Open Access Article
Open Access ArticleAl doping effects on LiCrTiO4 as an anode for lithium-ion batteries
Xiang Li ,
Yangyang Huang,
Yuyu Li,
Shixiong Sun,
Yi Liu,
Jiahuan Luo,
Jiantao Han and
Yunhui Huang
,
Yangyang Huang,
Yuyu Li,
Shixiong Sun,
Yi Liu,
Jiahuan Luo,
Jiantao Han and
Yunhui Huang
School of Materials Science and Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei 430074, China. E-mail: jthan@hust.edu.cn; huangyh@hust.edu.cn
First published on 17th January 2017
Abstract
Al-Doped LiCrTiO4 anode materials are successfully synthesized by a conventional solid-state reaction. Their structural and electrochemical properties are systematically investigated. With increasing the Al doping level (x), the lattice parameters of LiAlxCr1−xTiO4 get smaller. Meanwhile, asymmetric polarization was significantly reduced during the charge/discharge process, in contrast to an enhanced compatibility of electrode materials with organic electrolyte. The Al-doped LiAl0.2Cr0.8TiO4 anode can still keep a discharge capacity of 123 mA h g−1 at 1C for 100 cycles and 109 mA h g−1 at 2C. More importantly, the Al-doped LiAl0.2Cr0.8TiO4 anode exhibits remarkable electrochemical properties at a high-temperature of 60 °C with a very stable capacity of about 145 mA h g−1 at 1C, and is promising as a high-performance anode.
Introduction
Lithium-ion batteries (LIBs) have been extensively used in portable electronic devices, and electric and electric hybrid vehicles. Considering the profound and lasting influence on human life, the safety and cycle life of LIBs are important, and largely depend on the electrode materials used.1–4 Graphite is the most widely commercial anode material for LIBs, but suffers from poor rate capability and serious safety issues related to lithium dendritic growth especially at low temperature.4–15 Therefore, replacing graphite by other alternative anode materials is urgently required.Among various candidates, spinel Li4Ti5O12 is regarded as one of promising anode materials. It possesses many advantages over graphite. For example, Li4Ti5O12 has a stable and flat operating voltage at 1.5 V, which can prevent the growth of lithium dendrites to achieve safe and reliable high-power LIBs.16–22 Meanwhile, it is a zero-train insertion material, which results in a brilliant cycling performance.23–26 However, its poor electronic conductivity, low Li-ion diffusion coefficient and especially the phenomenon of battery bloating seriously limit the applications at high rate and/or at high temperature.
More recently, LiCrTiO4 has been comprehensively investigated due to its similar characteristics to Li4Ti5O12.27 LiCrTiO4 also gives a similar potential at 1.5 V vs. Li/Li+ and a small volume change (less than 0.7%) during electrochemical cycling. Compared with Li4Ti5O12, LiCrTiO4 has a much higher electronic conductivity of 4 × 10−6 S cm−1 and Li-ion diffusion coefficient of 10−9 cm2 s−1. We can image that LiCrTiO4 should be very promising as a high-rate and long-cycle anode for LIBs. To date, many efforts have been devoted to improving the electrochemical performance by using carbon coating, polymer incorporation and novel microstructure design.27–37 As we know, cationic doping is an effective method to enhance electrochemical performance of electrode materials in LIBs.38–40 For example, the Al substitution can significantly increase the reversible capacity and cycling stability of the LiNi1−x−yCoxMnyO2 system due to the stable crystal structure after Al doping. Introduction of Al3+ has been proven to be effective to prevent the capacity fade.41–51
In order to achieve better rate and cycle performance of LiCrTiO4, we employ Al doping in LiCrTiO4 and try to understand its role in electrochemical performance. Al-Doped LiCrTiO4 samples have been successfully synthesized by a traditional solid-state reaction. The Al-doped LiCrTiO4 exhibits an excellent rate performance and capacity retention. Comparing the electrochemical performances between Al-doped and pristine LiCrTiO4, we find that a small amount of Al doping can significantly improve rate capability and high-temperature performance of LiCrTiO4 anode.
Experimental
In a typical solid-state reaction, stoichiometric starting materials Li2CO3, TiO2, Al2O3 and Cr2O3 were thoroughly mixed, ground in a mortar and pressed into pellets under a pressure of 20 MPa. Subsequently, the pellets were heated in a box furnace at 850 °C for 16 h in air and cooled down to room temperature naturally.The phases of LiAlxCr1−xTiO4 were determined by X-ray diffraction (XRD, PANalytical B.V., Holland) with Cu Kα radiation of λ = 1.5405 Å. Rietveld refinement was carried out using the GSAS suite of programs with the EXPGUI interface. The morphology was observed by scanning electron microscopy (SEM, Nova nanoSEM 450) under 10 kV accelerating voltage. The high-resolution transmission electron microscopy (HR-TEM) images and selected area electron diffraction (SAED) patterns were recorded on a transmission electron microscope (JEM-2100).
Electrochemical performances were tested on CR2032 coin cells. The working electrodes were made from a slurry containing 80 wt% active material, 10 wt% C-black and 10 wt% polyvinylidene difluoride (PVDF) binder mixed in N-methyl pyrrolidinone (NMP). The slurry was pasted onto a copper foil. After being dried at 120 °C for 12 h in a vacuum oven, the foil was roll pressed and punched into round disks with a diameter of 8 mm. The loading of active material on each disk was ∼1.2 mg cm−2. The counter electrode was lithium metal. The electrolyte was 1 mol L−1 LiPF6 in a mixed solvent of ethylene carbonate and dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio). A thin sheet of microporous polyethylene (Celgard 2400) served as separator. The cells were assembled in an argon-filled glove box. Cyclic voltammetry (CV) was measured by an electrochemical workstation (PARSTAT MC, Princeton Applied Research, US) at a scan rate of 0.1 mV s−1 within a voltage range of 1.0–2.5 V. Electrochemical impedance spectroscopy (EIS) was also carried out on the PARSTAT MC with a potential amplitude of 5 mV in a frequency range of 105 to 10−1 Hz. Charge/discharge measurement was performed on a battery testing system (Land CT2001A, China) at 25 °C with various rates (0.1–5C).
1 volume ratio). A thin sheet of microporous polyethylene (Celgard 2400) served as separator. The cells were assembled in an argon-filled glove box. Cyclic voltammetry (CV) was measured by an electrochemical workstation (PARSTAT MC, Princeton Applied Research, US) at a scan rate of 0.1 mV s−1 within a voltage range of 1.0–2.5 V. Electrochemical impedance spectroscopy (EIS) was also carried out on the PARSTAT MC with a potential amplitude of 5 mV in a frequency range of 105 to 10−1 Hz. Charge/discharge measurement was performed on a battery testing system (Land CT2001A, China) at 25 °C with various rates (0.1–5C).
Results and discussion
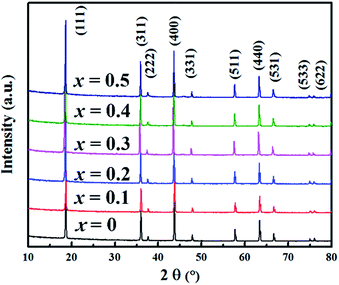
Al-Doped LiCrTiO4 can be readily synthesized via a conventional solid-state reaction. The XRD patterns of LiAlxCr1−xTiO4 (x = 0–0.5) are shown in Fig. 2. The diffraction peaks are indexed to the cubic spinel phase [space group 227, JCPDS no. 47-0139]. At a low-level Al doping, the difference in XRD patterns is not obvious. With increasing x, the diffractions slightly shift to high angles, demonstrating that Al substitution happens in the crystal lattice. Fig. 1a and c show the Rietveld refinement and the obtained lattice parameters, respectively. Table 1 lists the results of the refinements for LiAlxCr1−xTiO4 by assuming that Al ions are located at Ti sites. The best refinement model is based on F![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. With increasing x, lattice parameters a, b and c shrink while the cell volume becomes smaller. Since the ionic radius of Al3+ is smaller than that of Cr3+, it is reasonable that the lattice parameters enlarged with increasing x. Corresponding to the Rietveld refinements in Table 1, Fig. 1b shows that Al3+, Cr3+ and Ti4+ ions share the 16c position and Li+ ions sit at 8b position, and the spinel structure keeps stable after the introduction of Al3+ ions. The reliability factor Rp of all the Rietveld refinements is below 10%, indicating the reliability of the refinement results. The lattice change of LiAl0.1Cr0.9TiO4 is further identified by TEM and SAED, as shown in Fig. 3. The results agree well with the above description. The interplanar spacing of [111] is ∼4.95 Å, much close to that of pristine LiCrTiO4.
m space group. With increasing x, lattice parameters a, b and c shrink while the cell volume becomes smaller. Since the ionic radius of Al3+ is smaller than that of Cr3+, it is reasonable that the lattice parameters enlarged with increasing x. Corresponding to the Rietveld refinements in Table 1, Fig. 1b shows that Al3+, Cr3+ and Ti4+ ions share the 16c position and Li+ ions sit at 8b position, and the spinel structure keeps stable after the introduction of Al3+ ions. The reliability factor Rp of all the Rietveld refinements is below 10%, indicating the reliability of the refinement results. The lattice change of LiAl0.1Cr0.9TiO4 is further identified by TEM and SAED, as shown in Fig. 3. The results agree well with the above description. The interplanar spacing of [111] is ∼4.95 Å, much close to that of pristine LiCrTiO4.
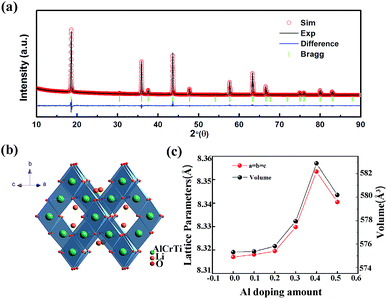 | ||
| Fig. 1 (a) Rietveld refinement of XRD patterns of LiAl0.2Cr0.8TiO4, (b) crystallographic arrangement of LiAl0.2Cr0.8TiO4, (c) lattice parameters variation with Al doping amount. | ||
| Sample | x = 0.1 | x = 0.2 | x = 0.3 | x = 0.4 | x = 0.5 |
|---|---|---|---|---|---|
| a = b = c/Å | 8.3181(3) | 8.3195(1) | 8.3298(1) | 8.3534(2) | 8.3405(1) |
| V/Å3 | 575.53(7) | 575.82(3) | 577.96(5) | 582.89(5) | 580.19(2) |
| Rp | 11.34% | 9.12% | 10.26% | 10.6% | 12.47% |
| Rwp | 8.34% | 6.4% | 7.36% | 7.2% | 8.54% |
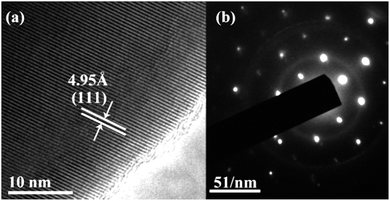 | ||
| Fig. 3 (a) Transmission electron microscopic image of LiAl0.1Cr0.9TiO4, (b) selected area electron diffraction (SAED) pattern of LiAl0.1Cr0.9TiO4. | ||
Fig. 4 and 5 give SEM images of LiAlxCr1−xTiO4 samples. It is very clear that all the samples present irregular particles. Elemental mappings in Fig. 5 show that Al, Cr and Ti elements are uniformly distributed with less Al dispersion due to a small amount of dopant, which demonstrates that Al exists in the material proportionately.
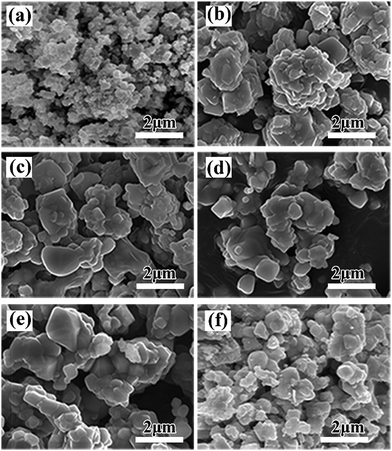 | ||
| Fig. 4 SEM images of LiAlxCr1−xTiO4: (a) x = 0.0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3, (e) x = 0.4, (f) x = 0.5. | ||
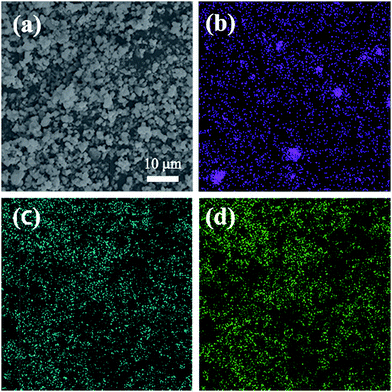 | ||
| Fig. 5 Elemental mapping for the particles of the LiAl0.2Cr0.8TiO4: (a) SEM image, (b) Al, (c) Cr, (d) Ti. | ||
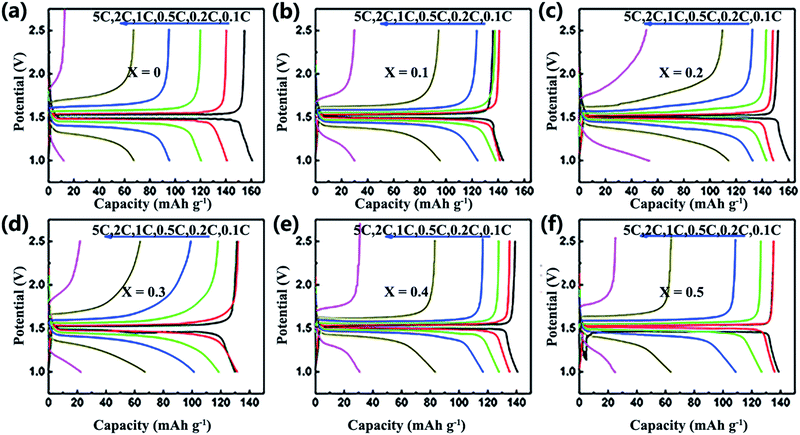
Fig. 6a shows the discharge/charge capacities of the LiAlxCr1−xTiO4 electrodes at rates of 0.1–10C from 1.0 to 2.5 V. Apparently all the samples exhibited almost the same specific capacity close to the theory capacity at lower rate, as an increasing current, pure samples showed a rapid fading capacity of 95 mA h g−1 at 150 mA g−1. The specific discharge capacities are 101, 132, 122, 113 and 109 mA h g−1 for x = 0.1, 0.2, 0.3, 0.4 and 0.5, respectively. All samples deliver extremely low capacity at 1.5 A g−1 owing to the structure collapse. As shown in Fig. 6b, the LiAl0.2Cr0.8TiO4 displays much better cyclability with a stable and broad charge/discharge plateau than LiCrTiO4, and its columbic efficiency is nearly 100% at 1C during 200 cycles. It is obvious that the electrochemical performances of LiCrTiO4 can be remarkably improved by Al doping.
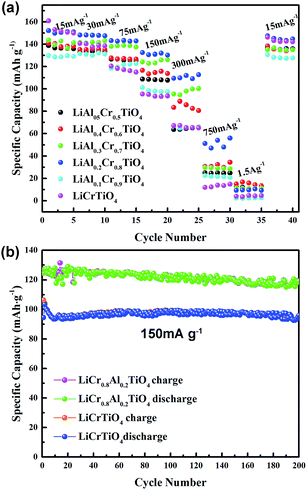 | ||
| Fig. 6 Electrochemical properties of LiAlxCr1−xTiO4: (a) rate performance at different current rate from 0.1C to 10C, (b) specific capacity for 200 cycles at 150 mA h g−1. | ||
Fig. 7 displays the initial charge/discharge curves of the LiAlxCr1−xTiO4 electrodes measured at different current densities within a voltage range of 1–2.5 V. Compared with pristine LiCrTiO4, the capacities of Al-doped LiCrTiO4 are greatly enhanced, especially at high current densities. The improved rate capability can be ascribed to a kinetic effect that Al-doped LiCrTiO4 has a high ionic conductivity as a host for Li-ion intercalation/extraction.
In order to investigate the comprehensive performances of Al-doped LiCrTiO4 anodes, the cells were tested in a broad temperature range from 0 to 60 °C at 1C. As shown in Fig. 8a, the gap between charge and discharge curves of LiCrTiO4 becomes broader and the voltage plateau becomes shorter and shorter with decreasing temperature. Both the capacity and the discharge voltage plateau drop obviously as temperature decreases, which is ascribed to slow conductivity and high charge-transfer resistance of the electrode/electrolyte interface. Compared with LiCrTiO4, LiAl0.2Cr0.8TiO4 delivers a better electrochemical performance at a broad temperature range (Fig. 8b). A possible mechanism might be that the Al doping improves the compatibility of the electrode materials with the organic electrolyte especially at high temperature and significantly reduces the asymmetric polarization in charge/discharge process at high rate.50–52 What's more, many practical applications require LIBs to be capable of operating within a lager temperature range even more than 60 °C.
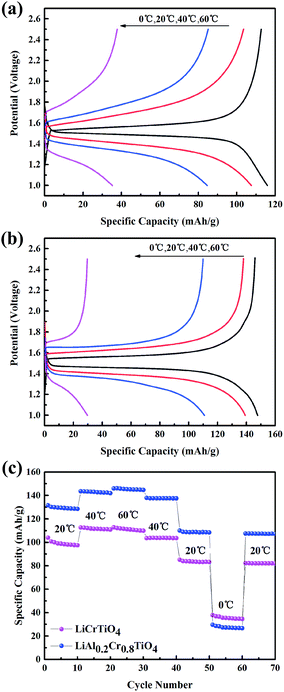 | ||
| Fig. 8 (a) Galvanostatic charge and discharge curves of LiAlCrTiO4 (b) LiAl0.2Cr0.8TiO4, (c) the electrochemical performance of Al-doped and pristine samples at different temperature at 1C. | ||
Fig. 9 shows the CV curves of the pristine LiCrTiO4 and the LiAl0.2Cr0.8TiO4 at different scan rates from 0.1 to 1 mV s−1 within 1.0–2.5 V. It can be seen obviously that there is one couple of reversible redox peaks, corresponding to the lithium insertion/extraction reactions. Fig. 9c displays the relationship between the cathodic peak current and the square root of the scan rate. It can be expressed by the classical Randles–Sevick equation: ip = 2.69 × 105n3/2AC0D1/2ν1/2, where ip is the peak current, n is the number of electrons per molecule during the intercalation, A is the surface area of the anode, C0 is the concentration of lithium ions [mol cm−3], D is the diffusion coefficient of lithium ions, and ν is the scan rate. Based on the above equation and the slopes of ip vs. ν1/2 plots, the calculated Li-ion diffusion coefficients of LiCrTiO4 and LiAl0.2Cr0.8TiO4 are 1.533 × 10−11 and 2.046 × 10−11 cm2 s−1, respectively. The results demonstrate that the Al-doped LiCrTiO4 allows a higher Li-ion diffusion, which is in good agreement with the rate capabilities in Fig. 6.
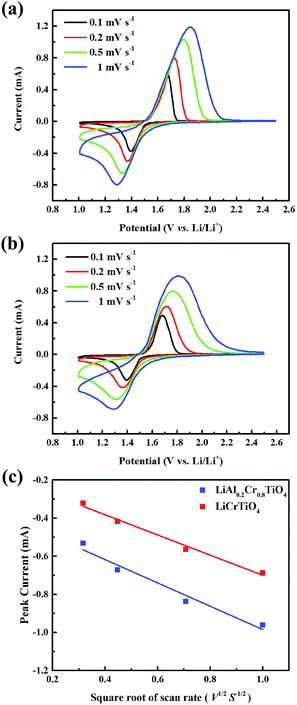 | ||
| Fig. 9 CV curves at different rates for (a) LiAl0.2Cr0.8TiO4 and (b) LiCrTiO4; (c) relationship between the peak current (ip) and square root of scan root (ν1/2). | ||
We evaluated the internal resistance of lithium-half cells with EIS. In Fig. 10c. The resistance R1, R2 and R3 refer to the electrolyte resistance, charge transfer resistance and ionic migration through solid electrolyte interface (SEI), respectively. The CPE1 and CPE2 describe the capacitive behaviors between electrode and SEI, SEI and electrolyte, respectively. The Wo1 represents the Warburg diffusion impedance. In Fig. 10a, the Nyquist plots reveal that the resistance varies with the Al doping level. The sloping line in low frequency corresponding to Rct indicates that Li-ion diffusion resistance decreases due to gradual Al substitution, which suggests that the Al doping is beneficial to Li+-ion diffusion in the LiAlxCr1−xTiO4 system. The Fig. 10b shows that the Rct reduces gradually after 100 cycles, which also is in accord with the above results.
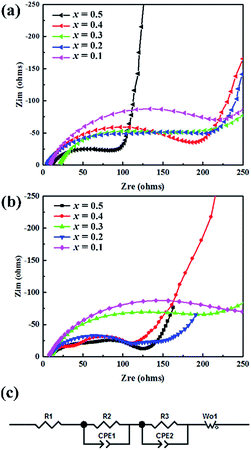 | ||
| Fig. 10 AC impedance spectra of LiAlxCr1−xTiO4/Li half-cell: (a) fresh cells, (b) after 100 cycles, (c) equivalent circuit of LiAl0.2Cr0.8TiO4 electrode. | ||
Conclusions
Al-Doped LiCrTiO4 samples were synthesized by one-step solid-state reaction. The Rietveld refinement analysis shows that the Al-doped LiCrTiO4 samples maintain the original spinel structure. The LiAl0.2Cr0.8TiO4 exhibits a high capacity of 150 mA h g−1 at a current density of 25 mA g−1, which is close to its theoretical capacity of 157 mA h g−1. It keeps a discharge capacity of 123 mA h g−1 at 1C and 109 mA h g−1 at 2C for 200 cycles. The Al-doped LiCrTiO4 electrodes show a stable cycle performance, especially at high temperature, with less asymmetric polarization. EIS results demonstrate that both electrode kinetics and ionic transport in LiCrTiO4 are facilitated by Al doping. Consequently, the Al doping could be responsible for the high rate capacity and outstanding cycling stability. Our results show that LiCrTiO4 is a promising anode material with high-rate and high-temperature performances for lithium-ion batteries.Acknowledgements
This work is supported by the National High-Tech R&D Program of China (No. 2015AA034600, 2016YFB010030X and 2016YFB0700600), the China Postdoctoral Science Foundation (2016M590691). In addition, the authors thank Analytical and Testing Center of Huazhong University of Science and Technology for XRD, SEM and TEM measurement.Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- L. Zhao, Y.-S. Hu, H. Li, Z. Wang and L. Chen, Adv. Mater., 2011, 23, 1385–1388 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- B. Luo and L. Zhi, Energy Environ. Science., 2015, 8, 456–477 RSC.
- S. Han, D. Wu, S. Li, F. Zhang and X. Feng, Adv. Mater., 2014, 26, 849–864 CrossRef CAS PubMed.
- D. Aurbach, B. Markovsky, I. Weissman, E. Levi and Y. Ein-Eli, Electrochim. Acta, 1999, 45, 67–86 CrossRef CAS.
- R. Yazami, Electrochim. Acta, 1999, 45, 87–97 CrossRef CAS.
- A. Funabiki, M. Inaba, Z. Ogumi, S. Yuasa, J. Otsuji and A. Tasaka, J. Electrochem. Soc., 1998, 145, 172–178 CrossRef CAS.
- M. Winter, P. Novak and A. Monnier, J. Electrochem. Soc., 1998, 145, 428–436 CrossRef CAS.
- M. Yoshio, H. Y. Wang, K. Fukuda, Y. Hara and Y. Adachi, J. Electrochem. Soc., 2000, 147, 1245–1250 CrossRef CAS.
- D. Aurbach, E. Zinigrad, Y. Cohen and H. Teller, Solid State Ionics, 2002, 148, 405–416 CrossRef CAS.
- K. Takada, T. Inada, A. Kajiyama, H. Sasaki, S. Kondo, M. Watanabe, M. Murayama and R. Kanno, Solid State Ionics, 2003, 158, 269–274 CrossRef CAS.
- B. Fang, M.-S. Kim, J. H. Kim, S. Lim and J.-S. Yu, J. Mater. Chem., 2010, 20, 10253–10259 RSC.
- M.-S. Kim, B. Fang, J. H. Kim, D. Yang, Y. K. Kim, T.-S. Bae and J.-S. Yu, J. Mater. Chem., 2011, 21, 19362–19367 RSC.
- M.-S. Kim, D. Bhattacharjya, B. Fang, D.-S. Yang, T.-S. Bae and J.-S. Yu, Langmuir, 2013, 29, 6754–6761 CrossRef CAS PubMed.
- L. Zhao, Y. S. Hu, H. Li, Z. X. Wang and L. Q. Chen, Adv. Mater., 2011, 23, 1385–1388 CrossRef CAS PubMed.
- L. Aldon, P. Kubiak, M. Womes, J. C. Jumas, J. Olivier-Fourcade, J. L. Tirado, J. I. Corredor and C. P. Vicente, Chem. Mater., 2004, 16, 5721–5725 CrossRef CAS.
- A. S. Prakash, P. Manikandan, K. Ramesha, M. Sathiya, J. M. Tarascon and A. K. Shukla, Chem. Mater., 2010, 22, 2857–2863 CrossRef CAS.
- E. M. Sorensen, S. J. Barry, H. K. Jung, J. R. Rondinelli, J. T. Vaughey and K. R. Poeppelmeier, Chem. Mater., 2006, 18, 482–489 CrossRef CAS.
- H. G. Jung, S. T. Myung, C. S. Yoon, S. B. Son, K. H. Oh, K. Amine, B. Scrosati and Y. K. Sun, Energy Environ. Science., 2011, 4, 1345–1351 RSC.
- K. Zaghib, M. Simoneau, M. Armand and M. Gauthier, J. Power Sources, 1999, 81, 300–305 CrossRef.
- K. S. Park, A. Benayad, D. J. Kang and S. G. Doo, J. Am. Chem. Soc., 2008, 130, 14930–14931 CrossRef CAS PubMed.
- T.-F. Yi, S.-Y. Yang and Y. Xie, J. Mater. Chem. A, 2015, 3, 5750–5777 CAS.
- H. H. Xu, X. L. Hu, W. Luo, Y. M. Sun, Z. Yang, C. C. Hu and Y. H. Huang, ChemElectroChem, 2014, 1, 611–616 CrossRef.
- H. Xu, X. Hu, Y. Sun, W. Luo, C. Chen, Y. Liu and Y. Huang, Nano Energy, 2014, 10, 163–171 CrossRef CAS.
- T. Ohzuku, A. Ueda and N. Yamamoto, J. Electrochem. Soc., 1995, 142, 1431–1435 CrossRef CAS.
- D. Liu, J. Han, M. Dontigny, P. Charest, A. Guerfi, K. Zaghib and J. B. Goodenough, J. Electrochem. Soc., 2010, 157, A770 CrossRef CAS.
- M. A. K. L. Dissanayake, R. P. Gunawardane, H. H. Sumathipala and A. R. West, Solid State Ionics, 1995, 76, 215–220 CrossRef CAS.
- M. A. Arillo, M. L. López, M. T. Fernández, M. L. Veiga and C. Pico, J. Solid State Chem., 1996, 125, 211–215 CrossRef CAS.
- M. Nakayama, Y. Ishida, H. Ikuta and M. Wakihara, Solid State Ionics, 1999, 117, 265–271 CrossRef CAS.
- A. Kuhn, M. Martin and F. Garcia-Alvarado, Z. Anorg. Allg. Chem., 2008, 634, 880–886 CrossRef CAS.
- C. V. Rao and B. Rambabu, Solid State Ionics, 2010, 181, 839–843 CrossRef CAS.
- V. Aravindan, W. Chuiling and S. Madhavi, J. Mater. Chem., 2012, 22, 16026–16031 RSC.
- V. Aravindan, W. C. Ling and S. Madhavi, ChemPhysChem, 2012, 13, 3263–3266 CrossRef CAS PubMed.
- X. Y. Feng, C. Shen, N. Ding and C. H. Chen, J. Mater. Chem., 2012, 22, 20861–20865 RSC.
- L. Wang, Q. Z. Xiao, L. J. Wu, G. T. Lei and Z. H. Li, Solid State Ionics, 2013, 236, 43–47 CrossRef CAS.
- J. W. Yang, B. Yan, J. Ye, X. Li, Y. S. Liu and H. P. You, Phys. Chem. Chem. Phys., 2014, 16, 2882–2891 RSC.
- M. Abbate, S. M. Lala, L. A. Montoro and J. M. Rosolen, Electrochem. Solid-State Lett., 2005, 8, A288–A290 CrossRef CAS.
- J. H. Lee, J. K. Hong, D. H. Jang, Y. K. Sun and S. M. Oh, J. Power Sources, 2000, 89, 7–14 CrossRef CAS.
- M. R. Yang and W. H. Ke, J. Electrochem. Soc., 2008, 155, A729–A732 CrossRef CAS.
- P. F. Wang, P. Li, T. F. Yi, X. T. Lin, Y. R. Zhu, L. Y. Shao, M. Shui, N. B. Long and J. Shu, J. Power Sources, 2015, 293, 33–41 CrossRef CAS.
- M. M. Lao, P. Li, P. F. Wang, X. Zheng, W. J. Wu, M. Shui, X. T. Lin, N. B. Long and J. Shu, Electrochim. Acta, 2015, 176, 694–704 CrossRef CAS.
- J. Y. Lin, C. C. Hsu, H. P. Ho and S. H. Wu, Electrochim. Acta, 2013, 87, 126–132 CrossRef CAS.
- H. L. Zhao, Y. Li, Z. M. Zhu, J. Lin, Z. H. Tian and R. L. Wang, Electrochim. Acta, 2008, 53, 7079–7083 CrossRef CAS.
- S. H. Park, K. S. Park, Y. K. Sun, K. S. Nahm, Y. S. Lee and M. Yoshio, Electrochim. Acta, 2001, 46, 1215–1222 CrossRef CAS.
- A. R. Cho, J. N. Son, V. Aravindan, H. Kim, K. S. Kang, W. S. Yoon, W. S. Kim and Y. S. Lee, J. Mater. Chem., 2012, 22, 6556–6560 RSC.
- S. T. Myung, N. Kumagai, S. Komaba and H. T. Chung, Solid State Ionics, 2001, 139, 47–56 CrossRef CAS.
- A. B. Yuan, L. Tian, W. M. Xu and Y. Q. Wang, J. Power Sources, 2010, 195, 5032–5038 CrossRef CAS.
- S. T. Myung, S. Komaba and N. Kumagai, J. Electrochem. Soc., 2001, 148, A482–A489 CrossRef CAS.
- T. Kakuda, K. Uematsu, K. Toda and M. Sato, J. Power Sources, 2007, 167, 499–503 CrossRef CAS.
- L. F. Xiao, Y. Q. Zhao, Y. Y. Yang, Y. L. Cao, X. P. Ai and H. X. Yang, Electrochim. Acta, 2008, 54, 545–550 CrossRef CAS.
- T. Yi, X. Hu and K. Gao, J. Power Sources, 2006, 162, 636–643 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |