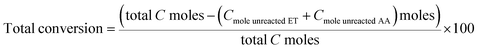Open Access Article
Open Access ArticleEthanol/acetaldehyde conversion into butadiene over sol–gel ZrO2–SiO2 catalysts doped with ZnO
Yuchao Xuab,
Zongzhang Liuab,
Zheng Hanab and
Minhua Zhang*ab
aKey Laboratory for Green Chemical Technology of Ministry of Education, R&D Center for Petrochemical Technology, Tianjin University, Tianjin 300072, P. R. China. E-mail: mhzhang@tju.edu.cn; Fax: +86-22-27401826; Tel: +86-22-27401826
bCollaborative Innovation Center of Chemical Science and Engineering, Tianjin, China
First published on 20th January 2017
Abstract
ZnO promoted ZrO2–SiO2 catalysts synthesized by a sol–gel method were investigated in the two-step ethanol transforming to 1,3-butadiene process. The influence of promoters and the preparation method of the catalysts on the catalytic performance were studied in detail and the reaction conditions were optimized. The as-prepared catalysts were characterized by BET, X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Fourier Transform Infrared Resonance (FT-IR), X-ray photoelectron spectroscopy (XPS), FT-IR spectroscopy of adsorbed pyridine (Py-IR) and temperature-programmed desorption of NH3 (NH3-TPD). ZnO added ZrO2–SiO2 catalysts show the best catalytic activity and the catalysts prepared by the hybrid sol–gel method were superior to those prepared by the sol–gel coupled with impregnation method. The addition of promoters in the ZrO2–SiO2 system decreased the total acidity and lowered the selectivity to dehydration products. The best performance with ethanol/acetaldehyde conversion of 40.7% and 1,3-butadiene selectivity of 83.3% was reached at 310 °C, ethanol/acetaldehyde mole ratio of 3.5 and WHSV of 1.4 h−1 using a 0.5 wt% ZnO doped ZrO2–SiO2 catalyst.
1. Introduction
1,3-Butadiene (BD) is one of the most important bulk chemicals in the oil industry, and has been widely used in various fields of synthesis of rubber elastomers and resins. Generally, the ethanol to BD route is divided into two different parts, the one-step process and the two-step process. The former was first introduced by Lebedev, a USSR chemist, in the 1920s. The latter was a refined process by using an ethanol and acetaldehyde mixture as the feed, and was first commercialized by a US company, the Carbide and Carbon Chemicals Corporation.In recent years, the supply and the price of BD largely depend on the supply of ethylene, one of bulk chemicals in oil industry. With the improvement on the technology of exploiting syngas in America and west Europe, there would be an shortage of the supply of BD in the near future.1 Meanwhile, the technology of bio-ethanol has been improved dramatically and used widely all over the world. The bio-ethanol can be easily obtained not only from crops such as corn and sugarcane but also from inedible raw materials such as fibre and lignin.2 In 2011, the output of ethanol worldwide was over 100 billion litres, which showed a fast growth. The production of high value-added products from bio-ethanol has been attracting interest from all over the world.
Ever since the commercialization of ethanol into BD, researchers have been studying the mechanism of this process for decades. Up to now, the mechanism is still in debate among studies, and a generally accepted reaction network was used in dozens of studies as reported in ref. 3–6. It's also believed that the mechanisms for one-step process and two-step process show no difference.7 The generally accepted mechanism contains: (1) ethanol dehydrogenation into acetaldehyde; (2) aldol condensation of acetaldehyde into acetaldol; (3) dehydration of acetaldol into crotonaldehyde; (4) Meerwein–Ponndorf–Verley reaction of crotonaldehyde by ethanol; and (5) dehydration of crotyl alcohol into BD.
As reported in former literatures,8,9 MgO–SiO2 and ZrO2–SiO2 were regarded as the two most promising catalytic system in ethanol to BD process, each for one-step and two-step, respectively. Dozens of studies were performed on MgO–SiO2 catalytic system.5,10–13 While, only a few studies were conducted using ZrO2–SiO2 or ZrO2–SiO2 based catalysts in ethanol to BD process before 2010. Afterwards, systematic studies have been conducted by Vitaly L. Sushkevich et al. in order to choose the most active component, accommodate acid–base properties, and figure out the function of promoters. Many studies have been conducted on ZrO2–SiO2 or metal oxides promoted ZrO2–SiO2 system in the past five years, as reported in ref. 7, 14 and 15 Sushkevich et al.7 revealed the excellent catalytic capacity of Ag promoted Zr-containing SiO2 molecular sieve (Zr-MCM-41 and Zr-BEA) in the ethanol transforming to BD process. The best performance was obtained with the ethanol conversion of 48% and BD selectivity of 56% using 1 wt% Ag doped Zr-BEA catalyst. Jian et al.15 reported a Zr/MCF catalyst of high performance, which showed the highest 1,3-butadiene yield and selectivity at the WHSV of 3.7 h−1 and 1.5 h−1, respectively. The former studies conducted by other researchers showed a possibility of industrial application of Zr–Si catalytic system in ethanol conversion to BD process.
