Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nano-hydroxyapatite reinforced polyphenylene sulfide biocomposite with superior cytocompatibility and in vivo osteogenesis as a novel orthopedic implant†
Yi Denga,
Yuanyi Yangd,
Yuan Mac,
Kexia Fanc,
Weizhong Yang*b and
Guangfu Yinb
aSchool of Chemical Engineering, Sichuan University, Chengdu 610065, China
bCollege of Materials Science and Engineering, Sichuan University, Chengdu 610065, China. E-mail: scuywz@139.com
cDepartment of Neurosurgery, Chengdu Military General Hospital, Chengdu 610083, China
dDepartment of Materials Engineering, Sichuan College of Architectural Technology, Deyang 618000, China
First published on 3rd January 2017
Abstract
The design of novel functional biomaterials that possess similar mechanical attributes as human bones, accompanied with admirable osteogenesis to replace conventional metallic implants would be an intriguing accomplishment, especially in the orthopedic, craniomaxillofacial and dental fields where biointerfaces with outstanding osseointegration are in high demand. Guided by this purpose, in the current study, nano-hydroxyapatite reinforced polyphenylene sulfide (PPS/nano-HA) biocomposites via a process of compounding and injection-molding, in an attempt to elevate the bioactivity and osteogenic properties of PPS, were successfully developed for the first time. The resultant binary composites were characterized in terms of topological structure, chemical composition, hydrophilicity, and water uptake capacity. Mechanical property evaluation revealed that the elastic modulus of the PSS/nano-HA composites was closer to that of natural bones. Besides, in vitro cytotoxicity, cell proliferation, alkaline phosphatase activity, osteocalcin expression and calcium mineral deposition all disclosed that the PSS/nano-HA bioactive composites evoked better cell viability and osteo-differentiation of osteoblasts on account of the contribution of the doped nano-HA. To our delight, in vivo assessment of the calvarial defect model by means of soft X-ray, histological observation, and real-time PCR analysis after 8 weeks confirmed the dramatically accelerated osteogenesis and osteointegration. Overall, our findings demonstrated that the nano-HA enriched PPS biocomposites with impressive cytocompatibility and osteogenic functions hold large potential in load-bearing orthopedic and dental applications. In addition, this work will, as expected, offer a crucial scientific basis and experimental fundamentals to support the adoption of PPS-based biomaterials as new hard tissue repair materials for further clinical therapy.
1. Introduction
Bone defects resulting from disease, aging, trauma, congenital abnormalities and surgical resections remain a serious fast-growing challenge in the medical area worldwide, and the associated annual healthcare expenditures are estimated to be tens of billions of dollars with a prominent increase over the coming decades.1,2 When irreparable bone damage occurs, and it cannot be regenerated by the in-house self-healing process of human body, orthopedic and dental implants are much-needed in clinic. Currently, the popular orthopedic biomaterials are still traditional metals such as titanium (Ti) alloys, stainless steels, cobalt-based alloys and so on, because of their unique characteristics in chemical constitution, mechanics, and biocompatibility.3,4 There are concerns, nevertheless, regarding potential release of harmful metal ions and radiopacity of metal alloys in vivo. Moreover, the mismatched elasticity between adjacent bone tissues and metals leads to implant failure and even bone resorption.5,6 To overcome these limitations, the design of innovative synthetic polymer with the goal of subrogating for metals is extensively pursued, and encourages an emerging area in the fields of orthopaedics, dentistry, and neurosurgery over the recent years.Some high-performance engineering plastics (HPEPs) including polyetheretherketone (PEEK),7–10 and polyphenylene sulfide (PPS), have a tremendous potential to replace metallic implants worthy of serious consideration for the following reasons: (1) different from metallic materials which present a large elastic modulus of over 100 GPa, whereas the two HPEPs has an elastic moduli between 2–4 GPa (PEEK ≈ 3–4 GPa;5,11 PPS ≈ 2–4 GPa (ref. 12 and 13)) closer to that of human cortical bone (about 18 GPa), which can mitigate concerns over the potential metal ion release and the risks of osteanabrosis and bone resorption caused by stress shielding.14 (2) The outstanding chemical resistance of PEEK and PPS can avoid the degradation caused by bio-corrosion, which often occurs on metallic implants. (3) PEEK and PPS, non-resorbable thermoplastic polymer, displayed the natural radiolucency, even magnetic resonance imaging (MRI) compatibility, and bioinertness nature, eliciting no positive response in the body.15 In the orthopaedic clinic, PEEK implants has been fabricated into spine cages for vertebral fusion, patient specific craniomaxillofacial implants such as skull plates and as arthroscopic suture anchors to repair anterior cruciate ligaments,16,17 since receiving USA Food and Drug Administration (FDA) approval in the late 1980s, but there is no publication focusing on PPS as potential biomaterials used in biomedical fields and medical devices.
PPS, a semi-crystalline polymer, that possesses an approximate crystallinity (Xc) of 29–36%, glass transition temperature (Tg) of about 85 °C, and melting point (Tm) at ∼272 °C,18 has harvested a considerable interest for numerous challenging applications ranging from aerospace science to industrial use.19,20 From the processing perspective, it is more readily manufactured by conventional plastic processing equipments, and shaped by machining and heat contouring to fit the shape of bones due to lower melting point and processing temperature compared with PEEK (melting point = 334 °C). In addition to abundant yield, lower production cost and comparative mechanical characteristics to PEEK can make PPS become a more potent candidate as bone-grafting materials than PEEK. However, some drawbacks such as bioinertness and inferior osteoconduction of PPS likewise impede its further application in clinic. Combining polymers with bioactive inorganic materials, such as β-tricalcium phosphate (β-TCP),21,22 hydroxyapatite (HA)23,24 and titanium dioxide (TiO2)25,26 through blending is considered a fascinating and advisable strategy to built biocomposite with appropriate properties for orthopedic purposes, because native bone per se is an organic–inorganic biocomposite organized on micro- and nanoscale.27
In view of the immanent osteoconduction and osseointegration potence,28 nano-hydroxyapatite (nano-HA, Ca10(PO4)6(OH)2), the chief mineral component of bone matrix, is one of the most appealing inorganic materials for applications in bone regenerative medicine, and therefore has been widely employed in hard tissue engineering to promote biological properties of bioinert polymer via compound approach.29,30 For instance, Yubao Li and his group previously constructed polyamide 66/nano-HA (PA66/nano-HA) composites by incorporating nano-HA particles within PA66 matrix to improve its stiffness, biocompatibility, and osteogenic differentiation activity.31,32 A PEEK/nano-HA biocomposite through twin screw extruder and injection molding was lately prepared in our lab as dental implant material. Compared to pure PEEK, the composite containing nano-HA exhibited enhanced mechanical behaviors, and favorable adhesion, proliferation and osteogenic conversion of human mesenchymal stem cells (hMSCs), as well as superior in vivo osseointegration in canine alveolar bone.5 Despite the attractive merits and progress in development of various high-performance biocomposites, the employment of HA as nano-reinforcement in PPS-based composites bestowing osteogenic activity and osseointegration on PPS for load-bearing orthopedic applications, to the best of our current knowledge, has not yet been investigated and reported. Herein, the aims of the present work are (1) to develop and characterize the foregoing PPS/nano-HA binary composite; (2) to investigate in vitro how osteoblast-like MG-63 cells responded on the nano-HA doped PPS interfaces in terms of proliferation, alkaline phosphatase (ALP) activity, osteocalcin (OCN) secretion, and extracellular matrix (ECM) mineralization; (3) to assess in vivo osteogenesis ability of bony tissue exposed to the biocomposite in rat calvarial defect model. This study is intended to help broaden the clinic application and expedite the pace of PPS-based materials to orthopedic/dental implants.
