CrossRef CAS.
- K. Naemchanthara, S. Meejoo and W. Onreabroy, Adv. Mater. Res., 2008, 55, 333–336 CrossRef.
- M. J. Ramos, A. Casas, L. Rodríguez, R. Romero and Á. Pérez, Appl. Catal., A, 2008, 346, 79–85 CrossRef CAS.
- X. Deng, Z. Fang, Y. H. Liu and C. L. Yu, Energy, 2011, 36, 777–784 CrossRef CAS.
- H. Li, S. L. Niu and C. M. Lu, Fuel, 2016, 176, 63–71 CrossRef CAS.
- C. K. Lambert and R. D. Gonzalez, Microporous Mater., 1997, 12, 179–188 CrossRef CAS.
- C. S. Castro, L. C. F. G. Júnior and J. M. Assaf, Fuel Process. Technol., 2014, 125, 73–78 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |