Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
AgSCF3-mediated trifluoromethylthiolation of α,α-diaryl allylic alcohols via radical neophyl rearrangement†
Kai Liu,
Qiao Jin,
Shuang Chen and
Pei Nian Liu*
Shanghai Key Laboratory of Functional Materials Chemistry, Key Lab for Advanced Materials, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Meilong Road 130, Shanghai, 200237, China. E-mail: liupn@ecust.edu.cn
First published on 5th January 2017
Abstract
A novel example of AgSCF3-mediated oxidative radical trifluoromethylthiolation of α,α-diaryl allylic alcohols is presented, producing various α-aryl-β-trifluoromethylthiolated carbonyl ketones via radical neophyl rearrangement under mild conditions. This protocol involves formation of C(Ar)–C(sp3) and C(sp3)–S bonds in one step and tolerates a wide range of symmetrical and nonsymmetrical α,α-diaryl allylic alcohols.
Introduction
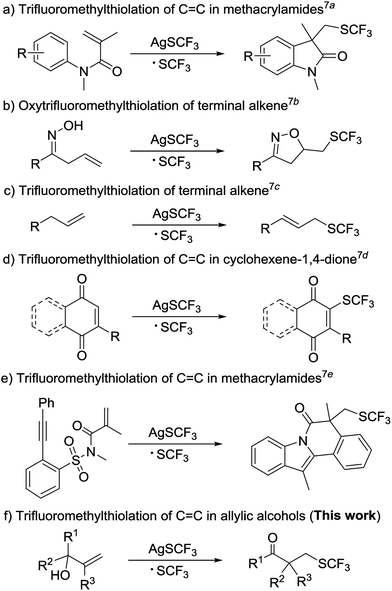
Fluorine-containing functional groups have achieved great application in many pharmaceuticals and agrochemicals because of their remarkable potential for modulating molecular chemical, physical, and biochemical properties.1 Among these intriguing fluorine-containing groups, the trifluoromethylthio (SCF3) group has attracted increasing attention because of its special biological properties such as enhancement of membrane permeability and absorption rate and improvement of the stability of parent molecules, due to its high electronegativity and lipophilicity.2 This has led to growing interest in developing new routes to incorporating the trifluoromethylthio group into organic molecules of interest.3 To date, numerous nucleophilic and electrophilic SCF3 reagents have been developed to construct C–SCF3, and these reagents are widely used because they require mild conditions and show satisfied substrate scope.4,5 Recently, the efficient radical-type protocols for constructing C–SCF3 using a combination of AgSCF3 and oxidant have drawn great attention, but only a few protocols focused on the difunctionalization of alkene have been reported.6–8In 2014, the first radical silver-mediated oxidative aryltrifluoromethylthiolation of activated alkenes was reported by Wang group (Scheme 1a).7a Subsequently, an intramolecular oxytrifluoromethylthiolation of unactivated alkenes was reported (Scheme 1b).7b Soon afterward, the Qing group demonstrated direct trifluoromethylthiolation of an unactivated terminal alkene, affording various allyl trifluoromethyl sulfides (Scheme 1c).7c In the following year, the same group reported another radical protocol for the trifluoromethylthiolation of quinones (Scheme 1d).7d Recently, the Nevado group has reported a silver-catalyzed radical cascade trifluoromethylthiolation affording highly functionalized heterocyclic scaffolds (Scheme 1e).7e Despite these achievements, new methods involving difunctionalization-type trifluoromethylthiolation of alkenes remain highly desirable.
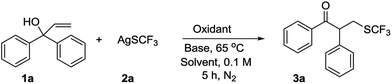
Recently, the neophyl rearrangement as an efficient and ingenious strategy to constructing multiple chemical structures and reorganizing molecular skeletons has attracted increasing attention.9–14 Inspired by the Tu semipinacol rearrangement protocol15 and recent literatures for forming β-trifluoromethyl ketones9 and previous advances in silver-mediated radical additions,16 we speculated that a neophyl-type rearrangement could be triggered after adding SCF3 radical to α,α-diaryl allylic alcohols, generating α-aryl-β-trifluoromethylthiolated carbonyl ketones which suffered from poor yield in previous literature.17 As part of our continuing efforts at introducing fluorine-containing groups into organic molecules,18 we report here a novel SCF3 radical-initiated radical neophyl rearrangement of α,α-diaryl allylic alcohols to afford trifluoromethylthiolated carbonyl ketones under mild conditions (Scheme 1f).
Result and discussion
Previous study showed that the SCF3 radical could be efficiently generated from AgSCF3 using K2S2O8 as an oxidant.6–8 Therefore we began our studies using α,α-diphenylprop-2-en-1-ol (1a) as substrate in the presence of AgSCF3 (1.5 equiv.) in CH3CN at 65 °C. A wide range of oxidants, including PhI(OAc)2, TBHP (tert-butyl hydroperoxide), NFSI (N-fluorobenzenesulfonimide), Mn(OAc)3, (NH4)2S2O8, Na2S2O8 and K2S2O8 were investigated for their ability to promote the reaction by generating the SCF3 radical. To our delight, the desired product 3a was obtained using persulfate salts as the oxidants, and 2 equiv. K2S2O8 gave the best yield of 26% (Table 1, entries 1–7). Decreasing the load of K2S2O8 improved the yield of the reaction (Table 1, entry 8). A brief survey of various representative solvents showed that CH3CN gave the best yield (Table 1, entries 9–13). A series of inorganic and organic bases were investigated to improve the yield further. While the inorganic or organic bases such as K2CO3, HMPA (hexamethylphosphoramide), DABCO (1,4-diazabicyclo[2.2.2]octane), and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) did not appreciably affect the transformation (Table 1, entries 14–19), pyridine (1 equiv.) increased the yield to 82% (entry 19). Changing the amount of pyridine did not improve yield (Table 1, entries 20 and 21). In a control experiment, we confirmed that no desired product was obtained when K2S2O8 was omitted from the reaction system (entry 22).| Entry | Oxidant | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reactions were performed in sealed tubes containing 1a (0.20 mmol), 2a (0.30 mmol), oxidant (2 equiv.), base (1.0 equiv.), solvent (2 mL) under N2 at 65 °C for 5 h. N. D. = not detected.b Isolated yield.c K2S2O8 (1.5 equiv.) was used.d Pyridine (2 equiv.) was used.e Pyridine (0.5 equiv.) was used. | ||||
| 1 | PhI(OAc)2 | None | CH3CN | N. D. |
| 2 | TBHP | None | CH3CN | N. D. |
| 3 | NFSI | None | CH3CN | N. D. |
| 4 | Mn(OAc)3 | None | CH3CN | N. D. |
| 5 | (NH4)2S2O8 | None | CH3CN | 15 |
| 6 | Na2S2O8 | None | CH3CN | 22 |
| 7 | K2S2O8 | None | CH3CN | 26 |
| 8c | K2S2O8 | None | CH3CN | 30 |
| 9c | K2S2O8 | None | DCE | Trace |
| 10c | K2S2O8 | None | EA | 11 |
| 11c | K2S2O8 | None | NMP | 20 |
| 12c | K2S2O8 | None | DMF | 13 |
| 13c | K2S2O8 | None | DMSO | 10 |
| 14c | K2S2O8 | K2CO3 | CH3CN | Trace |
| 15c | K2S2O8 | HMPA | CH3CN | Trace |
| 16c | K2S2O8 | DABCO | CH3CN | Trace |
| 17c | K2S2O8 | DBU | CH3CN | Trace |
| 18c | K2S2O8 | (i-Pr)2NEt | CH3CN | 45 |
| 19c | K2S2O8 | Pyridine | CH3CN | 82 |
| 20c,d | K2S2O8 | Pyridine | CH3CN | 74 |
| 21c,e | K2S2O8 | Pyridine | CH3CN | 76 |
| 22 | None | Pyridine | CH3CN | 0 |
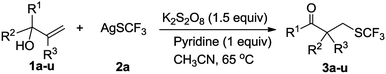
After determining the standard reaction conditions, we explored the scope of this difunctionalization protocol with various α,α-diaryl allylic alcohols (1), and the corresponding products 3a–3u were obtained in moderate to good isolated yields (Table 2). Note that the minor regioisomers 3f′, 3g′, 3i′, 3j′, 3l′, 3m′ and 3n′, in which R1 migrated instead of R2, were observed as byproducts in the reactions of corresponding substrates and the yields are indicated in the parentheses. When the symmetric allylic alcohols containing para-substituted bromo-group or chloro-group underwent rearrangement, the corresponding α-aryl-β-trifluoromethylthiolated carbonyl ketones 3b and 3c were obtained in respective yields of 72% and 63%. Similarly, the symmetric allylic alcohol substituted by para-OPh gave moderate 51% yield. When allylic alcohol substituted by meta-OMe was used as the substrate, no obvious substitution effect was observed and 3e was obtained in 79% yield.
| a Reaction conditions: 1a (0.20 mmol), 2a (0.30 mmol), K2S2O8 (0.30 mmol), pyridine (0.20 mmol), CH3CN (2 mL), 5 h, 65 °C under N2.b Isolated yields of products 3.c The yields of minor regioisomer 3′, in which R1 migrated instead of R2, were shown in the parentheses (determined by 1H NMR).d 3j and its isomer 3j′ were not separable. |
|---|
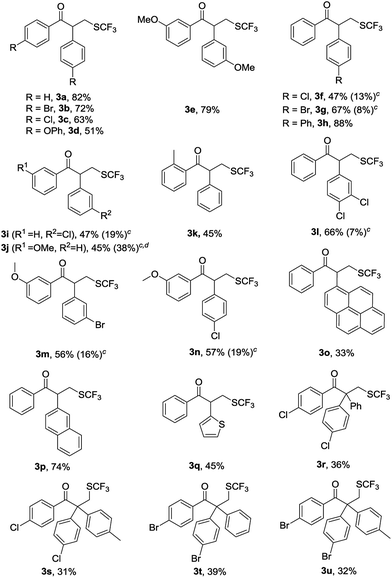 |
The allylic alcohols with a Ph group and a p-substituted-Ph group underwent p-substituted-Ph group migration as the major process, giving the corresponding products 3f–3h in 47–88% yield. Interestingly, allylic alcohols with a Ph group and a m-Cl–Ph group or a m-OMe–Ph group proceeded via the migration of the more electron-deficient aryl group, delivered the products 3i and 3j in respective yields of 47% and 45%. Allylic alcohols with a Ph group and an o-methylphenyl group delivered the Ph group-migration product 3k in 45% yield. Interestingly, allylic alcohols containing two different substituted aryl groups, also worked as substrates in the reaction, which selectively generated products 3l via migration of the 3,4-disubstituted-Ph group. For substrates with meta or para and meta substituents, the more electron-deficient aryl group migrated preferentially to afford the desired products 3m and 3n in respective yields of 56% and 57%. This unique selectivity suggested that the reaction might involve a radical neophyl rearrangement process rather than a simple semipinacol rearrangement.9 In the reactions generating 3o and 3p, migration of the Ph group took precedence over the pyren-2-yl and the naphthalene-2-yl, giving the respective yields of 33% and 74%. Gratifyingly, the Ph- and thiophen-2-yl-substituted allylic alcohol also worked well and afforded mainly the thiophen-2-yl-migration product 3q in good yield. Notably, the allylic alcohols with a tolyl or Ph attached to the vinyl group were also tolerated, affording the target products 3r–3u in 31–36% yield.
To gain further understanding of the reaction mechanism, the control experiments were performed (Scheme 2). When 2 equiv. of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT) were added to the reaction, the desired transformation was completely inhibited (Scheme 2a and b). This observation is consistent with the hypothesis that the reaction proceeds via a radical pathway through single-electron transfer. When CuSCF3 was used as the SCF3 source, the reaction led to no product, indicating that silver plays a vital role in this transformation (Scheme 2c).
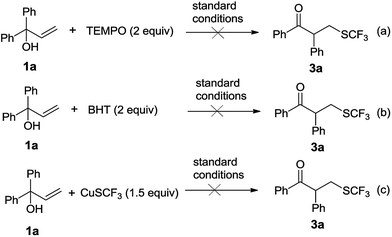 | ||
| Scheme 2 Mechanistic studies of radical addition and radical neophyl rearrangement cascade reaction. | ||
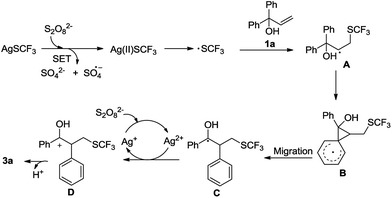
On the basis of the experimental results and previous reports,6,10–14 a plausible mechanism is proposed as depicted in Scheme 3. AgSCF3 is oxidized by K2S2O8 firstly to generate the SCF3 radical.6 Subsequent intermolecular addition of the SCF3 radical to the double bond of allylic alcohol 1a affords radical intermediate A. Within radical intermediate A, neophyl rearrangement of the aryl group occurs via spiro[2,5]octadienyl radical B to produce intermediate C.10–14 Another single-electron transfer from intermediate C to Ag(II) oxidant generates intermediate D, which affords product 3a by release of a proton.
Conclusions
In summary, we have developed a AgSCF3-mediated trifluoromethylthiolation of α,α-diaryl allylic alcohols for the synthesis of various α-aryl-β-trifluoromethylthiolated carbonyl ketones. The procedure involves the formations of C(Ar)–C(sp3) and C(sp3)–S bonds in one step via SCF3 radical addition and a radical neophyl rearrangement cascade process under mild conditions. The procedure tolerates a wide range of symmetrical and nonsymmetrical α,α-diaryl allylic alcohols. Given the frequent use of fluorine-containing compounds as bioactive agents, the convenience of this method affords an efficient approach in the future drug development and synthesis of fine chemicals.Experimental
General
All manipulations were carried out in a sealed tube under a nitrogen atmosphere using standard Schlenk techniques. The solvents were distilled under nitrogen from sodium–benzophenone (THF, toluene, dioxane) or calcium hydride (DMF, MeCN, 1,2-DCE) before used. The α,α-diaryl allylic alcohols19 and AgSCF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 were prepared according to the literature methods. Other chemicals were obtained from commercial sources, and were used without further purification. Chemical shifs (δ, ppm) in the 1H NMR spectra were recorded using TMS as internal standard. Chemical shifts in 13C{1H} NMR spectra were internally referenced to CHCl3 (δ = 77.16 ppm).
20 were prepared according to the literature methods. Other chemicals were obtained from commercial sources, and were used without further purification. Chemical shifs (δ, ppm) in the 1H NMR spectra were recorded using TMS as internal standard. Chemical shifts in 13C{1H} NMR spectra were internally referenced to CHCl3 (δ = 77.16 ppm).
General procedure for the synthesis of α-aryl-β-trifluoromethylthiolated carbonyl ketones
1a–u (0.2 mmol), 2a (62.7 mg, 0.3 mmol), K2S2O8 (81.1 mg, 0.3 mmol), pyridine (15.8 mg, 0.2 mmol), and CH3CN (2 mL) were mixed in an oven-dried sealed tube under N2. The tube was sealed and heated at 65 °C for 5 h. The resulting mixture was cooled to room temperature and the solvent was evaporated under vacuum. The crude product was purified by column chromatography on silica gel to afford the product 3a–u.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.22 (1H, dd, J = 5.6, 14.0 Hz), 3.64 (1H, dd, J = 9.2, 14.0 Hz), 4.92 (1H, dd, J = 5.6, 9.2 Hz), 7.24–7.40 (5H, m), 7.36–7.40 (2H, m), 7.47–7.51 (2H, m), 7.92–7.95 (1H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 54.6, 128.2, 128.3, 128.8, 129.0, 129.6, 131.4 (q, J = 304 Hz) 133.6, 135.9, 137.2, 197.5; 19F NMR (CDCl3, 376 MHz) δ −41.3; HRMS (EI, TOF) calcd for C16H13F3OS+ [M]+: 310.0639, found: 310.0638.
1). 1H NMR (400 MHz, CDCl3) δ 3.22 (1H, dd, J = 5.6, 14.0 Hz), 3.64 (1H, dd, J = 9.2, 14.0 Hz), 4.92 (1H, dd, J = 5.6, 9.2 Hz), 7.24–7.40 (5H, m), 7.36–7.40 (2H, m), 7.47–7.51 (2H, m), 7.92–7.95 (1H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 54.6, 128.2, 128.3, 128.8, 129.0, 129.6, 131.4 (q, J = 304 Hz) 133.6, 135.9, 137.2, 197.5; 19F NMR (CDCl3, 376 MHz) δ −41.3; HRMS (EI, TOF) calcd for C16H13F3OS+ [M]+: 310.0639, found: 310.0638.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.18 (1H, q, J = 5.7, 14.2 Hz), 3.59 (1H, q, J = 8.9, 14.2 Hz), 4.80 (1H, J = 6.8 Hz), 7.46 (2H, d, J = 7.8 Hz), 7.46 (2H, d, J = 7.7 Hz), 7.54 (2H, d, J = 7.9 Hz), 7.76 (2H, d, J = 7.8 Hz); 13C NMR (100 MHz, CDCl3) δ 32.5, 53.9, 122.7, 129.2, 129.8, 130.4, 130.7 (q, J = 304 Hz), 132.3, 132.9, 134.3, 135.8, 196.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C15H10Br2O+ [M − H − SCF3]+: 363.9087, found: 363.9105.
1). 1H NMR (400 MHz, CDCl3) δ 3.18 (1H, q, J = 5.7, 14.2 Hz), 3.59 (1H, q, J = 8.9, 14.2 Hz), 4.80 (1H, J = 6.8 Hz), 7.46 (2H, d, J = 7.8 Hz), 7.46 (2H, d, J = 7.7 Hz), 7.54 (2H, d, J = 7.9 Hz), 7.76 (2H, d, J = 7.8 Hz); 13C NMR (100 MHz, CDCl3) δ 32.5, 53.9, 122.7, 129.2, 129.8, 130.4, 130.7 (q, J = 304 Hz), 132.3, 132.9, 134.3, 135.8, 196.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C15H10Br2O+ [M − H − SCF3]+: 363.9087, found: 363.9105.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.18 (1H, dd, J = 5.8, 14.2 Hz), 3.60 (1H, dd, J = 8.9, 14.2 Hz), 4.82 (1H, t, J = 7.2 Hz), 7.20 (2H, d, J = 8.1 Hz), 7.31 (2H, d, J = 7.9 Hz), 7.38 (2H, d, J = 8.1 Hz), 7.85 (2H, d, J = 8.1 Hz); 13C NMR (100 MHz, CDCl3) δ 32.6, 53.8, 129.3, 129.5, 129.9, 130.3, 131.3 (q, J = 304 Hz), 133.9, 134.5, 135.3, 140.4, 196.1; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C15H11Cl2O+ [M − SCF3]+: 277.0181, found: 277.0168.
1). 1H NMR (400 MHz, CDCl3) δ 3.18 (1H, dd, J = 5.8, 14.2 Hz), 3.60 (1H, dd, J = 8.9, 14.2 Hz), 4.82 (1H, t, J = 7.2 Hz), 7.20 (2H, d, J = 8.1 Hz), 7.31 (2H, d, J = 7.9 Hz), 7.38 (2H, d, J = 8.1 Hz), 7.85 (2H, d, J = 8.1 Hz); 13C NMR (100 MHz, CDCl3) δ 32.6, 53.8, 129.3, 129.5, 129.9, 130.3, 131.3 (q, J = 304 Hz), 133.9, 134.5, 135.3, 140.4, 196.1; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C15H11Cl2O+ [M − SCF3]+: 277.0181, found: 277.0168.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 5.7, 14.0 Hz), 3.62 (1H, dd, J = 9.0, 14.0 Hz), 4.80 (1H, dd, J = 5.7, 9.0 Hz), 7.13 (2H, d, J = 8.2 Hz), 7.47 (2H, d, J = 8.2 Hz), 7.55 (1H, d, J = 8.4 Hz), 7.76 (2H, d, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 32.9, 53.5, 117.2, 119.3, 119.5, 120.5, 123.9, 124.9, 129.5, 129.9, 130.2, 130.3, 131.4 (q, J = 304 Hz), 131.8, 155.2, 156.5, 157.5, 162.5; 19F NMR (CDCl3, 376 MHz) δ −41.2; HRMS (EI, TOF) calcd for C28H21F3O3S+ [M]+: 494.1163, found: 494.1165.