In our previous work,16 we reported the application of sol–gel ZrO2–SiO2 catalysts and gained a relatively high performance compared with the results in other references. Meanwhile, the selectivity to dehydration products remains high and the catalysts show a relatively poor durability on the long-time stability test. As reported,5,10,17–19 the addition of promoters into MgO–SiO2 system would be beneficial for elevating BD selectivity as well as prolonging catalyst life.
Thus, in this study, metal or metal oxide promoted ZrO2–SiO2 catalysts prepared via sol–gel method were characterized and their catalytic performances for the formation of BD from bioethanol and acetaldehyde were investigated. The effects of addition of promoters and preparation method of catalysts on the structure and acid properties were studied. The mass content of the best promoter was also optimized. The experimental conditions, i.e., the reaction temperature, the mole ratio of ethanol to acetaldehyde and the WHSV were optimized. The long-time durability study was also conducted as to figure out the stability of the catalysts. Different characterization methods were used to explore the relevance of the type of promoters and acid properties to the catalytic performances.
2. Experimental
2.1 Chemicals and materials
Zirconium oxynitrate (AR), acetaldehyde (97%), magnesium nitrate hexahydrate (AR) were obtained from Aladdin Industrial Corporation, Shanghai, China. Tetraethyl orthosilicate (AR), zinc nitrate hexahydrate (AR), manganese nitrate (50% solution, AR), silver nitrate (AR) were purchased from Tianjin Guangfu Chemical Reagent Co., Ltd, Tianjin, China. Nitric acid (AR) was purchased from Beijing Chemical Works. Dry ethanol (AR) and pyridine were purchased from Tianjin Jiangtian Chemical Technology Co., LTD.2.2 Catalyst preparation
All ZrO2/SiO2 catalysts were prepared by using sol–gel method as described in ref. 16. The ZrO2 mass loading for all samples was 2%. Promoter ZnO was added by using two different methods: hybrid sol–gel method (HSG) and sol–gel, and impregnation method (SGI). The catalysts prepared by the former method were referred to as Zr–Si-A and the latter referred to as Zr–Si-B. In general, the HSG catalysts were synthesized through the same process as described in our previous work.16 In particular, the precursor of the dopant, Zn(NO3)2·6(H2O), with a mass content of 0.5%, and ZrO(NO3)2 were added simultaneously into the solvent before gelation process. The SGI catalysts were synthesized by incipient wetness impregnation of ZrO2/SiO2 catalysts with an aqueous solution of zinc nitrate hexahydrate to attain a ZnO mass content of 0.5%. After impregnation process, the catalyst was dried at 383 K for 6 h and calcined at 923 K for 6 h with a heating rate of 5 °C min−1.2.3 Catalyst characterization
N2 adsorption–desorption isotherms (BET) were measured at −196 °C using a Micromeritics Tristar 3000 surface area and pore size analyser. Before measurement, all samples were pretreated at 300 °C for 6 h. The Brunauer–Emmett–Teller (BET) method was used here to calculate the surface areas.Powder X-ray diffraction patterns (XRD) of the catalysts were taken using a Rigaku D/Max 2500 type X-ray powder diffractometer with Cu Kα radiation at a wavelength of 1.5456 Å with the scan range of 5–90° at the scan rate of 5° s−1.
Scanning Electron Microscope (SEM) images were taken using a Hitachi S-4800 with the resolution of 1 nm. All the samples were ground thoroughly and treated with conductive coating before measurement, TEM images were obtained using a Tacnai G2 F20 electron microscope operating at 200 kV. All the samples were suspended in absolute ethanol with an ultrasonic dispersion for 0.5 h. The suspension was doped on a copper grid coated with amorphous carbon film before measurement.
The infrared spectra were obtained using a Nicolet 6700 spectrometer with the scanning range from 4000 to 400 cm−1 and at a resolution of 4 cm−1. All the catalysts were prepared with the addition of KBr at the weight proportion of 99%.
X-ray photoelectron spectroscopy (XPS) spectra were taken on a PerkinElmer PHI-1600 spectrometer using an Mg Kα X-ray radiation source at the pressure of 3.0 × 10−7 Mbar. The collected binding-energy values were referenced to the C 1s line at 284.6 eV.
The acidic properties were studied using FT-IR spectroscopy of adsorbed pyridine. IR spectra were taken on a Nicolet 6700 FT-IR spectrometer with the optical resolution of 4 cm−1. Before measurement, the samples were pretreated at 200 °C under a vacuum of 10−3 Pa for 2 h. Adsorption of pyridine was carried out at 110 °C, difference spectra were obtained by subtraction of the blank spectra of the catalyst samples at 110 °C from the spectra of the samples with adsorbate at correlated temperatures.
The quantity and strength of acidity were determined by NH3-TPD method using a Micromeritic Autochem II 2920. All samples were pre-treated at 500 °C for two hours under a helium (99.999%) flow rate of 50 mL min−1. After being cooled to 70 °C, the sample was saturated with ammonia (argon 99%, ammonia 1%) at a flow rate of 20 mL min−1 for 50 min and subsequently purged with He (30 mL min−1) for 1 h to remove the physically adsorbed NH3. Temperature programming process was then conducted at the range from 70 °C to 400 °C with a temperature ramp rate of 15 °C min−1 in helium at a flow rate of 50 mL min−1. The signal of desorbed NH3 was recorded by a Thermal Conductivity Detector (TCD).
2.4 Catalyst evaluation
The catalytic conversion of ethanol and acetaldehyde mixture into BD was performed on a fixed bed reactor. Before experiment, the samples were ground and sieved into 20–40 mesh. The mole ratio range of ethanol to acetaldehyde was 2.5–4.5. The WHSV varied from 0.2 to 2.2 h−1. The reaction temperature range was 290–340 °C. The dry gas was analysed online by Agilent 7890A gas chromatograph using a 30 m HP-PLOT-Q column. The products contain 1,3-butadiene (BD), ethylene (EL), propylene (PL), butylene (BL), ethyl acetate (EA), and diethyl ether (DE).The conversion of ethanol and acetaldehyde mixture, and selectivity towards the products were calculated as follows:20
3. Results and discussion
3.1 Catalyst characterization
| Line | Sample | SBET (m2 g−1) | d (Å) | V (cm3 g−1) |
|---|---|---|---|---|
| 1 | Zr–Si | 601 | 26.1 | 0.39 |
| 2 | Cu1.0–Zr–Si-A | 544 | 24.3 | 0.33 |
| 3 | Ag1.0–Zr–Si-A | 614 | 23.9 | 0.37 |
| 4 | Mn1.0–Zr–Si-A | 448 | 24.1 | 0.27 |
| 5 | Mg1.0–Zr–Si-A | 561 | 25.0 | 0.34 |
| 6 | Zn1.0–Zr–Si-B | 514 | 23.1 | 0.30 |
| 7 | Zn0.25–Zr–Si-A | 607 | 25.6 | 0.38 |
| 8 | Zn0.5–Zr–Si-A | 594 | 25.5 | 0.36 |
| 9 | Zn1.0–Zr–Si-A | 570 | 25.2 | 0.36 |
| 10 | Zn2.0–Zr–Si-A | 580 | 25.4 | 0.37 |
| 11 | Zn4.0–Zr–Si-A | 568 | 25.1 | 0.36 |
| 12 | Zn8.0–Zr–Si-A | 548 | 24.5 | 0.36 |
XRD was performed in order to study the difference of the structure of two methods, as shown in Fig. 1. No typical peaks of SiO2 or ZrO2 were found for three samples, only a halo at around 2θ = 23.0° was obvious in all patterns, which can be explained as a well dispersed state of ZrO2 on SiO2 surface.21,22 As to ZnO promoted Zr–Si catalysts, a typical halo was also shown at 2θ = 23.0°. At lower ZnO loadings, no typical peaks of ZnO were recorded, which means a well dispersed state of ZnO species on SiO2 surface.23,24 When ZnO loading increased up to 4.0 wt%, some typical peaks at 2θ = 31.767°, 34.421°, 36.252°, 56.592°, 62.856° and 67.945° were observed, which were assigned to (100), (002), (101), (110), (103), (112) crystal face of ZnO (JCPDS 36-1451), respectively.
 | ||
| Fig. 1 XRD patterns for the catalysts (1) catalyst samples prepared by different method (a) Zr–Si; (b) Zn1.0–Zr–Si-A; (c) Zn1.0–Zr–Si-B; (2) catalyst samples with different ZnO contents. | ||
SEM and TEM characterizations were conducted to confirm the information resulted from XRD patterns. Comparing the SEM images in Fig. 2a and b, it's obvious that the particles size in Fig. 2a was smaller and smoother than that in Fig. 2b, which indicated a well-dispersed state of ZrO2 and ZnO on SiO2 through hybrid sol–gel method. TEM images in Fig. 2c and d exhibited a better sight of the morphology of two types of catalysts. In Fig. 2c, no observable ZrO2 or ZnO particles were found at the scale of 200 nm, which elucidated a well dispersed state of amorphous ZrO2 and ZnO on silica support. While in Fig. 2d, the agglomeration of the particles can be seen at the same scale of Fig. 2c. But no crystal ZrO2 or ZnO was found in the map, implying that ZrO2 and ZnO were at amorphous state. The results obtained from SEM and TEM images were in good agreement with that of XRD patterns.
 | ||
| Fig. 2 SEM image (5 μm) of (a) Zn1.0–Zr–Si-A, (b) Zn1.0–Zr–Si-B. TEM image of (c) Zn1.0–Zr–Si-A (50 nm), (d) Zn1.0–Zr–Si-B (200 nm). | ||
In order to verify the interaction between ZnO, ZrO2 and SiO2 phase, FT-IR studies of SiO2, ZrO2/SiO2, Zn1.0–Zr–Si, Zn1.0–Zr–Si-B were performed. In Fig. 3, some typical wavenumbers were shown at 400–2200 cm−1. In particular, wavenumbers at 1082 cm−1 and 1223 cm−1 were assigned to νas(Si–O–Si),25,26 wavenumbers at 804 cm−1 and 463 cm−1 were assigned to νs(Si–O–Si)26,27 and wavenumber at 1631 cm−1 was assigned to physisorbed water on silica phase.28
It has been noted in ref. 29 that typical adsorption of Si–OH at 960 cm−1 can be observable on silica surface at low calcination temperatures, while at high temperatures, it disappears. This can be explained as the dehydration of Si–OH groups forming Si–O–Si moieties. But in the present work, a different condition was observed, even the samples were calcinated at a relatively high temperature (650 °C), and a similar band at 964 cm−1 remained distinct for SiO2 sample (line a in Fig. 3).
For SiO2 supported metal oxides, the vibration peaks at 962–970 cm−1 band is assigned to the metals incorporated into the framework of the mesoporous silica materials.30 When metals are incorporated into silica phase, the intensity of this band increases, which is generally considered as a proof of the incorporation of hetero-atoms into the silica framework. In the present study, the wavenumber, at around 964 cm−1, can be described as the formation of Zr–O–Si structure with the transition of Zr atoms into silica framework.21,31
XPS was conducted in order to study the chemical states of ZnO and ZrO2 on silica surface and the forms it combined between ZnO, ZrO2 and SiO2. In the present work, ZnO was added as the promoter. Meanwhile, as depicted in the XRD patterns, no characteristic peaks of ZnO were detected, which may indicate a strong interaction between ZnO and SiO2. Considering this point of view, XPS profiles of Zn 2p were shown in Fig. 4A. As for Zn1.0–Zr–Si-B, the binding energy of Zn 2p was 1022.7 eV, which means ZnO was more likely presented as bulk ZnO32 (binding energy varies from 1021.8 eV to 1022.5 eV) on SiO2 surface. On the contrary, the binding energy of Zn 2p for Zn1.0–Zr–Si-A sample was significantly higher than that of bulk ZnO. The shift of the binding energy of Zn 2p was reported in ZnO–SiO2 catalysts.23,33 Upon ZnO loading, the binding energy of Zn 2p in ZnO–SiO2 catalysts shifted to lower values compared with that of bulk ZnO, because the valence electron density of Zn in the Si–O–Zn bond is lower than that in the Zn–O–Zn bond.23 Meanwhile, in the present study, this shift towards higher values was due to the strong interaction between ZnO and ZrO2, which may be presented by Zn–O–Zr bonds, rather than the interaction between ZnO and SiO2. The results indicated the existence of a Zn–Zr synergistic interaction in these catalysts.
 | ||
| Fig. 4 (1) XPS profiles of Zn 2p (a) Zn1.0–Zr–Si-B; (b) Zn1.0–Zr–Si-A; (2) XPS profiles of Zr 3d for: (a) Zr–Si; (b) Zn1.0–Zr–Si-B; (c) Zn1.0–Zr–Si-A. | ||
Generally, synergistic effect of the promoter and loading components may affect binding energy in XPS results. As shown in Fig. 4B, the binding energy of Zr 3d for three samples was higher than that of bulk ZrO2. According to Sushkevich, Vitaly L. et al.,7 this phenomenon can be explained by the formation of Zr–O–Si bonds upon ZrO2 loading through sol–gel process, which confirmed the result of FT-IR study. Interestingly, when comparing the result of curve (a) and curve (b) or curve (b) and curve (c), one may find that the binding energy of Zn1.0–Zr–Si-B was similar to that of the blank sample Zr–Si. Meanwhile, binding energy of Zn1.0–Zr–Si-A was much higher than that of the counterparts, which also implied the interaction between ZnO and ZrO2 in sol–gel process. As a result, binding energies of both ZnO and ZrO2 were shifted to higher values.
| Line | Sample | Quantity of acidity (mmol g−1) | Temperature of NH3 desorption peaks (°C) |
|---|---|---|---|
| 1 | Zr–Si | 0.18 | 119 |
| 2 | Mn1.0–Zr–Si-A | 0.13 | 128 |
| 3 | Mg1.0–Zr–Si-A | 0.12 | 136 |
| 4 | Cu1.0–Zr–Si-A | 0.07 | 125 |
| 5 | Ag1.0–Zr–Si-A | 0.05 | 120 |
| 6 | Zn1.0–Zr–Si-B | 0.08 | 127 |
| 7 | Zn0.25–Zr–Si-A | 0.12 | 119 |
| 8 | Zn0.5–Zr–Si-A | 0.08 | 119 |
| 9 | Zn1.0–Zr–Si-A | 0.09 | 119 |
| 10 | Zn2.0–Zr–Si-A | 0.07 | 121 |
| 11 | Zn4.0–Zr–Si-A | 0.06 | 124 |
| 12 | Zn8.0–Zr–Si-A | 0.03 | 120 |
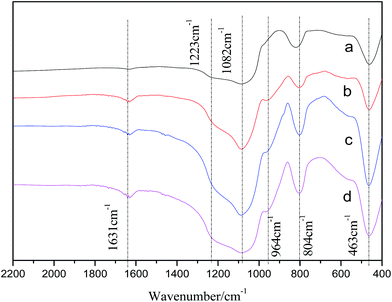
As for the strength of acidity, temperatures vary from 119 °C to 136 °C, Mg1.0–Zr–Si-A shows the highest strength of acidity with a temperature of peak at 136 °C. No change of Zn1.0–Zr–Si-A was found compared with blank catalyst Zr–Si in terms of temperature of peaks. The preparation method of catalyst often shows impact on the acid–base properties of the catalyst, i.e., quantity and strength of acidity. A comparison of quantity and strength of acidity between Zn1.0–Zr–Si-A and Zn1.0–Zr–Si-B (line 9 and line 6, Table 2) was shown. No big difference was recorded on the quantity of acidity, while the temperature of NH3 desorption peaks for Zn1.0–Zr–Si-B sample was higher than that for Zn1.0–Zr–Si-A sample.
Upon increasing ZnO loadings, quantity of acidity decreased lineally from 0.12 mmol g−1 to 0.03 mmol g−1 (line 7–12), which means the majority of acidity was suppressed by ZnO. While as to the strength of acidity, no big changes were found in different ZnO loading samples. However, as we can see, the temperature of NH3 desorption peaks were shifted to higher values, implying a relatively higher strength of acidity resulted from large amount of ZnO (bulk crystal) in the ZrO2–SiO2 system, as revealed in XRD patterns and TEM images.
In conclude, the importance of acid properties has been revealed in former literatures,17,18 but few systematic study has been conducted as to the relevance and weight between the quantity and strength of acidity in ZrO2–SiO2 catalytic system. Combining the results of the NH3-TPD with catalytic performances of catalysts in this work, we predicted boldly that the strength of acidity was more important than quantity of acidity as to improving catalytic performance. A moderate strength of acidity should be essential for high catalytic performance of the catalyst.
In this work, an ordered study was performed by Py-IR to analyze the acid type of acid site of as-prepared catalysts. As reported,35 the adsorption at 1450 cm−1 and 1608 cm−1 were typical bands of pyridine adsorbed on Lewis acid sites, 1490 cm−1 was assigned to pyridine adsorbed on both Lewis and Bronsted acid sites. For all promoter added catalysts, two bands at ca. 1590 cm−1 and 1445 cm−1 were observed, which showed a little gap from the typical bands of Lewis acid sites. Flanigen et al.36 described this shifts to lower wavenumbers as the change of the diameter or positive charge of cations forming acid sites. As can be seen in Fig. 6a, acid types for different promoter added samples were mostly presented as Lewis acid sites. No distinct Bronsted acid, which shown typical adsorption peak at around 1540 cm−1, was observable in the spectra of Zr–Si, Zn1.0–Zr–Si-A, Cu1.0–Zr–Si-A and Ag1.0–Zr–Si-A samples. Meanwhile, the band at 1490 cm−1, was distinguishable in the spectra of Mn and Mg promoted samples. For ZnO promoted samples, as shown in Fig. 6b, the bands at ca. 1445 cm−1 and 1590 cm−1 were evident. No obvious bands of Bronsted acid sites were recorded at low ZnO loadings. Meanwhile, some observable bands at ca. 1490 cm−1, 1565 cm−1 and 1580 cm−1 were found when ZnO loading was higher than 4 wt%, which means new acid sites were formed on bulk crystal ZnO surface,37,38 as analysed by XRD patterns. Interestingly, upon increasing ZnO loadings the band of Lewis acid sites at ca. 1590 cm−1 was shifted toward higher wavenumbers. This was in agreement with the study performed by Baylon et al.35 They have explained this phenomenon as an increase of Lewis acid strength, which was in good agreement NH3-TPD results in the present work.
3.2 Catalyst evaluation in ethanol conversion into butadiene
| Line | Sample | Con (%) | Selectivity (C, mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EL | PL | BL | BD | DE | EA | BU | C6+a | |||
| a Unidentified heavier compounds in GC chromatography. | ||||||||||
| 1 | Zr–Si | 42.2 | 13.5 | 1.5 | 0.8 | 66.0 | 12.6 | 1.6 | 0.8 | 3.0 |
| 2 | Cu1.0–Zr–Si-A | 22.3 | 3.8 | 0 | 20.3 | 38.8 | 7.4 | 6.5 | 3.6 | 19.6 |
| 3 | Ag1.0–Zr–Si-A | 15.9 | 10.2 | 0.9 | 0.6 | 70.0 | 10.9 | 1.6 | 0 | 5.8 |
| 4 | Mn1.0–Zr–Si-A | 27.9 | 5.9 | 0.3 | 0.5 | 76.8 | 9.0 | 1.1 | 0.3 | 6.2 |
| 5 | Mg1.0–Zr–Si-A | 27.0 | 11.1 | 1.5 | 0.7 | 75.3 | 8.1 | 0.8 | 0 | 2.6 |
| 6 | Zn1.0–Zr–Si-A | 33.2 | 6.3 | 1.5 | 0.7 | 83.0 | 3.6 | 1.4 | 0.6 | 3.1 |
| 7 | Zn1.0–Zr–Si-B | 19.1 | 4.8 | 0.7 | 0.7 | 72.2 | 13.3 | 1.8 | 1.1 | 5.5 |
| 8 | Zn0.25–Zr–Si-A | 34.3 | 5.5 | 1.8 | 0.4 | 82.1 | 2.0 | 1.3 | 1.5 | 5.6 |
| 9 | Zn0.5–Zr–Si-A | 36.8 | 5.1 | 1.4 | 0.4 | 83.5 | 3.7 | 1.1 | 0.2 | 4.8 |
| 10 | Zn2.0–Zr–Si-A | 30.4 | 5.7 | 1.2 | 0.6 | 80.9 | 5.0 | 1.9 | 0.8 | 4.3 |
| 11 | Zn4.0–Zr–Si-A | 35.0 | 9.8 | 1.3 | 0.6 | 78.1 | 5.9 | 1.4 | 0.3 | 2.7 |
| 12 | Zn8.0–Zr–Si-A | 36.1 | 11.6 | 1.0 | 0.5 | 74.7 | 6.9 | 1.6 | 0.4 | 3.5 |
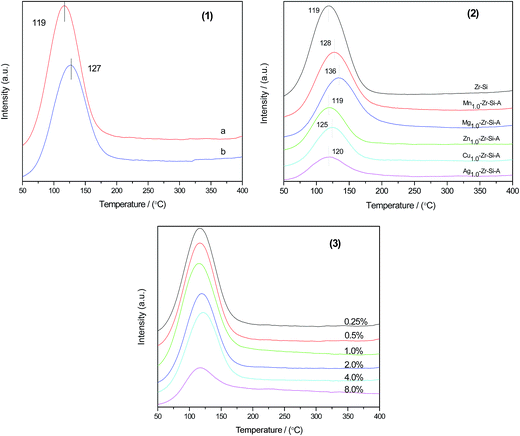
As shown in Table 3, among the testing results for different metal oxide promoted catalysts (line 2–6), the highest BD selectivity, 83.0%, was achieved using Zn1.0–Zr–Si-A catalyst. Moreover, the conversion of Zn1.0–Zr–Si-A was also the best. The addition of ZnO can boost the dehydrogenation ability in ethanol conversion to acetaldehyde process as well as MPV reaction of crotonaldehyde. Combining the analysis results above, the addition of ZnO into Zr–Si catalytic system did decrease the quantity of acidity to some extent, though, no changes were observed as to the acid strength and acid type. It can be inferred that moderate acid type and strength was critical to the process of ethanol conversion to BD. The addition of Mn and Mg into ZrO2–SiO2 system didn't inhibit the formation of dehydration products such as EL and DE effectively (line 4 and 5). Combining the result of NH3-TPD and Py-IR with the catalytic performance of due catalysts, one can predict that the increase of the quantity of acidity and the formation of Bronsted acid sites for Mn and Mg added samples may cause the decrease on the activity. Generally, Cu and Ag were regarded as dehydrogenation promoters, as reported in ref. 17, 19 and 39 which in turn, boosted BD selectivity. However, in this study, Cu and Ag showed inferior catalytic performances to that of other promoters, the BD selectivity was even lower than that of blank ZrO2–SiO2. The addition of Cu introduces redox-active sites which can boost dehydrogenation process of ethanol into acetaldehyde and inhibit the dehydration of ethanol. By the meantime, CuO can also poison acid site of the catalyst, which lowers the ethanol/acetaldehyde conversion and the selectivity to dehydration products (as shown in line 2).19 The dehydrogenation ability of Ag was inferior to that of CuO, and more dehydration products (EL and DE) were formed at the expense of lower BD selectivity. Therefore, as analysed above, ZnO was chosen as the best promoter in this study.
As known to all, the method of promoter addition also had a great impact on the conversion of ethanol and acetaldehyde and BD selectivity. Obviously, the conversion of ZnO added ZrO2–SiO2 fresh sample through SGI process (line 7) dropped dramatically compared with ZrO2–SiO2 counterpart (line 1). This can be explained by the formation of bulk crystal of ZnO in SGI samples, which caused the blockage of pores, as analysed by BET, XRD, TEM and XPS. Whereas highly dispersed ZnO species obtained by HSG method on ZrO2–SiO2 surface can effectively decrease the quantity of acidity of Zr–Si without much difference on the strength of acid sites, which hindered the dehydration property of Zr–Si catalyst and boosted BD selectivity by the meantime (line 6).
It's also eye-catching that upon different promoters loading on Zr–Si system, the conversion of promoted samples dropped to some extent compared with that of Zr–Si counterpart. This can be explained as the decrease of acidity of those promoted samples. A relatively low acidity of the catalyst means some amount of active sites of Zr–Si system was reduced or covered by those metal oxides.