2. Materials and methods
2.1. Materials
Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), ammonium phosphate dibasic ((NH4)2HPO4), tetramethylene sulfone (C4H8O2S), dimethylsilicone oil ((C2H6OSi)n, ρ = 0.963 g cm−3), and absolute ethyl alcohol (C2H5OH) were provided by Chengdu Kelong Reagent Co., Ltd. (China). Ammonium hydroxide (NH3·H2O, 25–28%) was purchased from Chongqing Maoye Reagent Co., Ltd. (China). Polyphenylene sulfide (PPS, (–C6H4-4-S–)n, Mn ≈ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) powders with the density of about 1.34 g cm−3 were supplied from Sichuan Deyang Chemical Co., Ltd. (China). (3-Aminopropyl)triethoxysilane (APTES, H2N(CH2)3Si(OC2H5)3) was obtained from Sichuan Huatong Co., Ltd. (China). All other chemicals were of analytical reagent grade and were used as received unless noted. All aqueous solutions were prepared with de-ionized water (D.I. water).
000 g mol−1) powders with the density of about 1.34 g cm−3 were supplied from Sichuan Deyang Chemical Co., Ltd. (China). (3-Aminopropyl)triethoxysilane (APTES, H2N(CH2)3Si(OC2H5)3) was obtained from Sichuan Huatong Co., Ltd. (China). All other chemicals were of analytical reagent grade and were used as received unless noted. All aqueous solutions were prepared with de-ionized water (D.I. water).
2.2. Synthesis of nano-HA particles
Nano-HA was synthesized in our laboratory via chemical precipitation method: firstly, Ca(NO3)2 and (NH4)2HPO4 were dissolved in D.I. water separately according to a Ca/P molar ratio of 1.67/1. Then, (NH4)2HPO4 solution was slowly dropped into Ca(NO3)2 solution with continuous stirring. Apatite growth occurred when kept at 40 °C for 6 h in an oil-bath, and the pH value of supernatant was adjusted and maintained to approximate 10 by addition of NH3·H2O during the whole experiment. The reaction of HA can be expressed by the reaction:| 10Ca2+ + 6HPO42− + 8OH− → Ca10(PO4)6(OH)2 + 6H2O | (1) |
After reaction, HA slurry was aged for 24 h at room temperature, and the precipitate was obtained after washing with D.I. water and ethanol at least three times, respectively. Finally, the prepared nano-HA particles was air-dried overnight in an oven at 80 °C for future use and characterization.
2.3. Preparation of PPS/nano-HA biocomposites
PPS/nano-HA binary composite containing 30 wt% and 40 wt% nano-HA powders were fabricated by two different approaches: (1) solid–solid blending (physical blending) of PPS and nano-HA: in briefly, a defined amount of PPS, nano-HA powders, and APTES (1 wt%) were blended in a QM-3B high-speed vibrating ball mill (Nanjing T-Bota Scietech Instruments & Equipment Co., Ltd., China) at a mixing speed of 500 rpm for 1 h. APTES was used as coupling agent to increase the dispersity and bonding strength of nano-HA and PPS in the present study. Following this, the resulting mixtures were then dried at 80 °C for 12 h. (2) Liquid–liquid compounding of PPS and nano-HA: the PPS powder was first dissolved in tetramethylene sulfone at a concentration of 20 w/v%, and the obtained nano-HA was re-suspended in D.I. water by ultrasonic and vigorous stirring to yield a nano-HA slurry. Then, PPS solution, nano-HA slurry and 1 wt% APTES were put into a three-necked bottle and compounded at 120 °C in an oil-bath under nitrogen (N2) atmosphere for 4 h to remove the water. The use of N2 was to prevent the oxidation of PPS. After complete dehydration, the temperature of sample was elevated to 174 °C for 5 h. The PSS/nano-HA mixture was rinsed with hot D.I. water and ethanol at least three times respectively, and dried at 80 °C for 12 h.Finally, these PPS/nano-HA binary composites prepared from solid–solid blending and liquid–liquid compounding methods were all producted with a CJ-150M2 injection-molding machine (Zhengxiong Injection Molding Machine Co., Ltd., Shenzhen, China) at an injecting and molding temperature of 300 °C under a load of 30 MPa. After reaching the target temperature, the temperature and pressure were held for 10 min. Then the die and samples were air cooled to 150 °C, and the samples were removed from the molds. All samples were cut into 2 mm thick disks with diameter of 10 mm for surface characterization and in vitro testing, and 1 mm thick disks with diameter of 6 mm for in vivo measurement. Bare PPS samples were also prepared in the light of the same process and cut into the same shapes as control group. The specific synthetic condition of each sample was listed in Table 1.
| Sample name | PPS content (wt%) | Nano-HA content (wt%) | Method |
|---|---|---|---|
| s-PPS7/nano-HA3 | 70 | 30 | Solid–solid blending |
| s-PPS6/nano-HA4 | 60 | 40 | Solid–solid blending |
| l-PPS7/nano-HA3 | 70 | 30 | Liquid–liquid compounding |
| l-PPS6/nano-HA4 | 60 | 40 | Liquid–liquid compounding |
2.4. Morphological and physic-chemical characterization
The crystalline phase of as-prepared nano-HA was examined by X-ray diffraction (XRD, XpertTRO MPD, Philips, Netherlands) using a Cu target as radiation source (λ = 1.540598 Å) at 40 kV. The diffraction angles (2θ) were set between 10° and 70°, with an incremental step size of 4° min−1. The phase identification was achieved by comparing the sample diffraction pattern with standard cards in ICDD-JCPDS database.The microstructure of nano-HA crystals was carried out using transmission electron microscopy (TEM, Tecnai G2 F20, FEI, USA) with an operating voltage of 100 kV. Samples for TEM imaging were dispersed into ethanol by ultrasonic waves, and the suspension was dropped onto carbon-coated copper grids, air-dried before observation.
Nitrogen adsorption–desorption isotherms of samples were collected on micromeritics porosimeter (Tristar 3000, Micrometrics Instrument Corp., USA) at 77 K under a continuous adsorption condition.
Fourier transform infrared (FT-IR, Magna-IR 750, Nicolet, USA) spectra was collected to identify the functional groups of the HA powders, PPS and PPS/nano-HA composites in the range of 400–4000 cm−1.
The surface hydrophilicity was determined by a contact angle goniometry (JC200C1, Shanghai Zhongchen Digital Technic Apparatus Co., Ltd., China) based on the sessile drop method using 2 mL of D.I. water droplets under ambient temperature and humidity. Measurements were taken until droplets were well settled on samples and repeated in triplicate, at six different positions per substrate type.
The surface topologies of pure PPS, and PPS/nano-HA powders were observed by field-emission scanning electron microscopy (FE-SEM, JSM-7500F, JEOL, Japan). Before observation, the samples were sputtered with gold for 60 s and examined at an accelerating voltage of 5 kV.
Water absorption assay of the PPS/nano-HA biocomposites was performed by incubating dry samples into D.I. water for 3, 5, and 7 days at 37 °C. At noted intervals, specimens were taken out, and any visible surface moisture was wiped off using filter papers. These samples were weighed using a digital balance (Jinghai Instruments Co., Ltd., Shanghai, China) with a precision of 0.1 mg. The water uptake percentage at any given time (Wt) in samples was determined from:
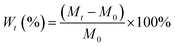 | (2) |
Mechanical property evaluation including ultimate tensile strength and bending strength of pristine PPS and PPS/nano-HA composites was performed by universal testing machine (AGS-J, Strider Instruments, Shanghai, China) at a loading velocity of 3 cm min−1. Before measurement, the samples were cut to be 4 mm in thickness, 10 mm in width, and 6 mm in length for test. The elastic modulus and flexural modulus of samples were obtained from stress–strain curves. Six pieces of samples were used to improve the statistics.
The thermal analysis including thermogravimetry and differential scanning calorimeter (TG-DSC) was carried out to supply the information of phase transformation of the final composites. A small fragment weighing about 11 mg cut from the material was heated from 30 °C to 1000 °C with a heating rate of 10 °C min−1 under a nitrogen atmosphere.
2.5. Immersion test in SBF
The 1× SBF (Table S1†) was prepared by dissolving the following chemicals in the sequence of NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2, and Na2SO4 in D.I. water and buffering to pH 7.4 with (CH2OH)3CNH2 (Tris) and 1 M HCl at 37 °C. Pure PPS and PSS/nano-HA samples were immersed in SBF solution at 37 °C in a static condition for 10 days. After soaking at scheduled time, the pH of the solution was monitored by a pH meter (Sartorius, pb-10, Germany) with an accuracy of ±0.02. Six samples in each stage were measured to provide an average and standard deviation.2.6. Hemocompatibility assessments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was taken and diluted with normal saline (4
1 was taken and diluted with normal saline (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio by volume). The PPS/n-HA biocomposite and pure PPS (Φ10 mm × 2 mm) was dipped in a standard tube containing 10 mL of normal saline that was previously incubated at 37 °C for 30 min. Then 0.2 mL of diluted blood was added to this standard tube, and the mixtures were incubated at 37 °C for 60 min per ISO 10993-4: 2002. As a negative control, 0.2 mL of diluted blood was again diluted with normal saline, and distilled water was added to a standard tube containing diluted blood, which served as a positive control. After the incubation, all the tubes were centrifuged for 5 min at 2500 rpm, and the supernatant was carefully removed and transferred to a new 96-well plate for spectroscopic analysis by an ultraviolet-visible (UV-VIS) spectrophotometer (WFJ7200, UNIC, USA) at 545 nm. Six pieces of samples were used to improve the statistics.
5 ratio by volume). The PPS/n-HA biocomposite and pure PPS (Φ10 mm × 2 mm) was dipped in a standard tube containing 10 mL of normal saline that was previously incubated at 37 °C for 30 min. Then 0.2 mL of diluted blood was added to this standard tube, and the mixtures were incubated at 37 °C for 60 min per ISO 10993-4: 2002. As a negative control, 0.2 mL of diluted blood was again diluted with normal saline, and distilled water was added to a standard tube containing diluted blood, which served as a positive control. After the incubation, all the tubes were centrifuged for 5 min at 2500 rpm, and the supernatant was carefully removed and transferred to a new 96-well plate for spectroscopic analysis by an ultraviolet-visible (UV-VIS) spectrophotometer (WFJ7200, UNIC, USA) at 545 nm. Six pieces of samples were used to improve the statistics.
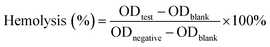 | (3) |
2.7. Cell culture and seeding
Human osteoblast-like MG-63 cells and murine fibroblast L929 cells obtained from American Type Culture Collection (ATCC, USA) were adopted for in vitro tests. Cells were cultured in high-glucose Dulbecco's modified eagle's medium (DMEM, Gibco, Carlsbad, Canada), supplemented with 10% fetal bovine serum (Gibco), 0.1 mg mL−1 streptomycin (Harbin pharmaceutical factory, China) and 100 U mL−1 penicillin (Harbin pharmaceutical factory) at 37 °C in humidified 5% CO2 incubator (Heraeus, Germany). Prior to cell experiments, the studied materials were sterilized using an autoclave at 121 °C for 30 min, followed by thorough rinse with disinfected D-Hanks buffer. When reaching 70–75% confluence, cells were dissociated with trypsin–EDTA (Gibco), counted by hemocytometer and seeded onto samples. The medium was refreshed every 2–3 days.2.8. Cytocompatibility evaluation of PPS/nano-HA biocomposites
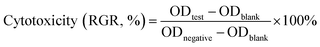 | (4) |
2.9. Osteogenic potential studies of PPS/nano-HA biocomposites
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 − lgGV. Six parallel measurements were used to provide an average and standard deviation.
225 − lgGV. Six parallel measurements were used to provide an average and standard deviation.2.10. In vivo experiments
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Sichuan University (NO. SKLODLL2013A177). All experiments were performed in compliance with animal protection law of the People's Republic of China, and followed IACUC guidelines. All the animals were maintained on a normal, solid lab diet and regular tap water.Nine Sprague-Dawley (SD) rats (provided from Third Military Medical University, half males and half females) aged 3 months and weighing about 200 g were randomly divided into two groups: PPS/nano-HA biocomposite, and bare PPS. After under general anesthesia (0.5% pentobarbital sodium, intravenous injection, 10 mL kg−1), the rats were dehaired on the back, then 0.1 mL of extracts were injected into the dorsal subcutaneous bilateral area at three different points. After 1 and 3 days post-injection, the rats were dehaired again, and the erythema and dropsy area on the back were observed.
2.11. Statistical analysis
All the quantitative data were expressed as mean ± standard deviation. Statistical analysis was done using SPSS 10.0 software. One-way analysis of variance (ANOVA) or Student's t-test was used to determine the significant differences among the groups, and p-values less than 0.05 were considered statistically significant.3. Results and discussion
3.1. Characterization of nano-HA crystals
The XRD pattern of the resulting powders was displayed in Fig. 1a. The Bragg diffraction peaks of the product, matched quite well with those of traditional nano-HA (PDF # 09-4032) at 2θ values of 25.9°, 31.7°, 32.9°, 39.6°, 49.4°, and 53.1°, which were indexed to (002), (211), (300), (222), (213), and (004) planes respectively,35 proving the formation of nano-HA phase. Fig. 1b showed the FT-IR spectra of HA powders, also confirming the presence of an apatite phase. The typical broad peak at 3442 cm−1 and 1641 cm−1 were associated with the adsorbed water (H2O). The weak band at 3576 cm−1 was attributed to the presence of OH− group from nano-HA. Existence of CO32− derived from atmosphere was recorded at around 1387 cm−1 (ν3) and 871 cm−1 (ν2), suggesting that trace amounts of PO43− were partially substituted by CO32− to form B-type carbon-substituted HA,36 which was similar to the apatite found in the bone.37 Moreover, several characteristic absorption peaks of PO43− were also observed: the non-degenerate symmetric stretching mode ν1 at 960 cm−1, doubly degenerate bending mode ν2 at about 464 cm−1, the triply degenerate antisymmetric stretching vibration ν3 at 1107 cm−1 and 1042 cm−1, the triply degenerate vibration ν4 at 608 cm−1 and 565 cm−1.35,38 Particularly, the peak at 960 cm−1 was a representative indication of crystalline HA.39 The morphology and surface area of the nano-HA crystals was characterized by TEM and nitrogen adsorption experiment. The typical needle-like particles with a specific surface area value of 6.89 m2 g−1 (Fig. S1†), which was the characteristic structure of nano-HA obtained from chemical precipitation process, were presented in Fig. 1c. Due to the high surface area and surface energy, the HA nanoparticles with a length of 82 ± 11 nm and a width of 9 ± 3 nm had a strong tendency to generate agglomerates.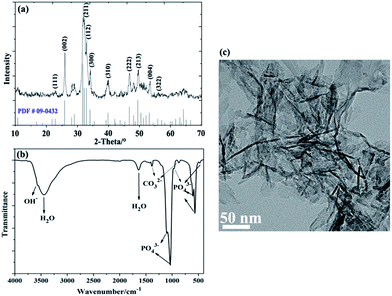 | ||
| Fig. 1 Chemical constituent and morphology of nano-HA particles: (a) XRD pattern, (b) FT-IR spectra, and (c) TEM image. | ||
3.2. Preparation and characterization of PPS/nano-HA composite
PPS is one a well-known semi-crystalline thermoplastic polymer for a set of outstanding traits, including quite high chemical resistance, low creep, reasonable cost allied with excellent mechanical properties analogous to PEEK.40,41 So it could be explored to engender new hard tissue repair biomaterial, but yet suffers from bioinertness and defective osteogenesis. Besides, the current stiffness and elastic modulus of pure PPS are insufficient to replace natural bone. On the other hand, although nano-HA particles displayed similar physico-chemical features such as chemical composition, crystalline structure, and morphology with mineral nanocrystals in bone tissue, their extremely brittle nature may hamper their use for loading-bearing applications. Therefore, the HA nanoparticles were then doped with PPS matrix via compounding and injection-molding processes to overcome HA's mechanical weaknesses and improve PPS's osteogenesis for bone repair implant. The steps in fabricating and biologically evaluating our developed PPS/nano-HA bioactive composite were illustrated in Fig. 2.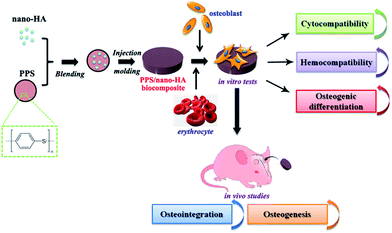 | ||
| Fig. 2 Schematic illustration of preparation and in vitro/in vivo evaluation of the PPS/nano-HA biocomposite. | ||
To explore the alterations of chemical composition and morphology after the blending of nano-HA and PPS, the prepared PPS/nano-HA biocomposites via two different methods were characterized by FT-IR, contact angle goniometry, SEM, water uptake analysis, variation of local pH value as well as TG-DSC. The FT-IR spectra of various PPS/nano-HA biocomposites were depicted in Fig. 3a. Pure PPS matrix exhibited some feature peaks: the strong peak at 811 cm−1 originated from C–H out-of-plane bending vibration of benzene, and the bands at 3063 cm−1 corresponded to stretching vibration band of C–H. Three typical bands of C–C vibration at 1390 cm−1, 1473 cm−1, and 1572 cm−1 belonged to the in-plane stretching vibration of benzene. The FT-IR absorption peaks at 1004 cm−1 and 1087 cm−1 were ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in-plane bending vibration of benzene and C–S in-plane stretching vibration, respectively.42,43 It was notable that the bands of P–O stretch and vibration at 558 cm−1, 602 cm−1, 961 cm−1, 1035 cm−1, and 1102 cm−1 resulted from the PO43− group and the peak of OH− group showed up in the s-PPS/nano-HA and l-PPS/nano-HA,35,38 reflecting the compound of HA and PPS. Whilst the intensity of peaks from PO43− groups for both s-PPS/nano-HA and l-PPS/nano-HA increased with nano-HA amount.
C in-plane bending vibration of benzene and C–S in-plane stretching vibration, respectively.42,43 It was notable that the bands of P–O stretch and vibration at 558 cm−1, 602 cm−1, 961 cm−1, 1035 cm−1, and 1102 cm−1 resulted from the PO43− group and the peak of OH− group showed up in the s-PPS/nano-HA and l-PPS/nano-HA,35,38 reflecting the compound of HA and PPS. Whilst the intensity of peaks from PO43− groups for both s-PPS/nano-HA and l-PPS/nano-HA increased with nano-HA amount.
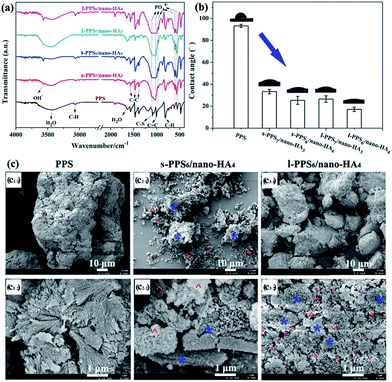 | ||
| Fig. 3 FT-IR spectra (a) and water contact angle (b) of s-PPS/nano-HA and l-PPS/nano-HA binary composite. SEM images (c) of s-PPS6/nano-HA4 and l-PPS6/nano-HA4. | ||
In biological systems, the hydrophilicity of the implant surface plays a fatal role in mediating cell adhesion and proliferation. The presence of nano-HA particles on PPS surfaces might help elevate the bioactive properties of the composite, especially the hydrophilicity. The wettability of bare and modified PPS was determined by contact angle measurements of sessile water drop. As presented in Fig. 3b, bare PPS substrate had hydrophobic feature with a contact angle of approximately 92° corresponding to the lowest surface hydrophilicity, owing to its hydrophobic aromatic ring and C–S functional groups. Numerous superhydrophobic materials, like polytetrafluoroethylene (PTFE),44 polyvinylidene fluoride (PVDF),45 and PEEK5 were reported to display increased wettability when blended with nano-HA, owing to the hydrophilicity of OH− group in HA crystals. Thereby, contact angle measurement showed that the introduction of HA nanoparticles significantly improved the hydrophilicity of PPS to a certain extent, with increase in HA concentration both for solid–solid blending and liquid–liquid compounding approaches. Moreover, compared with s-PPS7/nano-HA3 (33.3 ± 1.9°) and s-PPS6/nano-HA4 (25.1 ± 3.7°) composites, surface wettability of the corresponding l-PPS7/nano-HA3 (26.5 ± 3°) and l-PPS6/nano-HA4 (17.2 ± 2.3°) was enhanced at same nano-HA content, providing an indirect evidence for the more presence of HA on the outermost surface of l-PPS/nano-HA composite through liquid–liquid compounding process.
The effect of nano-HA and blend mode on the surface topology of PPS/nano-HA composite powders was examined by SEM. There were no detectable differences in the surface morphologies between PPS7/nano-HA4, and PPS6/nano-HA4 samples, therefore, PPS6/nano-HA4 as representative was observed under SEM. As shown in Fig. 3c, pure PPS powders possessed a rough surface morphology with petal shapes under high magnification. It could be seen that bulk PPS particles (*) and small HA powders (^) were visually separated in the s-PPS/nano-HA composite. Compared with s-PPS/nano-HA group, nonetheless, the bulk particles were comprised of PPS matrix and HA crystals. In the vision of high magnification, nano-HA particles (^) dispersed homogeneously in the compound powder and combined firmly with PPS matrix (*), suggesting that liquid–liquid compounding method was more beneficial to increase the dispersity and bonding strength of nano-HA in the compound than solid–solid blending approach.
Furthermore, the moisture absorption percentage of pure PPS and PPS/nano-HA samples within the initial 7 days was presented in Fig. 4a. The amount of water adsorption kept ascending quickly with the extension of time, except for pure PPS, which displayed no obvious water uptake during 7 days. The moisture is predominantly absorbed by composite component in the composites, because PPS matrix is water resistant. The numerous free hydroxyl (OH−) groups present in the nano-HA crystals are responsible for forming hydrogen bonding with water molecules, and the water absorption of the composites hinges on the availability of free hydroxyl functions on the surface. Therefore, the percentage of water absorption on the biocomposite enhanced with the augmentation of nano-HA content. Additionally, the synthetic method also had a conspicuous influence on the moisture absorption of biocomposites. Clearly, at same nano-HA content, the water adsorption of l-PPS/nano-HA was substantially higher than that of s-PPS/nano-HA, and among them l-PPS6/nano-HA4 showed the best the water stored ability with 8.69 ± 0.83%. It could be explained by the better homogeneous dispersion of nano-HA in the composite prepared from liquid–liquid strategy. These results were in coincidence with the hydrophilicity test. The incorporation of nano-HA crystal and proper blending approach conferred inert PPS with strong bonding with water and good water holding function.
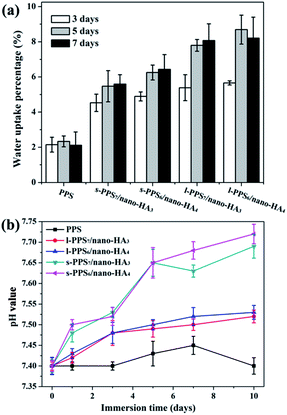 | ||
| Fig. 4 Water uptake property (a) and variation of pH value (b) with soaking time in SBF for the pure PPS, and PPS/nano-HA biocomposite. | ||
The local microenvironment of pH alteration resulted from materials degradation is an important parameter that should be considered, when designing materials for biomedical applications. The evolution of the pH values, hence, in SBF vs. soaking time for pure PPS and PPS/nano-HA biocomposites was shown in Fig. 4b. The pH values in SBF solution was improved, to different extents, for four PPS/nano-HA composites, whilst pure PPS exert no influence on the pH value maintaining at about 7.4 until 10 days. However, a drastic fluctuation in pH value increasing from 7.40 to 7.69–7.72 was seen in s-PPS/nano-HA composite owing to the partial ion exchange between H3O+ in SBF solution and Ca2+ cation from nano-HA on the surface. Moreover, the pH values of l-PPS/nano-HA exhibited a slight promotion from 7.40 to 7.52–7.53, and there was no evident difference in pH value between l-PPS7/nano-HA3 and l-PPS6/nano-HA4. The change of pH value for these PPS/nano-HA composites decreased in the order: s-PPS6/nano-HA4 > s-PPS7/nano-HA3 > l-PPS6/nano-HA4 ≥ l-PPS7/nano-HA3 > PPS. It was apparent that s-PPS/nano-HA groups induced more pH alteration in SBF than their counterpart (l-PPS/nano-HA) at same HA contents. This was because that weak binding between nano-HA crystals and PPS matrix through simple solid–solid blending contributed to rapid release of Ca2+, and finally led to a serious fluctuation in pH value. Ca2+ ions, nonetheless, released slowly from the surface of l-PPS/nano-HA on account of homogeneous dispersion of nano-HA in compound generating alkalescence condition (pH = 7.52–7.53) and weak undulation in pH value. Some earlier studies, at the same time, confirm that excessive dissolved Ca2+ led to drastic alteration of the microenvironment combined with the deteriorated mechanical properties of implant materials, disruption in the activity of host cells and creating adverse effects on adjacent tissues.46,47 In fact, tissue fluid (pH = 7.1–7.5) in human body is under a weak alkaline condition, and it is constantly circulating in the body to maintain homeostasis. And it was also verified that alkalescency derived from biomineralization was conducive to osteoblast proliferation and succedent osteogenic differentiation.48,49 Local alkalescent microenvironment and weak fluctuation of pH after immersion of specimens in SBF solution confirmed that l-PPS/nano-HA composite had a good stability in vitro.
3.3. Mechanical properties of PPS/nano-HA composite
Current treatments of large-sized bone defects prevailingly rely on the use of metal implants made of Ti and Ti alloys. Nevertheless, the ultimate tensile strength of Ti alloys (over 900 MPa) was approximately 6–20 times greater than that of cortical bones, which resulted in stress shielding. The use of materials stiffer than bony tissue leads to mechanical mismatch problems between the implant and the adjacent bone, where the integrity of the bone/implant interface may be compromised due to the resorption of bone tissue. Therefore, it is desired to fabricate novel material which mechanical performances of the repair material could be tailored to mimic human cortical bone avoiding stress shielding. Table 2 presented the mechanical properties of bare PPS and PPS/nano-HA composites with different contents of n-HA and synthetic methods. Obviously, extremely low elastic modulus (2.78 ± 0.58 GPa) and flexural modulus (3.29 ± 0.39 GPa) were detected in pristine PPS, while the s-PPS/nano-HA composite prepared from solid–solid blending exhibited a dramatic increase in elastic modulus and flexural modulus, but a reduce in tensile strength and bending strength as the nanoparticle concentration rose. With regard toward the l-PPS/nano-HA fabricated by liquid–liquid compounding, differing from s-PPS/nano-HA, the four mechanical indexes containing elastic modulus, flexural modulus, tensile strength, and bending strength were all improved with the n-HA content from 0 to 40 wt%, indicating enhancement in stiffness of the composites. Furthermore, it was remarkable that l-PPS/nano-HA composite, in contrast to s-PPS/nano-HA, displayed high mechanical properties at the same formulation, suggesting uniform decentralize of nano-HA and close linkage between nano-HA crystals and PPS matrix in the compound, contributing to improvement of mechanical properties. For developing a repairing prosthesis for bone tissue engineering, the material should be able to withstand dynamic mechanical loading under in vivo conditions. From the mechanical testing results, the composites prepared via liquid–liquid compounding method in our study harbored an elastic modulus in the range of 4–6 GPa, which endowed them with sufficient mechanical strength for load-bearing orthopedic and neurosurgical applications. Moreover, the enhanced elastic modulus was also believed to have a positive effect on the osteogenic differentiation of bone cells.50 It was expected that the l-PPS/nano-HA nanocomposites, especially for l-PPS6/nano-HA4, with such mechanical properties had a promising prospect used as load-bearing implant material.| Sample name | Tensile strength (MPa) | Elastic modulus (GPa) | Bending strength (MPa) | Flexural modulus (GPa) |
|---|---|---|---|---|
| PPS | 60.97 ± 2.32 | 2.78 ± 0.58 | 103.68 ± 1.67 | 3.29 ± 0.39 |
| s-PPS7/nano-HA3 | 45.95 ± 2.20 | 3.72 ± 0.21 | 93.29 ± 4.16 | 5.16 ± 0.24 |
| s-PPS6/nano-HA4 | 41.38 ± 1.71 | 4.29 ± 0.64 | 87.29 ± 3.18 | 6.45 ± 0.46 |
| l-PPS7/nano-HA3 | 72.13 ± 3.79 | 4.74 ± 0.73 | 135.62 ± 4.53 | 6.89 ± 1.04 |
| l-PPS6/nano-HA4 | 80.47 ± 3.38 | 6.10 ± 0.45 | 156.82 ± 5.27 | 8.37 ± 0.56 |
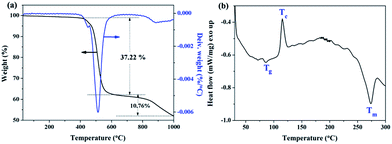
At last, the representative curves of TG and DSC analysis for the l-PPS6/nano-HA4 composite were depicted in Fig. 5. It showed that the final PPS/nano-HA had a two-step thermolysis. The sharp weight loss (about 37.22%) in a range of 400–650 °C was ascribed to thermal degradation and pyrolyzation of PPS molecules. There was a slow weight loss with 10.76% located at 650–1000 °C, probably corresponding to the release of OH− ions from the decomposition of HA, leaving oxyapatite (OAP) behind. According to the DSC results, the glass transition temperature (Tg), thermal crystallization temperature (Tc), and melting temperature (Tm) of new l-PPS6/nano-HA4 binary composite were 85.6 °C, 115.2 °C, and 273.5 °C respectively, which was similar to those of pure PPS.18
3.4. Blood compatibility of PPS/nano-HA biocomposite
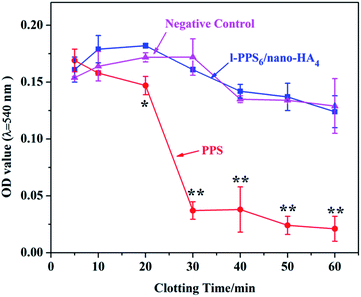
Hemocompatibility assessments including hemolysis test and dynamic blood coagulation testing were also evaluated in the present study, because for potential blood-contacting bone implant, the interplay between blood and biomaterials could affect bone formation and healing. The degree of hemolysis is a sensitive indicator of the extent of damage to erythrocytes. The degree of hemolysis in the presence of l-PPS6/nano-HA4 was 1.66 ± 0.29%, way below the safety-threatening threshold of 5%, suggesting that l-PPS6/nano-HA4 biocomposite provided an acceptable level of hemolysis. It was noteworthy, nonetheless, that the hemolysis rates for bare PPS sample (6.43 ± 0.76%) stayed higher than 5%, indicating that it had a risk of accelerating thrombosis and leading to further coagulation. The dynamic blood clotting time is an in vitro test that measures the degree of activation of intrinsic coagulation factors when surfaces interact with blood (“contact activation”). A curve providing a gentle slope usually implied low procoagulant properties in the implanted material.51,52 Fig. 6 presented that the similar curves for l-PPS6/nano-HA4 biocomposite and silylated glass (negative control), with both presenting slow and smooth downward inclination with coagulation time beyond 60 min; nevertheless, unmodified PPS displayed sharp blood coagulation cascade with procoagulant time at 20 min. Endosseous implants initially come into contact with blood. The interactions between blood and bone implants have a momentous impact on subsequent bone healing events in the peri-implant healing compartment. The finding of the study indicated that l-PPS6/nano-HA4 biocomposite with preferable hemocompatibility could have better potential to be used for orthopaedic implants.3.5. Cell toxicity
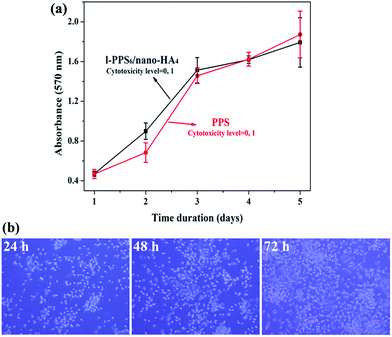
A good biocompatibility with the surrounding tissue cells is usually expected for implantable materials, therefore, the cytotoxicity of PPS/nano-HA biocomposite was probed by CCK-8 assay on the murine fibroblast L929 cells, which was widely used for cytotoxicity evaluation of biomaterials. Fig. 7a depicted the cell viability cultured in extracts of pristine PPS and l-PPS6/nano-HA4 for sequential 5 days, with pure DMEM media as control. It could be found that the OD value increased with time when L929 cells were co-cultured with the two extracts attested that PPS-based materials did not induce cytotoxic effect on cells. In addition, no significant differences in cell viabilities difference between l-PPS6/nano-HA4 and PPS, representing a similar non-toxicity feature. During 5 days of culture, the proliferation of L929 cells cultured in l-PPS6/nano-HA4 surface varied between 95.3% (day 5) and 103.56% (day 3), corresponding to the cytotoxicity level of 0 or 1 per the standard in Table S2.† Due to excellent biocompatibility and high mechanical behaviors, titanium and PEEK are two more used biomaterials as orthopedic implant. The cell viability and cytotoxicity level of the PPS-based materials were comparable to that of pure titanium (89–98% RGR)6,53 and PEEK (85–96% RGR, those data from previous literature)6,54 because of the bioinertness of these biomaterials, implying PPS-based materials had similar cytocompatibility to titanium and PEEK. These phenomena were further verified by optical microscopic observation. The morphologies of L929 cells cultured in extraction media from the l-PPS6/nano-HA4 group displayed healthy spindle-like shape, and the amount of cells rose with the extension of culture time (Fig. 7b), suggesting that l-PPS6/nano-HA4 composite imposed no suppression on the growth of cells. Good correspondence could be found between the direct observation and the indirect cell viability evaluation.3.6. Cell proliferation and osteogenic differentiation in vitro
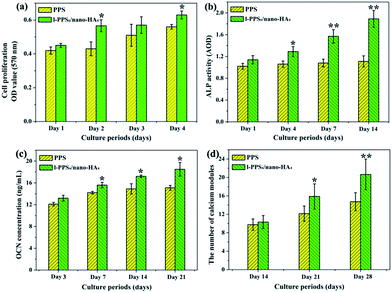
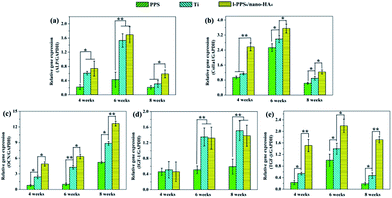
Initial cell adhesion is usually responsible for cellular functions and eventual tissue integration, while cell proliferation is closely correlated with the amount of new bone formation. Better cell proliferation probably produces a larger mass of bone tissues around the implant.55 To further explore MG-63 cells proliferation on PPS/nano-HA composite in the context of bone tissue engineering, CCK-8 assay was carried out. According to the viability data in Fig. 8a, all studied materials exhibited good time-dependent cell activity. Although cells at day 1 displayed no statistical difference among groups, nano-HA doped PPS possessed higher cell multiplication than pure PPS and kept better viability from day 2 to day 4, indicating that the exposure of nano-HA might have a positive impact on the cytocompatibility and improving the bioactivity of PPS materials. The osteogenic differentiation activity of cell to the biointerfaces, as far as bone-repair biomaterials concerned, is a key event in bone formation. Among the major osteogenic hallmarks, the up-regulation of ALP activity is a central event occurring during the early time points of osteogenesis.56 In vitro ALP activities of MG-63 cells cultivating with pure PPS and PPS/nano-HA biocomposite were evaluated at 1, 4, 7, and 14 days. As illustrated in Fig. 8b, when cells were cultured on PPS/nano-HA, the ALP activity expression was pronouncedly higher compared with that for pure PPS, whose ALP values almost kept unchanged.Concomitantly, OCN secretion and mineral deposition, being connatural characteristics of bone-like structures, were estimated as osteogenic markers at the late stage (Fig. 8c and d). OCN is regarded as the most specific marker for the mature osteoblast and mineralization during the course of osteogenesis, and it reaches the maximum expression during mineralization and accumulates in the mineralized bone due to its high affinity to HA crystals.57 As the osteogenic culture of MG-63 cells progressed to 21 days, cells started to aggregate together and form bone-like structures stained by ARS. Evidently, a drastic enhancement in the number of nodules and the amount of OCN protein were detected at most time points (14, 21 and 28 days) on PPS/nano-HA biocomposite compared with those on pure PPS, due to addition of nano-HA underlining the role of nano-HA crystals in the enhancement of osteogenic differentiation efficiency. As mentioned above, nano-HA crystals have been accepted to greatly up-regulate biological markers during osteogenesis and enhance osteogenic differentiation of bone cells,28,58 therefore facilitating the formation of bone. Besides that, the Ca ion released from nano-HA have been shown to stimulate osteoblast proliferation and differentiation by changing the expression of specific Ca2+ channel isoforms on osteoblasts.59 Overall, results from cell proliferation, ALP activity, calcium nodule deposits and OCN expression have clearly showcased that our developed PPS/nano-HA possessed robust osteoinductive capacity, and the addition of nano-HA has endowed bioinert PPS with both outstanding osteo-compatibility and osteogenesis potential in vitro.
3.7. In vivo biocompatibility evaluation
To verify the biocompatibility of l-PPS6/nano-HA4 biocomposite for clinical use, it is crucial to know its in vitro responses, therefore, the in vivo biocompatibility assessments including skin irritation test, acute toxicity evaluation and subcutaneous implant experiments were performed in the present work. First, a skin irritation test was conducted to determine whether l-PPS6/nano-HA4 biocomposite invoked inflammatory and allergic reactions. Although there was a little erythema on both dorsal areas of rats at the 1st day after injection of PPS and l-PPS6/nano-HA4 biocomposite extraction, after 3 days, the erythema disappeared and none of the tested parts of white rats treated with the extract medium displayed edema reactions (Fig. S2a†), implying that PPS-based materials were not irritant or traumatic to the skin. No death occurred during the whole 3 day observation period, and no toxic response was found in rats. The animals displayed normal energy, normal behavior, free movement, and shining hair. The animals' feces were in regular form and normal color, without mucus, pus, or blood. After sacrifice, no macroscopic pathological alterations and histopathological lesions attributed to the extracts of PPS and biocomposite were found in any rats at necropsy. Fig. S2b† presented the light microscopic image of the liver and kidney treated with the extracts. The classic structure of liver lobule with central vein was delineated, and no hepatocellular degeneration or necrosis was found. From the light micrograph of rat kidney, many renal tubes with normal shape were observed, with no degeneration, bleeding, or necrosis. In summary, these results showed that both PSS and l-PPS6/nano-HA4 materials had no short-term acute systemic toxicity, reflecting it might serve as a safe candidate for its potential applications in biomedicine fields.3.8. In vivo bone formation assessments
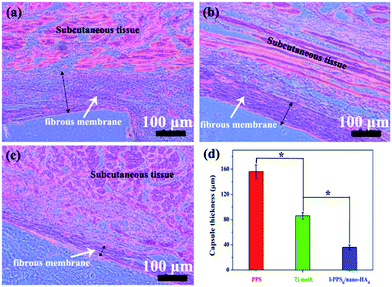
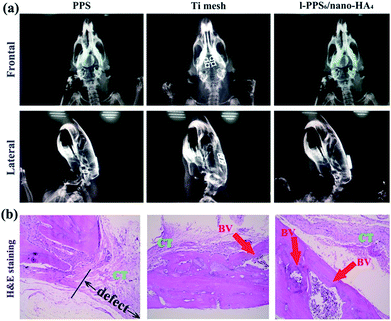
Histological analysis was used to observe interactions between host tissue and implanted biocomposite at defect sites. As illustrated in Fig. 10b, we only find limited newly-formed osteotylus at the periphery of the defect and a few inflammatory cell infiltration, but a plentiful of fibrillar connective tissue filled the defect for PPS group at 8 weeks after implantation. In Ti mesh-implanted group, Ti mesh was encapsulated with host cells and more organized tissue regeneration around the defect sites were detectable than those in the PPS-implant group. In mineralized tissue, the process of angiogenesis is a pre-requisite for normal osteogenesis. During bone repair angiogenesis precedes osteogenesis, since newly formed vessels drive the orientation of bone microcolumns.64 In reconstructive orthopaedic surgery, therefore, adequate blood supply is necessary for bone re-built and regeneration. As shown in Fig. 10b, blood vessels infiltrated throughout the defects in the two implanted materials groups, but the highest concentration of blood vessels was found in the PPS/nano-HA biocomposite. Nanocrystalline HA has been proved to promote angiogenesis via up-regulation of fibroblast growth factor 2 (FGF-2).65 One of the interesting results was that rats in the PPS/nano-HA-implanted group showed compact de novo bone, better bone healing, and regeneration of blood vessels (red arrow) in the newly-formed bone tissue. These results demonstrated that the quality of newly formed bone in contact with the implants of l-PPS6/nano-HA4 biocomposite group was drastically higher than that for bare PPS group. It seemed no significant discrepancy in bone healing and repairing between Ti mesh and l-PPS6/nano-HA4 biocomposite. It has been proved in vast previous literature that nano-HA modification of PEEK and Ti implants surfaces exhibited a prominently elevation in osteogensis-related gene expression levels and also exhibit higher degrees in the newly bone reconstruction.66,67 When PPS/nanoHA is in contact with bone after implantation, the exposure of nano-HA inevitably promotes the growth of osteoblasts and accelerates angiogenesis, resulting in bone formation. The results are closely correlated with osteoblast proliferation and differentiation in vitro as well. Thus, the PPS polymer after blending nano-HA crystals not only positively affects the osseointeration between implant and bone but also enhances bone maturation surrounding the implant.
4. Conclusion
In this paper, a unique PPS-based bioactive composite, PPS/nano-HA, was successfully developed through a process of compounding, and injection-molding, and its chemical constitutions, mechanical properties and biological performances were systematically evaluated both in vitro and in vivo. The mechanical property assessments proved that the elastic modulus of PPS/nano-HA samples was closer to that of human cortical bones. Cell experiment results demonstrated that the PPS/nano-HA composite was found to promote cell proliferation, osteogenic differentiation with higher ALP activity, and increased OCN secretion and calcium nodule-formation compared with bare PPS. More importantly, in the rat cranial defect implantation model, preferable tissue biocompatibility, better osteointegration, and accelerated osteogenesis could be observed around the PPS/nano-HA biocomposite implants in vivo than pure PPS and typical Ti mesh based on soft X-ray observation, histological analysis, and real-time PCR. This is the first time investigating the possibility of PPS and its composite as potential biomaterials. As consequence, our findings provide preliminary insights into the development of novel PPS-based biomaterials used in many challenging orthopedic/dental tissue engineering applications and enriched the library of PPS application in health issues.Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant 51173210), and the Fund of Health Department of Sichuan Province (No. 110467). Y. Deng and Y. Ma contributed equally.References
- M. Navarro, A. Michiardi, O. Castaño and J. A. Planell, J. R. Soc., Interface, 2002, 5, 1137 CrossRef PubMed.
- E. Gentleman, R. J. Swain, N. D. Evans, S. Boonrungsiman, G. Jell, M. D. Ball, T. A. Shean, M. L. Oyen, A. Porter and M. M. Stevens, Nat. Mater., 2009, 8, 763 CrossRef CAS PubMed.
- A. Dalal, V. Pawar, K. McAllister, C. Weaver and N. J. Hallab, J. Biomed. Mater. Res., Part A, 2012, 100, 2147 CrossRef PubMed.
- T. A. Schildhauer, E. Peter, G. Muhr and M. Köller, J. Biomed. Mater. Res., Part A, 2009, 88, 332 CrossRef CAS PubMed.
- L. Wang, S. He, X. Wu, S. Liang, Z. Mu, J. Wei, F. Deng, Y. Deng and S. Wei, Biomaterials, 2014, 35, 6758 CrossRef CAS PubMed.
- K. B. Sagomonyants, M. L. Jarman-Smith, J. N. Devine, M. S. Aronow and G. A. Gronowicz, Biomaterials, 2008, 29, 1563 CrossRef CAS PubMed.
- A. H. C. Poulsson, E. David, Z. Stefan, C. Karin, S. Christoph, A. Yash, N. Dirk, W. Joanne and R. G. Richards, Biomaterials, 2014, 35, 3717 CrossRef CAS PubMed.
- L. Ouyang, Y. Zhao, G. Jin, L. Tao, J. Li, Y. Qiao, C. Ning, X. Zhang, P. K. Chu and X. Liu, Biomaterials, 2016, 83, 115 CrossRef CAS PubMed.
- A. Xu, L. Zhou, Y. Deng, X. Chen, X. Xiong, F. Deng and S. Wei, J. Mater. Chem. B, 2016, 4, 1878 RSC.
- X. Wang, T. Lu, J. Wen, L. Xu, D. Zeng, Q. Wu, L. Cao, S. Lin, X. Liu and X. Jiang, Biomaterials, 2016, 83, 207 CrossRef CAS PubMed.
- L. Tao, W. Jin, Q. Shi, H. Cao, C. Ning, X. Pan, X. Jiang, X. Liu and P. K. Chu, Biomaterials, 2015, 51, 173 CrossRef PubMed.
- M. G. Garrell, B. M. Ma, A. J. Shih, E. Lara-Curzio and R. O. Scattergood, Mater. Sci. Eng., A, 2003, 359, 375 CrossRef.
- W. Luo, Q. Liu, Y. Li, S. Zhou, H. Zou and M. Liang, Composites, Part B, 2016, 91, 579 CrossRef CAS.
- S. M. Kurtz and J. N. Devine, Biomaterials, 2007, 28, 4845 CrossRef CAS PubMed.
- J. M. Toth, W. Mei, B. T. Estes, J. L. Scifert, H. B. S. Iii and A. S. Turner, Biomaterials, 2006, 27, 324 CrossRef CAS PubMed.
- E. Alonso-Rodriguez, J. L. Cebrián, M. J. Nieto, J. L. Del Castillo, J. Hernández-Godoy and M. Burgueño, Journal of Cranio-Maxillofacial Surgery, 2015, 43, 1232 CrossRef CAS PubMed.
- A. G. Kulkarni, H. T. Hee and H. K. Wong, Spine J., 2007, 7, 205 CrossRef PubMed.
- Y. Guo and R. D. Bradshaw, Polymer, 2009, 50, 4048 CrossRef CAS.
- A. M. Diez-Pascual, Manufacturing of Nanocomposites with Engineering Plastics, 2015, 127 Search PubMed.
- S. Pappadà, A. Salomi, J. Montanaro, A. Passaro, A. Caruso and A. Maffezzoli, Aerosp. Sci. Technol., 2015, 43, 314 CrossRef.
- M. Kikuchi, Y. Koyama, T. Yamada, Y. Imamura, T. Okada, N. Shirahama, K. Akita, K. Takakuda and J. Tanaka, Biomaterials, 2005, 25, 5979 CrossRef PubMed.
- Y. Takahashi, M. Yamamoto and Y. Tabata, Biomaterials, 2005, 26, 3587 CrossRef CAS PubMed.
- N. T. Khanarian, N. M. Haney, R. A. Burga and H. H. Lu, Biomaterials, 2012, 33, 5247 CrossRef CAS PubMed.
- Y.-P. Guo, J.-J. Guan, J. Yang, Y. Wang, C.-Q. Zhang and Q.-F. Ke, J. Mater. Chem. B, 2015, 3, 4679 RSC.
- X. Wu, X. Liu, J. Wei, J. Ma, F. Deng and S. Wei, Int. J. Nanomed., 2012, 7, 1215 CAS.
- R. D. Santis, M. Catauro, L. D. Silvio, L. Manto, M. G. Raucci, L. Ambrosio and L. Nicolais, Biomaterials, 2007, 28, 2801 CrossRef PubMed.
- M. J. Olszta, X. Cheng, S. J. Sang, R. Kumar, Y. Y. Kim, M. J. Kaufman, E. P. Douglas and L. B. Gower, Mater. Sci. Eng., R, 2007, 58, 77 CrossRef.
- H. Yang, H. Zeng, L. Hao, N. Zhao, C. Du, H. Liao and Y. Wang, J. Mater. Chem. B, 2014, 2, 4703 RSC.
- K. Yano, T. Namikawa, T. Uemura, M. Hoshino, S. Wakitani, K. Takaoka and H. Nakamura, J. Orthop. Sci., 2012, 17, 484 CrossRef CAS PubMed.
- L. Hua, W. X. Guo, Y. F. Wang, S. Z. Hong, X. Si, W. Yan, B. C. Heng, R. A. Cheng, H. Z. Gang and H. X. Ding, Biomaterials, 2015, 49, 103 CrossRef PubMed.
- H. Wang, Y. Li, Y. Zuo, J. Li, S. Ma and L. Cheng, Biomaterials, 2007, 28, 3338 CrossRef CAS PubMed.
- J. Li, Y. Man, Y. Zuo, L. Zhang, C. Huang, M. Liu and Y. Li, J. Biomater. Sci., Polym. Ed., 2011, 22, 263 CrossRef CAS PubMed.
- L. Xie, H. Yu, W. Yang, Z. Zhu and L. Yue, J. Biomater. Sci., Polym. Ed., 2016, 27, 1 CrossRef PubMed.
- F. Nie, Y. Zheng, Y. Wang and J. Wang, J. Biomed. Mater. Res., Part B, 2014, 102, 221 CrossRef CAS PubMed.
- Q. Li, M. Li, P. Zhu and S. Wei, J. Mater. Chem., 2012, 22, 20257 RSC.
- J. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132 CrossRef CAS.
- Y. Deng, Y. Sun, X. Chen, P. Zhu and S. Wei, Mater. Sci. Eng., C, 2013, 33, 2905 CrossRef CAS PubMed.
- M. C. Chang and J. Tanaka, Biomaterials, 2002, 23, 4811 CrossRef CAS PubMed.
- A. Antonakos, E. Liarokapis and T. Leventouri, Biomaterials, 2007, 28, 3043 CrossRef CAS PubMed.
- H. Quan, G. J. Zhong, Z. M. Li, M. B. Yang, B. H. Xie and S. Y. Yang, Polym. Eng. Sci., 2005, 45, 1303 CAS.
- L. G. Xia, A. J. Li, W. Q. Wang, Q. Yin, H. Lin and Y. B. Zhao, J. Power Sources, 2008, 178, 363 CrossRef CAS.
- D. Zhang, H. Yao, D. Zhou, L. Dai, J. Zhang and S. Yuan, Polym. Adv. Technol., 2014, 25, 1590 CrossRef CAS.
- G. Miao, Z. Yu, Q. Wu and Y. Chen, Eur. Polym. J., 1997, 33, 1401 CrossRef CAS.
- N. L. de Macedo, L. G. de Macedo, F. S. Matuda, S. M. Ouchi, A. S. Monteiro and Y. R. Carvalho, South Braz. Dent. J., 2003, 14, 119 Search PubMed.
- J. Sun, Z. Cao and L. Wu, Colloids Surf., B, 2015, 126, 265 CrossRef CAS PubMed.
- X. Lu, H. Yu, Y. Deng, W. Yang, L. Li and L. Qin, Mater. Sci. Eng., C, 2016, 59, 1007 CrossRef PubMed.
- S. Yamada, D. Heymann, J. M. Bouler and G. Daculsi, Biomaterials, 1997, 18, 1037 CrossRef CAS PubMed.
- L. Fei, C. C. Wang, X. Yang, K. Lin, C. Jiang and S. Jiao, J. Biomed. Mater. Res., Part B, 2012, 100, 1237 CrossRef PubMed.
- W. Zhai, H. Lu, C. Wu, L. Chen, X. Lin, K. Naoki, G. Chen and J. Chang, Acta Biomater., 2013, 9, 8004 CrossRef CAS PubMed.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677 CrossRef CAS PubMed.
- Y. Liu, D. Cai, J. Yang, Y. Wang, X. Zhang and S. Yin, Int. J. Clin. Exp. Med., 2014, 7, 1233 Search PubMed.
- E. A. Vogler, J. C. Graper, H. W. Sugg, L. M. Ler and W. J. Brittain, J. Biomed. Mater. Res., 1995, 29, 1017 CrossRef CAS PubMed.
- Z. Jia, P. Xiu, M. Li, X. Xu, Y. Shi, Y. Cheng, S. Wei, Y. Zheng, T. Xi and H. Cai, Biomaterials, 2016, 75, 203 CrossRef CAS PubMed.
- Y. Deng, P. Zhou, X. Liu, L. Wang, X. Xiong, Z. Tang, J. Wei and S. Wei, Colloids Surf., B, 2015, 136, 64 CrossRef CAS PubMed.
- Y. K. Jo, B.-H. Choi, C. Zhou, J.-S. Ahn, S. H. Jun and H. J. Cha, J. Mater. Chem. B, 2015, 3, 8102 RSC.
- A. K. Gaharwar, S. M. Mihaila, A. Swami, A. Patel, S. Sant, L. R. Rui, A. P. Marques, M. E. Gomes and A. Khademhosseini, Adv. Mater., 2013, 25, 3329 CrossRef CAS PubMed.
- G. Kaur, M. T. Valarmathi, J. D. Potts and Q. Wang, Biomaterials, 2008, 29, 4074 CrossRef CAS PubMed.
- Y. Huang, G. Zhou, L. Zheng, H. Liu, X. Niu and Y. Fan, Nanoscale, 2012, 4, 2484 RSC.
- C. Sarmento, Z. B. Luklinska, L. Brown, M. Anseau, P. N. D. Aza, S. D. Aza, F. J. Hughes and I. J. Mckay, J. Biomed. Mater. Res., Part A, 2004, 69, 351 CrossRef PubMed.
- K. L. Wong, C. T. Wong, W. C. Liu, H. B. Pan, M. K. Fong, W. M. Lam, W. L. Cheung, W. M. Tang, K. Y. Chiu, K. D. K. Luk and W. W. Lu, Biomaterials, 2009, 30, 3810 CrossRef CAS PubMed.
- E. Nejati, V. Firouzdor, M. B. Eslaminejad and F. Bagheri, Mater. Sci. Eng., C, 2009, 29, 942 CrossRef CAS.
- M. Kikuchi, S. Itoh, S. Ichinose, K. Shinomiya and J. Tanaka, Biomaterials, 2001, 22, 1705 CrossRef CAS PubMed.
- L. Fang, L. Yang and P. Gao, Biomaterials, 2005, 26, 347 CrossRef PubMed.
- U. Saran, P. S. Gemini and S. Chatterjee, Arch. Biochem. Biophys., 2014, 561, 109 CrossRef CAS PubMed.
- S. Pezzatini, L. Morbidelli, R. Solito, E. Paccagnini, E. Boanini, A. Bigi and M. Ziche, Bone, 2007, 41, 523 CrossRef CAS PubMed.
- J. H. Lee, H. L. Jang, K. M. Lee, H. R. Baek, K. Jin, K. S. Hong, J. H. Noh and H. K. Lee, Acta Biomater., 2013, 9, 6177 CrossRef CAS PubMed.
- M. Rocca, M. Fini, G. Giavaresi, N. N. Aldini and R. Giardino, Biomaterials, 2002, 23, 1017 CrossRef CAS PubMed.
- T. Lu, X. Liu, S. Qian, H. Cao, Y. Qiao, Y. Mei, P. K. Chu and C. Ding, Biomaterials, 2014, 35, 5731 CrossRef CAS PubMed.
- J. Fiedler, C. Brill, W. F. Blum and R. E. Brenner, Biochem. Biophys. Res. Commun., 2006, 345, 1177 CrossRef CAS PubMed.
- J. L. Crane, L. Xian and X. Cao, Methods Mol. Biol., 2016, 1344, 287 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra25526d |
| This journal is © The Royal Society of Chemistry 2017 |