1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 5.7, 14.0 Hz), 3.62 (1H, dd, J = 9.0, 14.0 Hz), 4.80 (1H, dd, J = 5.7, 9.0 Hz), 7.13 (2H, d, J = 8.2 Hz), 7.47 (2H, d, J = 8.2 Hz), 7.55 (1H, d, J = 8.4 Hz), 7.76 (2H, d, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 32.9, 53.5, 117.2, 119.3, 119.5, 120.5, 123.9, 124.9, 129.5, 129.9, 130.2, 130.3, 131.4 (q, J = 304 Hz), 131.8, 155.2, 156.5, 157.5, 162.5; 19F NMR (CDCl3, 376 MHz) δ −41.2; HRMS (EI, TOF) calcd for C28H21F3O3S+ [M]+: 494.1163, found: 494.1165.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.22 (1H, dd, J = 5.6, 14.0 Hz), 3.63 (1H, dd, J = 9.2, 14.0 Hz), 3.76 (3H, s), 3.8 (3H, s), 4.85 (1H, dd, J = 5.6, 9.2 Hz), 6.78–6.88 (3H, m), 7.03–7.06 (1H, m), 7.22–7.30 (2H, m), 7.47–7.52 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 54.7, 55.4, 55.5, 113.2, 113.3, 113.9, 120.1, 120.5, 121.6, 129.8, 130.6, 131.4 (q, J = 304 Hz), 137.2, 138.7, 159.9, 160.3, 197.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C18H17F3O3S+ [M]+: 370.0850, found: 370.0853.
1). 1H NMR (400 MHz, CDCl3) δ 3.22 (1H, dd, J = 5.6, 14.0 Hz), 3.63 (1H, dd, J = 9.2, 14.0 Hz), 3.76 (3H, s), 3.8 (3H, s), 4.85 (1H, dd, J = 5.6, 9.2 Hz), 6.78–6.88 (3H, m), 7.03–7.06 (1H, m), 7.22–7.30 (2H, m), 7.47–7.52 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 54.7, 55.4, 55.5, 113.2, 113.3, 113.9, 120.1, 120.5, 121.6, 129.8, 130.6, 131.4 (q, J = 304 Hz), 137.2, 138.7, 159.9, 160.3, 197.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C18H17F3O3S+ [M]+: 370.0850, found: 370.0853.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.19 (1H, dd, J = 5.9, 14.2 Hz), 3.61 (1H, dd, J = 8.8, 14.2 Hz), 4.89 (1H, dd, J = 5.9, 8.8 Hz), 7.21–7.23 (2H, d, J = 8.5 Hz), 7.29–7.31 (2H, m), 7.39–7.43 (2H, m), 7.51–7.55 (1H, m), 7.89–7.91 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 53.7, 128.9, 129.0, 129.6, 129.8, 131.3 (q, J = 304 Hz), 133.8, 134.3, 135.6, 135.7, 197.3; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C16H12ClF3OS+ [M]+: 344.0249, found: 344.0255.
1). 1H NMR (400 MHz, CDCl3) δ 3.19 (1H, dd, J = 5.9, 14.2 Hz), 3.61 (1H, dd, J = 8.8, 14.2 Hz), 4.89 (1H, dd, J = 5.9, 8.8 Hz), 7.21–7.23 (2H, d, J = 8.5 Hz), 7.29–7.31 (2H, m), 7.39–7.43 (2H, m), 7.51–7.55 (1H, m), 7.89–7.91 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 53.7, 128.9, 129.0, 129.6, 129.8, 131.3 (q, J = 304 Hz), 133.8, 134.3, 135.6, 135.7, 197.3; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C16H12ClF3OS+ [M]+: 344.0249, found: 344.0255.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.19 (1H, dd, J = 5.9, 14.2 Hz), 3.61 (1H, dd, J = 8.8, 14.2 Hz), 4.87 (1H, dd, J = 5.9, 8.8 Hz), 7.14–7.18 (2H, m), 7.39–7.47 (4H, m), 7.50–7.55 (1H, m), 7.89–7.91 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 53.8, 122.5, 128.9, 129.0, 129.9, 131.2 (q, J = 304 Hz), 132.8, 133.8, 135.6, 136.2, 197.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C15H12BrO+ [M − SCF3]+: 287.0072, found: 287.0039.
1). 1H NMR (400 MHz, CDCl3) δ 3.19 (1H, dd, J = 5.9, 14.2 Hz), 3.61 (1H, dd, J = 8.8, 14.2 Hz), 4.87 (1H, dd, J = 5.9, 8.8 Hz), 7.14–7.18 (2H, m), 7.39–7.47 (4H, m), 7.50–7.55 (1H, m), 7.89–7.91 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 53.8, 122.5, 128.9, 129.0, 129.9, 131.2 (q, J = 304 Hz), 132.8, 133.8, 135.6, 136.2, 197.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C15H12BrO+ [M − SCF3]+: 287.0072, found: 287.0039.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.25 (1H, dd, J = 5.6, 14.2 Hz), 3.68 (1H, dd, J = 9.1, 14.2 Hz), 4.96 (1H, t, J = 6.9 Hz), 7.32–7.36 (3H, m), 7.39–7.42 (4H, m), 7.49–7.55 (5H, m), 7.97 (2H, d, J = 7.7 Hz); 13C NMR (100 MHz, CDCl3) δ 32.8, 54.1, 127.1, 127.7, 128.2, 128.6, 128.8, 128.9, 129.0, 131.4 (q, J = 304 Hz), 133.6, 135.9, 136.1, 140.3, 141.2, 197.5; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C22H17F3OS+ [M]+: 386.0952, found: 386.0951.
1). 1H NMR (400 MHz, CDCl3) δ 3.25 (1H, dd, J = 5.6, 14.2 Hz), 3.68 (1H, dd, J = 9.1, 14.2 Hz), 4.96 (1H, t, J = 6.9 Hz), 7.32–7.36 (3H, m), 7.39–7.42 (4H, m), 7.49–7.55 (5H, m), 7.97 (2H, d, J = 7.7 Hz); 13C NMR (100 MHz, CDCl3) δ 32.8, 54.1, 127.1, 127.7, 128.2, 128.6, 128.8, 128.9, 129.0, 131.4 (q, J = 304 Hz), 133.6, 135.9, 136.1, 140.3, 141.2, 197.5; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C22H17F3OS+ [M]+: 386.0952, found: 386.0951.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 4.0, 16.0 Hz), 3.63 (1H, dd, J = 8.0, 16.0 Hz), 4.90 (1H, dd, J = 4.0, 8.0 Hz), 7.16–7.18 (1H, m), 7.23–7.29 (3H, m), 7.42 (2H, d, J = 8.0 Hz), 7.54 (1H, d, J = 8.0 Hz), 7.91–7.94 (1H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 53.9, 126.5, 128.3, 128.6, 128.9, 129.0, 130.8, 131.3 (q, J = 305 Hz), 133.9, 135.4, 135.6, 139.1, 197.0; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C16H12ClF3OS+ [M]+: 344.0249, found: 344.0251.
1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 4.0, 16.0 Hz), 3.63 (1H, dd, J = 8.0, 16.0 Hz), 4.90 (1H, dd, J = 4.0, 8.0 Hz), 7.16–7.18 (1H, m), 7.23–7.29 (3H, m), 7.42 (2H, d, J = 8.0 Hz), 7.54 (1H, d, J = 8.0 Hz), 7.91–7.94 (1H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 53.9, 126.5, 128.3, 128.6, 128.9, 129.0, 130.8, 131.3 (q, J = 305 Hz), 133.9, 135.4, 135.6, 139.1, 197.0; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C16H12ClF3OS+ [M]+: 344.0249, found: 344.0251.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.21 (2H, dd, J = 5.6, 14.0 Hz), 3.64 (2H, dd, J = 9.1, 14.0 Hz), 3.77 (2.68H, s), 3.80 (3.20H, s), 4.87 (2H, m), 6.78–6.80 (2H, m), 6.87 (1H, d, J = 7.6 Hz), 7.03–7.06 (1H, m), 7.22–7.41 (9H, m), 7.46–7.52 (3H, m), 7.93–7.95 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.75, 32.84, 54.6, 54.7, 55.4, 55.5, 113.2, 113.4, 113.9, 120.1, 120.6, 121.6, 128.2, 128.3, 128.8, 129.0, 129.6, 129.8, 130.6, 131.4 (q, J = 304 Hz), 133.6, 135.9, 137.2, 137.3, 138.7, 159.9, 160.4, 197.4; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C17H15F3O2S+ [M]+: 340.0745, found: 340.0749.
1). 1H NMR (400 MHz, CDCl3) δ 3.21 (2H, dd, J = 5.6, 14.0 Hz), 3.64 (2H, dd, J = 9.1, 14.0 Hz), 3.77 (2.68H, s), 3.80 (3.20H, s), 4.87 (2H, m), 6.78–6.80 (2H, m), 6.87 (1H, d, J = 7.6 Hz), 7.03–7.06 (1H, m), 7.22–7.41 (9H, m), 7.46–7.52 (3H, m), 7.93–7.95 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.75, 32.84, 54.6, 54.7, 55.4, 55.5, 113.2, 113.4, 113.9, 120.1, 120.6, 121.6, 128.2, 128.3, 128.8, 129.0, 129.6, 129.8, 130.6, 131.4 (q, J = 304 Hz), 133.6, 135.9, 137.2, 137.3, 138.7, 159.9, 160.4, 197.4; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C17H15F3O2S+ [M]+: 340.0745, found: 340.0749.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.37 (3H, s), 3.21 (1H, dd, J = 5.4, 14.0 Hz), 3.67 (1H, dd, J = 9.5, 14.0 Hz), 4.80 (1H, dd, J = 5.4, 9.5 Hz); 7.15–7.27 (4H, m), 7.28–7.32 (4H, m), 7.57 (1H, d, J = 7.7 Hz); 13C NMR (100 MHz, CDCl3) δ 21.4, 32.4, 57.0, 125.7, 128.2, 128.3, 128.5, 129.4, 131.4 (q, J = 304 Hz), 131.7, 132.0, 136.6, 137.3, 138.9, 201.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (ESI, TOF) calcd for C17H15F3OS+ [M]+: 324.0796, found: 324.0798.
1). 1H NMR (400 MHz, CDCl3) δ 2.37 (3H, s), 3.21 (1H, dd, J = 5.4, 14.0 Hz), 3.67 (1H, dd, J = 9.5, 14.0 Hz), 4.80 (1H, dd, J = 5.4, 9.5 Hz); 7.15–7.27 (4H, m), 7.28–7.32 (4H, m), 7.57 (1H, d, J = 7.7 Hz); 13C NMR (100 MHz, CDCl3) δ 21.4, 32.4, 57.0, 125.7, 128.2, 128.3, 128.5, 129.4, 131.4 (q, J = 304 Hz), 131.7, 132.0, 136.6, 137.3, 138.9, 201.2; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (ESI, TOF) calcd for C17H15F3OS+ [M]+: 324.0796, found: 324.0798.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 6.0, 14.2 Hz), 3.61 (1H, dd, J = 8.8, 14.2 Hz), 4.87 (1H, dd, J = 6.0, 8.8 Hz), 7.14 (1H, dd, J = 2.1, 8.3 Hz), 7.39–7.45 (4H, m), 7.53–7.57 (1H, m), 7.90–7.92 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 53.2, 127.6, 128.9, 129.0, 130.1, 130.5, 131.3 (q, J = 303 Hz), 131.5, 132.7, 133.7, 134.1, 135.5, 137.2, 196.8; 19F NMR (376 MHz, CDCl3) δ −41.2; HRMS (EI, TOF) calcd for C16H11Cl2F3OS+ [M]+: 377.9860, found: 377.9865.
1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 6.0, 14.2 Hz), 3.61 (1H, dd, J = 8.8, 14.2 Hz), 4.87 (1H, dd, J = 6.0, 8.8 Hz), 7.14 (1H, dd, J = 2.1, 8.3 Hz), 7.39–7.45 (4H, m), 7.53–7.57 (1H, m), 7.90–7.92 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 53.2, 127.6, 128.9, 129.0, 130.1, 130.5, 131.3 (q, J = 303 Hz), 131.5, 132.7, 133.7, 134.1, 135.5, 137.2, 196.8; 19F NMR (376 MHz, CDCl3) δ −41.2; HRMS (EI, TOF) calcd for C16H11Cl2F3OS+ [M]+: 377.9860, found: 377.9865.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 5.7, 14.2 Hz), 3.62 (1H, dd, J = 9.0, 14.2 Hz), 3.82 (3H, s), 4.86 (1H, dd, J = 5.7, 9.0 Hz), 7.06–7.09 (1H, m), 7.20–7.22 (2H, m), 7.31 (1H, t, J = 8.0 Hz), 7.39–7.42 (1H, m), 7.43–7.49 (3H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 54.0, 55.6, 113.2, 120.4, 121.6, 123.5, 126.9, 129.8, 129.9, 131.0, 131.1, 131.3 (q, J = 304 Hz), 131.5, 137.0, 139.4, 160.0, 196.8; 19F NMR (376 MHz, CDCl3) δ −41.2; HRMS (EI, TOF) calcd for C17H14BrF3O2S+ [M]+: 417.9850, found: 417.9854.
1). 1H NMR (400 MHz, CDCl3) δ 3.20 (1H, dd, J = 5.7, 14.2 Hz), 3.62 (1H, dd, J = 9.0, 14.2 Hz), 3.82 (3H, s), 4.86 (1H, dd, J = 5.7, 9.0 Hz), 7.06–7.09 (1H, m), 7.20–7.22 (2H, m), 7.31 (1H, t, J = 8.0 Hz), 7.39–7.42 (1H, m), 7.43–7.49 (3H, m); 13C NMR (100 MHz, CDCl3) δ 32.8, 54.0, 55.6, 113.2, 120.4, 121.6, 123.5, 126.9, 129.8, 129.9, 131.0, 131.1, 131.3 (q, J = 304 Hz), 131.5, 137.0, 139.4, 160.0, 196.8; 19F NMR (376 MHz, CDCl3) δ −41.2; HRMS (EI, TOF) calcd for C17H14BrF3O2S+ [M]+: 417.9850, found: 417.9854.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.19 (1H, dd, J = 5.9, 14.2 Hz), 3.60 (1H, dd, J = 8.8, 14.2 Hz), 3.81 (3H, s), 3.87 (1H, dd, J = 5.9, 8.8 Hz), 7.07 (1H, dd, J = 2.2, 7.9 Hz), 7.20–7.23 (2H, m), 7.28–7.32 (3H, m), 7.44–7.48 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 53.8, 55.6, 113.3, 120.2, 121.5, 129.6, 129.8, 129.9, 131.4 (q, J = 304 Hz), 134.3, 135.7, 137.0, 160.0, 197.1; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C17H14ClF3O2S+ [M]+: 374.0355, found: 374.0358.
1). 1H NMR (400 MHz, CDCl3) δ 3.19 (1H, dd, J = 5.9, 14.2 Hz), 3.60 (1H, dd, J = 8.8, 14.2 Hz), 3.81 (3H, s), 3.87 (1H, dd, J = 5.9, 8.8 Hz), 7.07 (1H, dd, J = 2.2, 7.9 Hz), 7.20–7.23 (2H, m), 7.28–7.32 (3H, m), 7.44–7.48 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.7, 53.8, 55.6, 113.3, 120.2, 121.5, 129.6, 129.8, 129.9, 131.4 (q, J = 304 Hz), 134.3, 135.7, 137.0, 160.0, 197.1; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C17H14ClF3O2S+ [M]+: 374.0355, found: 374.0358.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.37 (1H, dd, J = 4.4, 14.4 Hz), 3.84 (1H, dd, J = 10.5, 14.4 Hz), 5.97 (1H, dd, J = 4.4, 10.5 Hz), 7.21–7.26 (2H, m), 7.37 (1H, t, J = 7.4 Hz), 7.7 (1H, d, J = 8.0 Hz), 7.78–7.89 (2H, m), 7.98 (1H, d, J = 8.9 Hz), 8.03–8.09 (3H, m), 8.23 (1H, d, J = 7.4 Hz), 8.28 (1H, d, J = 7.6 Hz), 8.35 (1H, d, J = 9.3 Hz), 8.55 (1H, d, J = 9.3 Hz); 13C NMR (100 MHz, CDCl3) δ 32.4, 51.0, 121.3, 124.9, 125.1, 125.6, 125.7, 125.8, 126.1, 126.5, 127.5, 128.1, 128.2, 128.8, 128.9, 129.6, 130.8, 131.0, 131.3, 131.4, 131.7 (q, J = 303 Hz), 133.5, 135.9, 198.0; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C26H17F3OS+ [M]+: 434.0952, found: 434.0954.
1). 1H NMR (400 MHz, CDCl3) δ 3.37 (1H, dd, J = 4.4, 14.4 Hz), 3.84 (1H, dd, J = 10.5, 14.4 Hz), 5.97 (1H, dd, J = 4.4, 10.5 Hz), 7.21–7.26 (2H, m), 7.37 (1H, t, J = 7.4 Hz), 7.7 (1H, d, J = 8.0 Hz), 7.78–7.89 (2H, m), 7.98 (1H, d, J = 8.9 Hz), 8.03–8.09 (3H, m), 8.23 (1H, d, J = 7.4 Hz), 8.28 (1H, d, J = 7.6 Hz), 8.35 (1H, d, J = 9.3 Hz), 8.55 (1H, d, J = 9.3 Hz); 13C NMR (100 MHz, CDCl3) δ 32.4, 51.0, 121.3, 124.9, 125.1, 125.6, 125.7, 125.8, 126.1, 126.5, 127.5, 128.1, 128.2, 128.8, 128.9, 129.6, 130.8, 131.0, 131.3, 131.4, 131.7 (q, J = 303 Hz), 133.5, 135.9, 198.0; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C26H17F3OS+ [M]+: 434.0952, found: 434.0954.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.30 (1H, dd, J = 5.7, 14.1 Hz), 3.73 (1H, dd, J = 9.1, 141 Hz), 5.07 (1H, dd, J = 5.7, 9.1 Hz), 7.34–7.46 (3H, m), 7.46–7.49 (3H, m), 7.73 (1H, s), 7.77–7.84 (3H, m), 7.96–7.98 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.9, 54.7, 125.7, 126.6, 126.8, 127.5, 127.9, 128.0, 128.8, 129.0, 129.6, 131.5 (q, J = 304 Hz), 132.9, 133.6, 133.7, 134.7, 135.9, 197.5; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C20H15F3OS+ [M]+: 360.0796, found: 360.0797.
1). 1H NMR (400 MHz, CDCl3) δ 3.30 (1H, dd, J = 5.7, 14.1 Hz), 3.73 (1H, dd, J = 9.1, 141 Hz), 5.07 (1H, dd, J = 5.7, 9.1 Hz), 7.34–7.46 (3H, m), 7.46–7.49 (3H, m), 7.73 (1H, s), 7.77–7.84 (3H, m), 7.96–7.98 (2H, m); 13C NMR (100 MHz, CDCl3) δ 32.9, 54.7, 125.7, 126.6, 126.8, 127.5, 127.9, 128.0, 128.8, 129.0, 129.6, 131.5 (q, J = 304 Hz), 132.9, 133.6, 133.7, 134.7, 135.9, 197.5; 19F NMR (376 MHz, CDCl3) δ −41.3; HRMS (EI, TOF) calcd for C20H15F3OS+ [M]+: 360.0796, found: 360.0797.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 3.68 (2H, d, J = 12.8 Hz), 3.77 (1H, d, J = 12.8 Hz), 7.16 (2H, d, J = 8.9 Hz), 7.30–7.38 (9H, m), 7.56 (2H, d, J = 8.6 Hz); 13C NMR (100 MHz, CDCl3) δ 42.0, 64.8, 128.4, 128.9, 129.0, 129.1, 129.2, 130.8 (q, J = 304 Hz), 131.0, 132.0, 133.5, 134.3, 136.9, 138.2, 139.3, 198.1; 19F NMR (376 MHz, CDCl3) δ −42.8; HRMS (ESI, TOF) calcd for C22H15Cl2F3NaOS+ [M + Na]+: 477.0070, found: 477.0067.
1). 1H NMR (400 MHz, CDCl3) δ 3.68 (2H, d, J = 12.8 Hz), 3.77 (1H, d, J = 12.8 Hz), 7.16 (2H, d, J = 8.9 Hz), 7.30–7.38 (9H, m), 7.56 (2H, d, J = 8.6 Hz); 13C NMR (100 MHz, CDCl3) δ 42.0, 64.8, 128.4, 128.9, 129.0, 129.1, 129.2, 130.8 (q, J = 304 Hz), 131.0, 132.0, 133.5, 134.3, 136.9, 138.2, 139.3, 198.1; 19F NMR (376 MHz, CDCl3) δ −42.8; HRMS (ESI, TOF) calcd for C22H15Cl2F3NaOS+ [M + Na]+: 477.0070, found: 477.0067.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (3H, s), 3.65 (1H, d, J = 12.8 Hz), 3.77 (2H, d, J = 12.8 Hz), 7.14–7.18 (4H, m), 7.21 (2H, d, J = 8.4 Hz), 7.29–7.35 (4H, m), 7.55 (2H, d, J = 8.8 Hz); 13C NMR (100 MHz, CDCl3) δ 21.2, 42.0, 64.5, 128.5, 128.9, 129.1, 129.8, 131.0, 132.0, 133.7, 134.2, 135.1, 137.2, 138.4, 139.2, 199.2; 19F NMR (CDCl3, 376 MHz) δ −42.7; HRMS (EI, TOF) calcd for C22H17Cl2O+ [M]+: 367.0656, found: 367.0653.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (3H, s), 3.65 (1H, d, J = 12.8 Hz), 3.77 (2H, d, J = 12.8 Hz), 7.14–7.18 (4H, m), 7.21 (2H, d, J = 8.4 Hz), 7.29–7.35 (4H, m), 7.55 (2H, d, J = 8.8 Hz); 13C NMR (100 MHz, CDCl3) δ 21.2, 42.0, 64.5, 128.5, 128.9, 129.1, 129.8, 131.0, 132.0, 133.7, 134.2, 135.1, 137.2, 138.4, 139.2, 199.2; 19F NMR (CDCl3, 376 MHz) δ −42.7; HRMS (EI, TOF) calcd for C22H17Cl2O+ [M]+: 367.0656, found: 367.0653.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (3H, s), 3.67 (1H, d, J = 12.8 Hz), 3.77 (2H, d, J = 12.8 Hz), 7.24–7.27 (2H, m), 7.34–7.37 (7H, m), 7.46–7.51 (4H, m); 13C NMR (100 MHz, CDCl3) δ 41.9, 64.9, 122.5, 128.2, 128.5, 129.1, 129.2, 131.3, 131.5, 132.0, 132.1, 134.0, 137.5, 138.1, 198.2; 19F NMR (CDCl3, 376 MHz) δ −42.7; HRMS (EI, TOF) calcd for C21H15Br2O+ [M]+: 440.9490, found: 440.9491.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (3H, s), 3.67 (1H, d, J = 12.8 Hz), 3.77 (2H, d, J = 12.8 Hz), 7.24–7.27 (2H, m), 7.34–7.37 (7H, m), 7.46–7.51 (4H, m); 13C NMR (100 MHz, CDCl3) δ 41.9, 64.9, 122.5, 128.2, 128.5, 129.1, 129.2, 131.3, 131.5, 132.0, 132.1, 134.0, 137.5, 138.1, 198.2; 19F NMR (CDCl3, 376 MHz) δ −42.7; HRMS (EI, TOF) calcd for C21H15Br2O+ [M]+: 440.9490, found: 440.9491.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (3H, s), 3.67 (1H, d, J = 12.8 Hz), 3.77 (2H, d, J = 12.8 Hz), 7.15–7.18 (2H, m), 7.23–7.26 (4H, m), 7.32–7.34 (2H, m), 7.46–7.49 (4H, m); 13C NMR (100 MHz, CDCl3) δ 41.9, 64.9, 122.5, 128.2, 128.5, 129.1, 129.2, 131.3, 131.5, 132.0, 132.1, 134.0, 137.5, 138.1, 198.2; 19F NMR (CDCl3, 376 MHz) δ −42.7; HRMS (EI, TOF) calcd for C22H17Br2O+ [M]+: 454.9646, found: 454.9644.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (3H, s), 3.67 (1H, d, J = 12.8 Hz), 3.77 (2H, d, J = 12.8 Hz), 7.15–7.18 (2H, m), 7.23–7.26 (4H, m), 7.32–7.34 (2H, m), 7.46–7.49 (4H, m); 13C NMR (100 MHz, CDCl3) δ 41.9, 64.9, 122.5, 128.2, 128.5, 129.1, 129.2, 131.3, 131.5, 132.0, 132.1, 134.0, 137.5, 138.1, 198.2; 19F NMR (CDCl3, 376 MHz) δ −42.7; HRMS (EI, TOF) calcd for C22H17Br2O+ [M]+: 454.9646, found: 454.9644.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Project No. 21421004, 21561162003, 21672059 and 21372072), the Eastern Scholar Distinguished Professor Program, the Programme of Introducing Talents of Discipline to Universities (B16017), the NCET (NCET-13-0798) and the Fundamental Research Funds for the Central Universities.Notes and references
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (c) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed.
- (a) F. Leroux, P. Jeschke and M. Schlosser, Chem. Rev., 2005, 105, 827 CrossRef CAS PubMed; (b) B. Manteau, S. Pazenok, J. P. Vors and F. R. Leroux, J. Fluorine Chem., 2010, 131, 140 CrossRef CAS; (c) Y. Huang, X. He, X. Lin, M. Rong and Z. Weng, Org. Lett., 2014, 16, 3284 CrossRef CAS PubMed.
- (a) C. Chen, L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454 CrossRef CAS PubMed; (b) A. Tlili and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 6818 CrossRef CAS PubMed; (c) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS PubMed; (d) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed; (e) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed.
- (a) G. Teverobskiy, D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 7312 CrossRef PubMed; (b) C.-P. Zhang and D.-A. Vicic, J. Am. Chem. Soc., 2012, 134, 183 CrossRef CAS PubMed; (c) C. Chen, L.-L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454 CrossRef CAS PubMed; (d) Z. Weng, W. He, C. Chen, R. Lee, D. Dan, Z. Lai, D. Kong, Y. Yuan and K.-W. Huang, Angew. Chem., Int. Ed., 2013, 52, 1548 CrossRef CAS PubMed; (e) K.-P. Wang, S.-Y. Yun, P. Mamidipalli and D. Lee, Chem. Sci., 2013, 4, 3205 RSC; (f) G. Danoun, B. Bayarmagnai, M. F. Grünberg and J. L. Gooβen, Chem. Sci., 2014, 5, 1312 RSC; (g) L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237 CrossRef CAS PubMed; (h) C. Chen, X.-H. Xu, B. Yang and F.-L. Qing, Org. Lett., 2014, 16, 3372 CrossRef CAS PubMed; (i) Y. Huang, X. He, X. Lin, M. Rong and Z. Weng, Org. Lett., 2014, 16, 3284 CrossRef CAS PubMed; (j) C. Xu and Q. Shen, Org. Lett., 2014, 16, 2046 CrossRef CAS PubMed; (k) M. Hu, J. Rong, W. Miao, C. Ni, Y. Han and J. Hu, Org. Lett., 2014, 16, 2030 CrossRef CAS PubMed; (l) X.-X. Shao, X.-Q. Wang, Y. Yang, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2013, 123, 3541 CrossRef; (m) Y.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro and N. Shitaba, J. Am. Chem. Soc., 2013, 135, 8782 CrossRef CAS PubMed; (n) C.-F. Xu, B.-Q. Ma and Q. Shen, Angew. Chem., Int. Ed., 2014, 53, 9316 CrossRef CAS PubMed.
- (a) X. Xu, S. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed; (b) F. Tougloat, S. Alazet and T. Billard, Eur. J. Org. Chem., 2014, 2415 Search PubMed; (c) X. Shao, C. Xu and Q. Shen, Acc. Chem. Res., 2015, 48, 1227 CrossRef CAS PubMed; (d) X. Shao, C. Xu, L. Lu and Q. Shen, Acc. Chem. Res., 2014, 47, 1513 CrossRef PubMed; (e) Y. Yang, X. Jiang and F.-L. Qing, J. Org. Chem., 2012, 77, 7538 CrossRef CAS PubMed; (f) Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647 RSC; (g) J. Sheng, C. Fan and J. Wu, Chem. Commun., 2014, 50, 5494 RSC; (h) J. Sheng, S. Li and J. Wu, Chem. Commun., 2014, 50, 578 RSC; (i) Q. Xiao, H. Zhu, G. Li and Z. Chen, Adv. Synth. Catal., 2014, 356, 3809 CrossRef CAS.
- (a) Y.-F. Qiu, X.-Y. Zhu, Y.-X. Li, Y.-T. He, F. Yang, J. Wang, H.-L. Hua, L. Zheng, L.-C. Wang, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2015, 17, 3694 CrossRef CAS PubMed; (b) D.-P. Jin, P. Gao, D.-Q. Chen, S. Chen, J. Wang, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2016, 18, 3416 Search PubMed.
- (a) F. Yin and X.-S. Wang, Org. Lett., 2014, 16, 1128 CrossRef CAS PubMed; (b) L. Zhu, G. Wang, Q. Guo, Z. Xu, D. Zhang and R. Wang, Org. Lett., 2014, 16, 5390 CrossRef CAS PubMed; (c) K. Zhang, J.-B. Liu and F.-L. Qing, Chem. Commun., 2014, 50, 14157 RSC; (d) C. Li, K. Zhang, X.-H. Xu and F.-L. Qing, Tetrahedron Lett., 2015, 56, 6273 CrossRef CAS; (e) N. Fuentes, W. Kong, L. Fernández-Sánchez, E. Merino and C. Nevado, J. Am. Chem. Soc., 2015, 137, 964 CrossRef CAS PubMed.
- (a) F. Huang, Y. Wang, J. Sun, J. Xie, L. Qi and B. Zhang, RSC Adv., 2016, 6, 52710 RSC; (b) S. Guo, X. Zhang and P. Tang, Angew. Chem., Int. Ed., 2015, 54, 4065 CrossRef CAS PubMed; (c) H. Wu, Z. Xiao, J. Wu, Y. Guo, J. Xiao, C. Liu and Q. Chen, Angew. Chem., Int. Ed., 2015, 54, 4070 CrossRef CAS PubMed.
- (a) C. Rüchardt and R. Hecht, Chem. Ber., 1965, 98, 2471 CrossRef; (b) M. Tada, K. Inoue and M. Okabe, Bull. Chem. Soc. Jpn., 1983, 56, 1420 CrossRef CAS; (c) C. S. A. Antunes, M. Bietti, G. Ercolani, O. Lanzalunga and M. Salamone, J. Org. Chem., 2005, 70, 3884 CrossRef CAS PubMed; (d) H. Egami, R. Shimizu, Y. Usui and M. Sodeoka, Chem. Commun., 2013, 49, 7346 RSC.
- (a) Z. M. Chen, W. Bai, S. H. Wang, B. M. Yang, Y. Q. Tu and F. M. Zhang, Angew. Chem., Int. Ed., 2013, 52, 9781 CrossRef CAS PubMed; (b) H. Egami, R. Shimizu, Y. Usui and M. Sodeoka, Chem. Commun., 2013, 49, 7346 RSC; (c) X. Liu, F. Xiong, X. Huang, L. Xu, P. Li and X. Wu, Angew. Chem., Int. Ed., 2013, 52, 6962 CrossRef CAS PubMed.
- Y. Li, B. Liu, H.-B. Li, Q. Wang and J.-H. Li, Chem. Commun., 2015, 51, 1024 RSC.
- J. Zhao, H. Fang, R. Song, J. Zhou, J. Han and Y. Pan, Chem. Commun., 2015, 51, 599 RSC.
- R.-J. Song, Y.-Q. Tu, D.-Y. Zhu, F.-M. Zhang and S.-H. Wang, Chem. Commun., 2015, 51, 749 RSC.
- X. Q. Chu, Y. Zi, H. Meng, X. P. Xu and S. J. Ji, Chem. Commun., 2014, 50, 7642 RSC.
- (a) Y. Q. Tu, L. D. Sun and P. Z. Wang, J. Org. Chem., 1999, 64, 629 CrossRef CAS; (b) E. Zhang, Y.-Q. Tu, C.-A. Fan, X. Zhao, Y.-J. Jiang and S.-Y. Zhang, Org. Lett., 2008, 10, 4943 CrossRef CAS PubMed; (c) B. Wang and Y. Q. Tu, Acc. Chem. Res., 2011, 44, 1207 CrossRef CAS PubMed; (d) Z.-L. Song, C.-A. Fan and Y.-Q. Tu, Chem. Rev., 2011, 111, 7523 CrossRef CAS PubMed; (e) Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2015, 44, 5220 RSC.
- (a) F. Minisci, A. Citterio and C. Giordano, Acc. Chem. Res., 1983, 16, 27 CrossRef CAS; (b) A. Kumar and P. Neta, J. Am. Chem. Soc., 1980, 102, 7284 CrossRef CAS.
- S. Munavalli, D. I. Rossman, D. K. Rohrbaugh and H. D. Durst, J. Fluorine Chem., 1998, 89, 189 CrossRef CAS.
- (a) K. Liu, S. Chen, X. G. Li and P. N. Liu, J. Org. Chem., 2016, 81, 265 CrossRef CAS PubMed; (b) X. Y. Ji, H. X. Siyang, K. Liu, J. J. Jiao and P. N. Liu, Asian J. Org. Chem., 2016, 5, 240 CrossRef CAS.
- X. Liu, F. Xiong, X. Huang, L. Xu, P. Li and X. Wu, Angew. Chem., Int. Ed., 2013, 52, 6962 CrossRef CAS PubMed.
- (a) G. Teverovskiy, D. S. Surry and S. L. Buchward, Angew. Chem., Int. Ed., 2011, 50, 7312 CrossRef CAS PubMed; (b) C. Chen, L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure and characterization of products (H1, C13 and F19 NMR spectra). See DOI: 10.1039/c6ra25378d |
| This journal is © The Royal Society of Chemistry 2017 |