The catalytic data with different ZnO loading was shown in Table 3 (line 2, 3–8). When ZnO loading increased from 0.25 wt% to 8.0 wt%, no big change on the ethanol and acetaldehyde mixed conversion was observed, with a conversion range from 30% to 37%. Meanwhile, selectivity to dehydration products (EL and DE) increased at the expense of lower BD selectivity. Notably, the maximum of BD selectivity, 83.5%, was reached at the ZnO loading of 0.5 wt%.
4. Conclusions
A series of metal oxide doped ZrO2–SiO2 catalysts were prepared by sol–gel method and used in the conversion of ethanol and acetaldehyde into 1,3-butadiene process. The catalysts prepared by hybrid sol–gel method showed better catalytic performances compared with those prepared by sol–gel coupled with impregnation method. ZnO was chosen as the best promoter for ZrO2–SiO2 catalytic system and Zn0.5–Zr–Si-A catalyst showed the highest performance with 36.8% ethanol/acetaldehyde conversion and 83.5% BD selectivity. The reaction conditions were also optimized with 320 °C, 3.5 and 1.8 h−1 as the optimum reaction temperature, ethanol/acetaldehyde and WHSV, respectively. Acid studies showed the addition of ZnO into ZrO2–SiO2 system can decrease quantity of acidity yet didn't change the strength of acidity of the catalysts, which in turn lowered the selectivity to dehydration products and boosted BD selectivity simultaneously.Acknowledgements
The authors thank the Key Laboratory for Green Chemical Technology of Ministry of Education of Tianjin University for technical support and the large precision instrument platform for XRD, XPS, SEM and HR-TEM measurements.Notes and references
- G. O. Ezinkwo, T. V. Philippovich, A. Auwal and I. A. Mamadshoevich, ChemBioEng Rev., 2014, 1, 194–203 CrossRef CAS.
- C. A. Cardona and Ó. J. Sánchez, Bioresour. Technol., 2007, 98, 2415–2457 CrossRef CAS PubMed.
- T. De Baerdemaeker, M. Feyen, U. Müller, B. Yilmaz, F.-S. Xiao, W. Zhang, T. Yokoi, X. Bao, H. Gies and D. E. De Vos, ACS Catal., 2015, 5, 3393–3397 CrossRef CAS.
- S. Bhattacharyya and S. Sanyal, J. Catal., 1967, 7, 152–158 CrossRef CAS.
- M. Lewandowski, G. S. Babu, M. Vezzoli, M. D. Jones, R. E. Owen, D. Mattia, P. Plucinski, E. Mikolajska, A. Ochenduszko and D. C. Apperley, Catal. Commun., 2014, 49, 25–28 CrossRef CAS.
- Y. Wang and S. Liu, J. Bioprocess Eng. Biorefinery, 2012, 1, 33–43 CrossRef.
- V. L. Sushkevich, I. I. Ivanova and E. Taarning, Green Chem., 2015, 17, 2552–2559 RSC.
- B. Corson, H. Jones, C. Welling, J. Hinckley and E. Stahly, Ind. Eng. Chem., 1950, 42, 359–373 CrossRef CAS.
- S. Bhattacharyya and N. Ganguly, J. Appl. Chem., 1962, 12, 105–110 CrossRef CAS.
- Y. Sekiguchi, S. Akiyama, W. Urakawa, T.-r. Koyama, A. Miyaji, K. Motokura and T. Baba, Catal. Commun., 2015, 68, 20–24 CrossRef CAS.
- S. Kvisle, A. Aguero and R. P. A. Sneeden, Appl. Catal., 1988, 43, 117–131 CrossRef CAS.
- H. Niiyama, S. Morii and E. Echigoya, Bull. Chem. Soc. Jpn., 1972, 45, 655–659 CrossRef CAS.
- R. Ohnishi, T. Akimoto and K. Tanabe, J. Chem. Soc., Chem. Commun., 1985, 22, 1613–1614 RSC.
- X. G. Jun-Kun Lai, A. Chakrabarti, J. Baltrusaitis and I. E. Wachs, Catalytic Activity Testing from Ethanol to 1,3-butadiene on ZnO/ZrO2/SiO2, Salt Lake City, UT, 2015 Search PubMed.
- L. C. Jian, Y. Shao, S. J. R. Tan, X. Li, Y. Zhang and S. L. Su, ACS Sustainable Chem. Eng., 2016, 4, 4887–4894 CrossRef.
- Z. Han, X. Li, M. Zhang, Z. Liu and M. Gao, RSC Adv., 2015, 5, 103982–103988 RSC.
- W. Janssens, E. V. Makshina, P. Vanelderen, F. De Clippel, K. Houthoofd, S. Kerkhofs, J. A. Martens, P. A. Jacobs and B. F. Sels, ChemSusChem, 2015, 8, 994–1008 CrossRef CAS PubMed.
- O. V. Larina, P. I. Kyriienko and S. O. Soloviev, Catal Lett., 2015, 145, 1162–1168 CrossRef CAS.
- C. Angelici, M. E. Z. Velthoen, B. M. Weckhuysen and P. C. A. Bruijnincx, ChemSusChem, 2014, 7, 2505–2515 CrossRef CAS PubMed.
- H.-J. Chae, T.-W. Kim, Y.-K. Moon, H.-K. Kim, K.-E. Jeong, C.-U. Kim and S.-Y. Jeong, Appl. Catal., B, 2014, 150, 596–604 CrossRef.
- Y. Zhang, L. Pan, C. Gao and Y. Zhao, J. Sol-Gel Sci. Technol., 2011, 58, 572–579 CrossRef CAS.
- R. G. R. Avendaño, J. A. D. L. Reyes, J. A. Montoya and T. Viveros, J. Sol-Gel Sci. Technol., 2005, 33, 133–138 CrossRef.
- Q. Jiang, Z. Y. Wu, Y. M. Wang, Y. Cao, C. F. Zhou and J. H. Zhu, J. Mater. Chem., 2006, 16, 1536–1542 RSC.
- Q. Yuan, N. Li, J. Tu, X. Li, R. Wang, T. Zhang and C. Shao, Sens. Actuators, B, 2010, 149, 413–419 CrossRef CAS.
- S. K. Saha and P. Pramanik, J. Non-Cryst. Solids, 1993, 159, 31–37 CrossRef CAS.
- T. Lopez, J. Navarrete, R. Gomez, O. Novaro, F. Figueras and H. Armendariz, Appl. Catal., A, 1995, 125, 217–232 CrossRef CAS.
- D. M. Francisco, L. Willa and J. D. Mackenzie, J. Am. Ceram. Soc., 2000, 83, 1506–1512 Search PubMed.
- J. S. Loring, C. J. Thompson, Z. Wang, A. G. Joly, D. S. Sklarew, H. T. Schaef, E. S. Ilton, K. M. Rosso and A. R. Felmy, Environ. Sci. Technol., 2011, 45, 6204–6210 CrossRef CAS PubMed.
- H. S. Chen, Z. Y. Sun and J. C. Shao, Bull. Chin. Ceram. Soc., 2011, 30, 934–937 CAS.
- K. M. Parida and S. S. Dash, J. Mol. Catal. A: Chem., 2009, 306, 54–61 CrossRef CAS.
- B. Hua, G. Qian, M. Wang and K. Hirao, J. Sol-Gel Sci. Technol., 2005, 33, 169–173 CrossRef CAS.
- M. Chen, X. Wang, Y. H. Yu, Z. L. Pei, X. D. Bai, C. Sun, R. F. Huang and L. S. Wen, Appl. Surf. Sci., 2000, 158, 134–140 CrossRef CAS.
- Z. Fu, B. Yang, L. Li, W. Dong, C. Jia and W. Wu, J. Phys.: Condens. Matter, 2003, 15, 2867–2873 CrossRef CAS.
- J. Gao, J. Guo, D. Liang, Z. Hou, J. Fei and X. Zheng, Int. J. Hydrogen Energy, 2008, 33, 5493–5500 CrossRef CAS.
- R. A. Baylon, J. Sun and Y. Wang, Catal. Today, 2016, 259, 446–452 CrossRef CAS.
- E. M. Flanigen, in Zeolite Chemistry and Catalysis, ed. J. A. Rabo, ACS Monograph 171, American Chemical Society, Washington, DC, 1976, vol. 80 Search PubMed.
- A. Zecchina, C. Lamberti and S. Bordiga, Catal. Today, 1998, 41, 169–177 CrossRef CAS.
- T. H. Mahato, G. K. Prasad, B. Singh, J. Acharya, A. R. Srivastava and R. Vijayaraghavan, J. Hazard. Mater., 2009, 165, 928–932 CrossRef CAS PubMed.
- C. Angelici, B. M. Weckhuysen and P. C. A. Bruijnincx, ChemSusChem, 2013, 6, 1595–1614 CrossRef CAS PubMed.
- S. Freni, N. Mondello, S. Cavallaro, G. Cacciola, V. N. Parmon and V. A. Sobyanin, React. Kinet. Catal. Lett., 2000, 71, 143–152 CrossRef CAS.
- Y. Chen, Y. Wu, L. Tao, B. Dai, M. Yang, Z. Chen and X. Zhu, J. Ind. Eng. Chem., 2010, 16, 717–722 CrossRef CAS.
- S. H. Chai, H. P. Wang, Y. Liang and B. Q. Xu, Green Chem., 2007, 9, 1130–1136 RSC.
- M. D. Jones, C. G. Keir, C. D. Iulio, R. A. M. Robertson, C. V. Williams and D. C. Apperley, Catal. Sci. Technol., 2011, 1, 267–272 CAS.
- V. Ordomsky, V. Sushkevich and I. Ivanova, J. Mol. Catal. A: Chem., 2010, 333, 85–93 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |