Highly efficient one-pot multi-component synthesis of α-aminophosphonates and bis-α-aminophosphonates catalyzed by heterogeneous reusable silica supported dodecatungstophosphoric acid (DTP/SiO2) at ambient temperature and their antitubercular evaluation against Mycobactrium Tuberculosis†
Shafeek A. R. Mulla*a,
Mohsinkhan Y. Pathana,
Santosh S. Chavana,
Suwara P. Gampleb and
Dhiman Sarkarb
aChemical Engineering & Process Development Division, National Chemical Laboratory, Dr Homi Bhabha Road, Pune-411008, Maharashtra, India. E-mail: sa.mulla@ncl.res.in; Fax: +91-20-25902676; Tel: +91-20-25902316
bCombi. Chem. Bio. Resource Center, Organic Chemistry Division, National Chemical Laboratory, Pashan Road, Pune 411 008, India
First published on 26th November 2013
Abstract
A highly efficient one-pot multi-component reaction (MCR) protocol over DTP/SiO2 has been developed for the synthesis of α-aminophosphonate derivatives (4a–x) in excellent yields. The α-aminophosphonate derivatives were for the first time evaluated for their antitubercular activity against the M. tuberculosis H37Ra (MTB) strain. An evaluation of the data on the cytotoxicity and antimicrobial activity shows that 4n and 4v are promising antitubercular agents.
Introduction
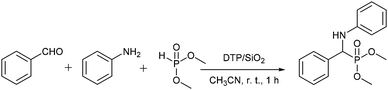
The synthesis of α-aminophosphonates has become the center of interest among world researchers due to their wide spectrum of biological and/or medicinal activities and structural analogy to natural α-amino acids and α-aminophosphoric acids. They have achieved a significant importance as a key moieties having wide applications not only in agriculture but also in biological/medicinal chemistry, as anti-cancer agents,1a–c inhibitors of synthase,1d HIV protease,1e antibiotics,1f enzyme inhibitors,1g anti-thrombotic agents,1h cytotoxicity,1c antibacterial activity,1i antifungal activity,1j antiproliferative activity,1j inhibitors of protein tyrosine phosphatases,1k herbicides, fungicides,1l insecticides,1m plant growth regulators,1n and as substrates in the synthesis of phosphonopeptides. Owing to the potential importance of α-aminophosphonate derivatives as a key moieties in life sciences, pharmaceuticals, agriculture, world-wide efforts have made in the last few decades by researchers and various protocols have been developed for their synthesis using various catalysts such as SnCl4,2 SnCl2,3 ZnCl2,4 BF3·OEt2,5 InCl3,6 Mg(ClO4)2,7 M(OTf)3,8 AlCl3,9 CF3CO2H,10 Montmorillonite Clay-MW,11 TiO2,12 scandium (tris-dodecyl sulfate),13 I2,14 Nano Fe3O4,15 EAN,16 H-beta zeolite,17 Nano ZnO,18 AIKIT-5,19 ZrOCl2·8H2O,20 SbCl3/Al2O3,21 and Amberlyst-15,22 which have been reported in the literature. The acid2–10,20 catalyzed synthesis of α-aminophosphonates by the nucleophilic addition reaction of phosphite to amines is convenient and the most preferred method, however, its practical approach is limited since the water formed during the course of the reaction either deactivates or decomposes the acid catalyst. Even though significant improvements and/or developments using either homogeneous or heterogeneous catalysts have been achieved, almost all of the methods reported so far lack general applicability and commercial scale implementation, and suffer from one or more limitations such as the use of excess or stoichiometric quantity, moisture sensitive, toxic, corrosive, expensive catalysts, which are non-recoverable and/or recoverable with tedious separation procedures involving lots of toxic waste generation besides a long reaction time, high temperature and low yield for the desired product.As α-aminophosphonate derivatives are the key constituent and structural backbone of many pharmaceutical and agricultural compounds, the development of more general and cost effective, one-pot multi-component protocols for their synthesis under much milder, more efficient, environment friendly conditions using recyclable, eco friendly catalysts is still a possibility to explore. As part of our continuous efforts to develop a green, ecofriendly, general and cost effective protocol for the organic transformation using DTP/SiO223a and recyclable catalysts,23b–f we report herein a highly efficient, cost effective, general, and much milder one-pot multi-component protocol for the synthesis of α-aminophosphonate and bis-α-aminophosphonate derivatives in excellent yields via a one-pot three component condensation of various aldehydes, amines and di or tri alkyl phosphites using a heterogeneous reusable silica supported dodecatungstophosphoric acid catalyst at ambient temperature in a short reaction time (Scheme 1).
 | ||
| Scheme 1 The synthesis of α-aminophosphonate derivatives via a one-pot three-component condensation reaction. | ||
TB is a chronic infectious disease24 and a serious threat to the public health worldwide. The world health organization (WHO) declared25 TB as an international public health crisis and appealed to develop the anti-TB drug or vaccine, which could be licensed by 2020. Even though α-aminophosphonate derivatives, which are constituents of various potent drugs,1a–k and their bioevaluation for anticancer activity are well reported,1a–c surprisingly its antitubercular (TB) activity has not been reported so far. Therefore, herein we report for the first time the preliminary results on the antitubercular activity of α-aminophosphonate derivatives against the Mycobactrium Tuberculosis H37Ra (MTB) strain.
To develop a one-pot multi-component protocol for synthesis of α-aminophosphonate derivatives, benzaldehyde (10 mmol), aniline (10 mmol), and dimethylphosphite (10 mmol), catalyzed by 50 mg (0.35 mol%) 20% DTP/SiO2 catalyst in a 5 ml solvent at ambient temperature for 1 h was selected as a model reaction to optimize the reaction conditions. Initially, the screening of different solvents such as methanol, ethanol, dichloromethane, acetonitrile, dimethylformamide and water were performed. However, the acetonitrile solvent gives the desired α-aminophosphonate product in a 98% yield (Table 1, entry 4) whereas methanol, ethanol and dichloromethane give 74%, 78%, and 40% yields, respectively (Table 1, entries 1–3). The formation of the desired product was not observed using dimethylformamide or water as the solvent (Table 1, entry 5, 6) and also in the absence of catalyst in an acetonitrile solvent (Table 1, entry 7). The promising results using acetonitrile as a solvent over a DTP/SiO2 catalyst allowed us to further optimize the DTP loading and catalyst loading, and the results in Table 1 (entry 8–11) reveal that the catalyst with 20% DTP loading and 50 mg catalyst shows excellent catalytic activity (Table 1, entry 4).
| Entry | Solvent | Catalyst | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: benzaldehyde (10 mmol), aniline (10 mmol), dimethylphosphite (10 mmol), catalyst 20% DTP/SiO2: 25–100 mg (0.18–0.7 mol% of DTP) in 5 ml solvent, room temperature 1 h.b Isolated yields after column chromatography.c 50 mg = 0.17 mol%.d 50 mg = 0.52 mol%. | |||
| 1 | Methanol | DTP/SiO2 | 74 |
| 2 | Ethanol | DTP/SiO2 | 78 |
| 3 | Dichloromethane | DTP/SiO2 | 40 |
| 4 | Acetonitrile | DTP/SiO2 | 98 |
| 5 | Dimethylformamide | DTP/SiO2 | N.R. |
| 6 | Water | DTP/SiO2 | N.R. |
| 7 | Acetonitrile | — | N.R. |
| 8 | Acetonitrile | DTP/SiO2 (25 mg) | 84 |
| 9 | Acetonitrile | DTP/SiO2 (100 mg) | 98 |
| 10c | Acetonitrile | 10% DTP/SiO2 | 76 |
| 11d | Acetonitrile | 30% DTP/SiO2 | 98 |
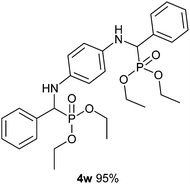
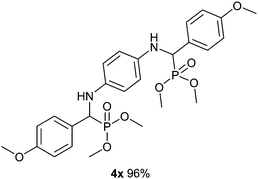
The excellent yield, using 20% DTP/SiO2 in an acetonitrile solvent, motivated us to investigate the scope of the one-pot multi-component protocol for the synthesis of the α-aminophosphonate and bis-α-aminophosphonate derivatives from various substituted aldehydes, amines and di or tri alkyl phosphites in the presence of a DTP/SiO2 catalyst at optimized reaction conditions. To establish the general applicability, a variety of substituted aldehydes, substituted amines and di/tri alkyl phosphites were subjected for a three-component condensation (Kabachnik-Fields) reaction. Interestingly, a wide range of aryl/heteroaromatics aldehydes. and amines possessing various electron donating and electron withdrawing functional groups reacted smoothly with di or tri alkyl phosphites over a DTP/SiO2 catalyst at ambient temperature for 1 h to give the desired products in excellent yields (Table 2, entries 4a–x). The aromatic/heteroaromatics aldehydes such as benzaldehyde, 4-methoxy benzaldehyde, 4-chloro benzaldehyde, 4-methyl benzaldehyde, 2,5-dimethoxy benzaldehyde and furfural reacted well with aniline/3-chloroaniline/2,4,6-trimethylaniline/1-naphthylamine/4-nitroaniline/4-methoxyaniline and dimethylphosphite to produce the corresponding α-aminophosphate in excellent yields (Table 2, entries 4a–o). To further elaborate the scope of a one-pot multi-component protocol over a DTP/SiO2 catalyst, the substituted aromatic amines such as aniline, 3-chroloaniline and 1-naphthylamine reacted smoothly with benzaldehyde/3,4,5-trimethoxybenzaldehyde/4-chloro benzaldehyde/4-methylbenzaldehyde/4-methoxy benzaldehyde, and dibenzylphosphite/triethylphosphite to obtain the corresponding α-aminophosphonate in an excellent yield (Table 2, entries 4p–u). The excellent performance of the DTP/SiO2 catalyst for the synthesis of α-aminophosphonate derivatives from the various combinations of aryl/heteroaromatics aldehydes/substituted aldehydes, amines/substituted amines and di or tri alkyl phosphites made us excited to investigate the dimer formation of α-aminophosphonates. Amazingly, benzaldehyde/4-methoxyaldehyde (20 mmol) reacted very well with dimethyl phosphite/triethylphosphite (20 mmol) and 4-amino aniline (10 mmol) to provide the corresponding bis-α-aminophosphonate as a dimer in a high yield (Table 2 entries 4v–x). The results in Table 2 clearly reveal that the one-pot three-component condensation reactions over the DTP/SiO2 catalyst show a remarkable and excellent performance irrespective to the presence of an electron donating/electron withdrawing groups on the aromatic/heterocyclic aldehydes and/or amines and hence the one-pot three-component protocol is highly effective, promising, and general for the synthesis of α-aminophosphonate and bis-α-aminophosphonate derivatives.
| a Reaction conditions: aldehyde (10 mmol), amine (10 mmol), phosphite (10 mmol), DTP/SiO2 (50 mg) in 5 ml CH3CN, room temperature 1 h.b Isolated yields after column chromatography.c IC90 for stander drugs.d IC50 against M. tuberculosis H37Ra for antitubercular activity.e IC50 against THP 1 cell line for cytotoxicity. | |||
|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
As per earlier literature,5,23a–c a probable mechanism is shown in Scheme 2 for the synthesis of α-aminophosphonate. The mechanism involves the activation of the carbonyl group of aldehyde by DTP/SiO2 (I) followed by the nucleophilic addition of amine to afford the imine (II) by the removal of water. The subsequent activation of imine (II) by DTP/SiO2 facilitated the addition of phosphite to give an activated phosphonium intermediate (III), which then gave the desired product IV (Scheme 2).
The recyclability and recovery of the DTP/SiO2 catalyst was investigated for the synthesis of α-aminophosphonates by a one-pot three-component condensation of benzaldehyde and aniline with dimethyl phosphite as a model substrate in an acetonitrile solvent at room temperature for 1 h, and the results are provided in Table 3. The DTP/SiO2 catalyst was recovered quantitatively from reaction mixture by filtration and reused several times without the loss of catalytic activity (Table 3, entries 2–6). The isolated yield obtained for the product at the end of the 5th recycle (Table 3, entry 6) is very much consistent with the fresh DTP/SiO2 catalyst (Table 3, entry 1). The consistent catalytic activity of the recovered and reused DTP/SiO2 catalyst indicates that the reused catalyst shows an excellent performance for the synthesis of α-aminophosphonates.
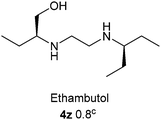
All of the synthesized α-aminophosphonate and bis-α-aminophosphonate derivatives (4a–x) were screened using 100 μg ml−1 concentrations for their in vitro antitubercular activity against the M. tuberculosis H37Ra (ATCC 25177) strain by XTT reduction menadione assay. As shown in Fig. 1, the 4e, 4f, 4g, 4i, 4j, 4n, 4o and 4v α-aminophosphonate derivatives exhibited inhibition. However, only the 4n and 4v derivatives exhibited more than 90% inhibition, and they were further screened using various concentrations for their in vitro antitubercular activity to achieve IC50 which is compared with standard drugs such as isoniazid, ethambutol and pyrazinamide (Table 2, entry 4y–aa).
 | ||
| Fig. 1 The analysis of the antitubercular activity of compounds using an XTT Reduction menadione assay. 100 μg ml−1 of the compounds were added to 2 M tuberculosis culture at 0 days after inoculation. The cell growth was estimated by monitoring the extent of the XTT reduction after 8 days of incubation with respect to the DMSO vehicle control and media as a blank. The percent inhibition of the compounds is as shown in a graph. Further details are provided in the ESI.† The results are the average of three identical experiments ± the standard deviation. | ||
The 4n and 4v α-aminophosphonate derivatives exhibited half maximal concentration (IC50) values of 8.62 and 7.51 μg ml−1 (Table 2 entry 4n and 4v), respectively, which indicate that the compounds are promising antitubercular agents. These findings inspired us to evaluate their cytotoxicity. Hence, all of the α-aminophosphonate derivatives (4a–x) were evaluated for their cytotoxicity using a THP 1 (Human acute monocytic leukemia cell line) in vitro MTT assay. Surprisingly, the 4n and 4v compounds were found to be non active towards cytotoxicity. However, a few compounds, 4b, 4c, 4p, 4q, 4r and 4s (Table 2), showed a good cytotoxicity.
The selectiveness of the 4n and 4v compounds towards antitubercular activity made us enthusiastic to evaluate these compounds for their antimicrobial activity. 4n and 4v were evaluated for their antimicrobial activity against gram-negative (Escherichia coli) and gram-positive (Staphylococcus aureus and Bacillus) bacteria (Table 4). Miraculously, 4n and 4v show no antibacterial activity towards gram-negative and gram-positive bacteria. The evaluation data on cytotoxicity using the THP 1 (Human acute monocytic leukemia) cell line (Table 2), antimicrobial activity against gram-positive and gram-negative bacteria (Table 4) clearly shows that 4n and 4v are highly selective towards antitubercular activity against the M. tuberculosis H37Ra (MTB) strain and were found to be promising antitubercular agents for further drug discoveries.
| Compounds | Concentration (μg ml−1) | % Inhibition | ||
|---|---|---|---|---|
| E. coli | S. aureus | Bacillus | ||
| 4n | 100 | 0.8 | 35 | 87.3 |
| 4v | 100 | 10.1 | −10.3 | 2.5 |
Conclusions
In conclusion, a novel, environment friendly, highly efficient, cost effective, one-pot multi-component protocol has been developed for the efficient synthesis of α-aminophosphonate derivatives in excellent yields via a one-pot three-component condensation of various substituted aldehydes, substituted amines and di or tri alkyl phosphites using an ecofriendly, heterogeneous, reusable silica supported dodecatungstophosphoric acid (DTP/SiO2) catalyst at ambient temperature in a short reaction time. The one-pot multi-component condensation reactions (MCR) over the DTP/SiO2 catalyst show a remarkable and excellent performance irrespective of the presence of electron donating/electron withdrawing groups on the aromatic/heterocyclic aldehydes and/or amines and hence the one-pot three-component protocol is highly effective, promising and general for the synthesis of α-aminophosphonate and bis-α-aminophosphonate derivatives.The catalyst was recycled several times without the loss of catalytic activity. These α-aminophosphonate derivatives were evaluated for the first time for the antitubercular activity against the M. tuberculosis H37Ra (MTB) strain by using an XTT reduction menadione assay (XRMA) protocol. However, the 4n and 4v α-aminophosphonate derivatives exhibited half maximal concentration (IC50) values of 8.62 and 7.51 μg ml−1 (Table 2 entry 4n and 4v), respectively. An evaluation of the data on the cytotoxicity and antimicrobial activity shows that 4n and 4v are highly selective towards antitubercular activity against the M. tuberculosis H37Ra (MTB) strain and were found to be promising antitubercular agents for further drug discoveries.
Experimental section
All chemicals and reagents were procured from Sigma Aldrich, S.D. Fine chemical and commercial suppliers and used without further purification. The products were characterized using 1H NMR, 13C NMR spectra. The NMR spectra of the product were obtained using a Bruker AC-200 MHz spectrometer with TMS as the internal standard. Column chromatography was performed on silica gel, Merck grade 60–120 mesh size. TLC was performed on 0.25 mm E. Merck precoated silica gel plates (60 F254).General experimental procedure for the 20% DTP/SiO2 mediated synthesis of α-aminophosphonates
The reaction mixture of aldehyde (10 mmol), amine (10 mmol) and di/tri alkyl phosphite (10 mmol) was stirred in a 10 ml round bottom flask containing 5 ml acetonitrile solvent in the presence of 50 mg DTP/SiO2 catalyst at room temperature for 1 h. The completion of the reaction was monitored by TLC. After the completion of the reaction, the reaction mixture was diluted with ethyl acetate (10 ml) and the catalyst was recovered by filtration. The filtrate was washed with aqueous NaHCO3 and then with water followed by the separation of an aqueous layer and organic layer. The organic layer is dried over anhydrous Na2SO4 and concentrated in a vacuum to give the crude product. The crude product was purified by silica gel column chromatography using a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio of pet ether
30 ratio of pet ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate to afford the pure α-amino phosphonate. The products obtained were characterized by NMR.
ethyl acetate to afford the pure α-amino phosphonate. The products obtained were characterized by NMR.
Acknowledgements
MYP and SSC are thankful to CSIR New Delhi for a SRF. The authors also thank Dr V. V. Ranade, Chair of CE-PD division for helpful discussion, encouragement and support.Notes and references
- (a) P. Kafarski and B. Lejczak, Curr. Med. Chem.: Anti-Cancer Agents, 2001, 1, 301–312 CrossRef CAS; (b) A. K. Bhattacharya, D. S. Raut, K. C. Rana, I. K. Polankia, M. S. Khan and S. Iramb, Eur. J. Med. Chem., 2013, 66, 146–152 CrossRef CAS PubMed; (c) Z. Rezaei, H. Firouzabadi, N. Iranpoor, A. Ghaderi, M. R. Jafari, A. A. Jafari and H. R. Zare, Eur. J. Med. Chem., 2009, 44, 4266–4275 CrossRef CAS PubMed; (d) J. A. Sikosrki, M. J. Miller and D. S. Braccolino, Phosphorus, Sulfur Silicon Relat. Elem., 1993, 76, 375–378 Search PubMed; (e) B. Stowasser, K. H. Budt, A. Peyman and D. Ruppert, Tetrahedron Lett., 1992, 33, 6625–6628 CrossRef CAS; (f) F. R. Atherton, C. H. Hassall and R. W. Lambert, J. Med. Chem., 1986, 29, 29–40 CrossRef CAS; (g) A. Peyman, K. H. Budt, J. S. Paning, B. Stowasser and D. Ruppert, Tetrahedron Lett., 1992, 33, 4549–4552 CrossRef CAS; (h) J. H. Meyer and P. A. Barlett, J. Am. Chem. Soc., 1998, 120, 4600–4609 CrossRef CAS; (i) S. N. Ali, S. Zakir, M. Patel and M. Farooqui, Eur. J. Med. Chem., 2012, 50, 39–43 CrossRef PubMed; (j) Z. Rezaei, S. Khabnadideh, K. Zomorodian, K. Pakshir, S. Nadali, N. Mohtashami and E. FaghihMirzaei, Int. J. Med. Chem., 2011, 11 Search PubMed; (k) Q. Wang, M. Zhu, R. Zhu, L. Lu, C. Yuan, S. Xing, Y. Mei and Q. Hang, Eur. J. Med. Chem., 2012, 49, 354–364 CrossRef CAS PubMed; (l) L. Maier, Phosphorus, Sulfur Silicon Relat. Elem., 1990, 53, 43–67 CrossRef CAS; (m) L. Maier and H. Spoerri, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 61, 69–75 CrossRef CAS; (n) J. Emsley and D. Hall, Chemistry of Phosphorus, Harper and Row, London, 1976, p. 494 Search PubMed.
- S. Laschat and H. Kunz, Synthesis, 1992, 90–95 CrossRef CAS.
- R. Gallardo-Macias and K. Nakayama, Synthesis, 2010, 57–62 CAS.
- S. Sobhani and A. Vafaee, Synthesis, 2009, 1909–1915 CrossRef CAS PubMed.
- J. Zon, Pol. J. Chem., 1981, 55, 643–646 CAS.
- B. C. Ranu, A. Hajra and U. Jana, Org. Lett., 1999, 1, 1141–1143 CrossRef CAS.
- S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2007, 72, 1263–1270 CrossRef CAS PubMed.
- H. Firouzabadi, N. Iranpoor and S. Sobhani, Synthesis, 2004, 2692–2696 CrossRef CAS PubMed.
- A. Manjula, B. V. Rao and P. Neelakantan, Synth. Commun., 2003, 33, 2963–2969 CrossRef CAS PubMed.
- T. Akiyama, M. Sanada and K. Fuchide, Synlett, 2003, 1463–1464 CrossRef CAS.
- J. S. Yadav, B. V. Subba Reddy and C. Madan, Synlett, 2001, 1131–1133 CrossRef CAS PubMed.
- M. Hosseini-Sarvari, Tetrahedron, 2008, 64, 5459–5466 CrossRef CAS PubMed.
- K. Manabe and S. Kobayashi, Chem. Commun., 2000, 669–670 RSC.
- S. Sobhani and A. Vafaee, J. Iran. Chem. Soc., 2010, 7, 227–236 CrossRef CAS.
- B. V. Subba Reddy, A. S. Krishna and A. V. Ganesh, Tetrahedron Lett., 2011, 52, 1359–1362 CrossRef PubMed.
- S. A. Dake, D. S. Raut, K. R. Kharat, R. S. Mhaske, S. U. Deshmukh and R. P. Pawar, Bioorg. Med. Chem. Lett., 2011, 21, 2527–2532 CrossRef CAS PubMed.
- V. H. Tillu, D. K. Dumbre, R. D. Wakharkar and V. R. Choudhary, Tetrahedron Lett., 2011, 52, 863–866 CrossRef CAS PubMed.
- M. Z. Kassaee, F. Movahedi and H. Masrouri, Synlett, 2009, 1326–1330 CrossRef CAS PubMed.
- A. Vinu, P. Kalita, V. V. Balasubramanian, H. Oveisi, T. Selvan, A. Mano, M. A. Chari and B. V. Subba Reddy, Tetrahedron Lett., 2009, 50, 7132–7136 CrossRef CAS PubMed.
- S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2008, 73, 6029–6032 CrossRef CAS PubMed.
- Ambica, S. Kumar, S. C. Taneja, M. S. Hundal and K. K. Kapoor, Tetrahedron Lett., 2008, 49, 2208–2212 CrossRef CAS PubMed.
- M. Tajbakhsh, A. Heydari, H. Alinezhad, M. Ghanei and S. Khaksar, Synthesis, 2008, 352–354 CrossRef CAS PubMed.
- (a) S. A. R. Mulla, M. Y. Pathan and S. S. Chavan, RSC Adv., 2013, 3, 20281 RSC; (b) S. A. R. Mulla, T. A. Salama, M. Y. Pathan, S. M. Inamdar and S. S. Chavan, Tetrahedron Lett., 2013, 54, 672–675 CrossRef CAS PubMed; (c) S. A. R. Mulla, A. Sudalai, M. Y. Pathan, S. A. Siddique, S. M. Inamdar, S. S. Chavan and R. S. Reddy, RSC Adv., 2012, 2, 3525–3529 RSC; (d) S. A. R. Mulla, S. M. Inamdar, M. Y. Pathan and S. S. Chavan, Tetrahedron Lett., 2012, 53, 1826–1829 CrossRef CAS PubMed; (e) S. A. R. Mulla, S. M. Inamdar, M. Y. Pathan and S. S. Chavan, RSC Adv., 2012, 2, 12818–12823 RSC; (f) S. A. R. Mulla, S. M. Inamdar, M. Y. Pathan and S. S. Chavan, Open J. Synth. Theory Appl., 2012, 1, 31–35 CrossRef CAS.
- B. M. Rothschild, L. D. Martin, G. Lev, H. Bercovier, C. Greenblatt, H. Donoghue, M. Spigelman and D. Brittain, Clin. Infect. Dis., 2001, 33, 305–311 CrossRef CAS PubMed.
- World Health Organization, Multidrug and Extensive Drug Resistant Tuberculosis: Global tuberculosis report 2012, http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
Footnote |
| † Electronic supplementary information (ESI) available: experimental details and spectral data of all the new compounds. See DOI: 10.1039/c3ra45853a |
| This journal is © The Royal Society of Chemistry 2014 |