Formal [3 + 2] cycloaddition of α-unsubstituted isocyanoacetates and methyleneindolinones: enantioselective synthesis of spirooxindoles†
Xiao-Jiao
Peng‡
a,
Yee Ann
Ho‡
b,
Zhi-Peng
Wang
a,
Pan-Lin
Shao
*a,
Yu
Zhao
*b and
Yun
He
*a
aSchool of Pharmaceutical Sciences and Innovative Drug Research Centre, Chongqing University, 55 Daxuecheng South Road, Shapingba, Chongqing 401331, P. R. China. E-mail: shaopl@cqu.edu.cn; yun.he@cqu.edu.cn
bDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543, Republic of Singapore. E-mail: zhaoyu@nus.edu.sg
First published on 20th October 2016
Abstract
The first enantioselective formal [3 + 2] cycloaddition of α-unsubstituted isocyanoacetates with protecting-group-free methyleneindolinones was developed. A variety of optically enriched 3,3′-pyrrolidinyl spirooxindoles were obtained in excellent yields, and diastereo- and enantioselectivities (up to 99% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 99% ee) with low catalyst loading under mild reaction conditions.
1 dr, 99% ee) with low catalyst loading under mild reaction conditions.
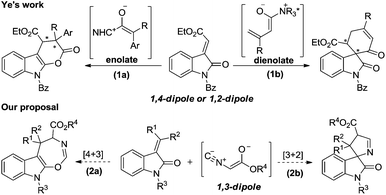
We have been pursuing the development of asymmetric enolate methodologies for the synthesis of heterocyclic compounds for several years.1 There have been many approaches to generate chiral enolate intermediates. For example, the nucleophilic addition of N-heterocyclic carbenes (NHCs)2 or cinchona alkaloid derivatives3 to ketenes or vinyl ketenes afforded 1,2-dipole enolates or dienolates, serving as 1,4-dipole enolates,4,2e respectively. Our recent efforts have focused on the use of functionalized 1,3-dipole enolates derived from isocyanoacetate, due to its isocyanide group and acidic α-CH fragment encountered chiral Brønsted base.1d,e
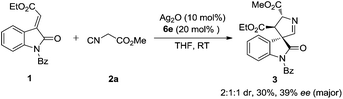
Recently, the cyclization of methyleneindolinones has emerged as a powerful tool for the construction of indole-fused heterocyclic compounds or spirooxindoles.4b,5 In 2010, Ye and co-workers reported the NHC-catalyzed formal [4 + 2] cycloaddition of ketenes with methyleneindolinones, as surrogates of “1,4-dipole”, to synthesize indole-fused dihydropyranones (Scheme 1, 1a).5g In 2013, they developed the cinchona alkaloid catalyzed [4 + 2] cyclization of α,β-unsaturated acyl chlorides with methyleneindolinones, as surrogates of “1,2-dipole”, to produce spirocarbocyclic oxindoles (Scheme 1, 1b).4b We reasoned that the 1,3-dipole enolate generated from isocyanoacetate may facilitate a [4 + 3] or [3 + 2] cycloaddition with methyleneindolinone to afford a indole-fused seven-membered heterocycle skeleton6 or spirooxindole,7 which are both privileged structure moieties found in many biologically active natural products and pharmaceutically important compounds (Scheme 1, 2a, 2b).
Initial exploration utilizing methyleneindolinone 1 and the commercially available methyl isocyanoacetate 2a employing Dixon's catalysis system led to a [3 + 2] cyclization affording 3,3′-pyrrolidinyl spirooxindole 3, whereas no [4 + 3] annulation product was observed (Scheme 2). The 3,3′-pyrrolidinyl spirooxindole scaffold is an important backbone in the spirooxindole family because of not only its interesting biological activities, but also its versatility as an intermediate in the synthesis of related and more sophisticated spirooxindole structures.5,7
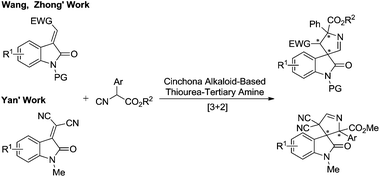
Recently, Wang,5a Zhong5b and Yan5cet al. developed stereoselective [3 + 2] cycloadditions of α-aryl isocyanoacetates with active protected methyleneindolinones to access 3,3′-pyrrolidinyl spirooxindoles catalyzed by cinchona alkaloid-based thiourea tertiary amines, respectively (Scheme 3). Notably, the isocyanoacetates bearing α-aryl substituents are crucial for achieving good reactivity and enantioselectivity in this catalytic system. Furthermore, to the best of our knowledge, the synthetic methodology to enantioselectively construct this rigid spiroarchitecture utilizing α-unsubstituted and α-alkyl isocyanoacetates is not available. Herein we report the first highly enantioselective formal [3 + 2] cycloaddition reaction of α-unsubstituted and α-alkyl isocyanoacetates with less active methyleneindolinones catalyzed by a chiral silver(I) complex developed by the group of Dixon.8
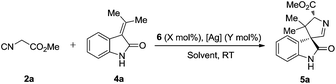
To further optimize the reaction conditions, the protecting-group-free methyleneindolinone 4a was chosen as the model substrate because of its easy synthesis.9 As shown in Table 1, several catalysts or ligands were screened to evaluate their ability to promote the Michael/cyclization reaction sequence of 2a and 4a at ambient temperature. To our disappointment, no desired [3 + 2] cyclization product 5a was obtained in the presence of quinine (6a) or the quinine-based thiourea tertiary amine (6b), respectively (entries 1 and 2). 6b proved to be an excellent catalyst in asymmetric cyclization of α-aryl isocyanoacetates,5a–c but was unable to activate the α-unsubstituted isocyanoacetates and hence was catalytically inactive for the reaction. Although a chiral silver complex with (S)-MOP 6c was the optimal catalyst in Gong's work of the enantioselective formal [3 + 2] cycloaddition reaction of α-aryl isocyanoacetates with 2-oxobutenoate esters,106c with AgOAc failed to promote the reaction (entry 3). Interestingly, 6c with Ag2O afforded the desired product in good yield though with poor diastereo- and enantioselectivities (entry 4). Inspired by the recent report from the group of Dixon on enantioselective isocyanoacetate cycloaddition catalyzed by a silver(I) complex with cinchona-derived amino phosphine precatalysts,8 we tested the related compounds 6d–f for our reaction. To our great delight, all these precatalysts with Ag2O led to excellent yields, diastereo- and enantioselectivities (entries 5–7). Lowering the loading of 6e and Ag2O had a negligible impact on the enantioselectivities (entries 8 and 9). A survey of solvents identified AcOEt as the most suitable media (entries 10–13). Increasing the concentration of the catalysts benefited the diastereoselectivity (entry 14), and much higher diastereoselectivity (trans/cis > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained via slow addition of methyl isocyanoacetate 2a to the mixture of the silver(I) complex and methyleneindolinone 4a over 5 hours.
1) was obtained via slow addition of methyl isocyanoacetate 2a to the mixture of the silver(I) complex and methyleneindolinone 4a over 5 hours.
| Entry | 6 (X) | [Ag] (Y) | Solvent | Yieldb [%] | trans/cisc | eed [%] |
|---|---|---|---|---|---|---|
a Reaction conditions: 2a (0.4 mmol), 4a (0.4 mmol), solvent (2 mL).
b Total yields of the isolated trans and cis-isomers.
c Determined by 1H NMR spectroscopy (400 MHz) of the reaction mixture.
d ee of the trans-isomer determined by HPLC analysis using a chiral stationary phase.
e AcOEt 0.4 mL.
f
2a (0.4 mmol) in AcOEt (0.4 mL) was added via a syringe pump over 5 h. 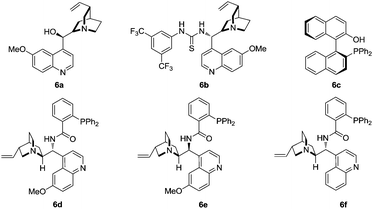
|
||||||
| 1 | 6a (10) | 0 | THF | 0 | — | — |
| 2 | 6b (10) | 0 | THF | 0 | — | — |
| 3 | 6c (12) | AgOAc (10) | THF | 0 | — | — |
| 4 | 6c (12) | Ag2O (5) | THF | 84 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31 |
| 5 | 6d (20) | Ag2O (10) | THF | 99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−93 |
| 6 | 6e (20) | Ag2O (10) | THF | 99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 7 | 6f (20) | Ag2O (10) | THF | 99 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−94 |
| 8 | 6e (10) | Ag2O (5) | THF | 99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 9 | 6e (5) | Ag2O (2.5) | THF | 99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 10 | 6e (5) | Ag2O (2.5) | CH2Cl2 | 99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 |
| 11 | 6e (5) | Ag2O (2.5) | MTBE | 93 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 12 | 6e (5) | Ag2O (2.5) | Toluene | 96 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 13 | 6e (5) | Ag2O (2.5) | AcOEt | 99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 14e | 6e (5) | Ag2O (2.5) | AcOEt | 99 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 15e,f | 6e (5) | Ag2O (2.5) | AcOEt | 99 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
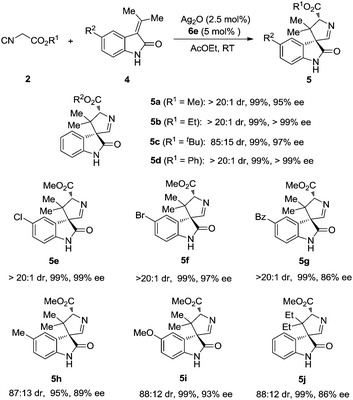
With the established optimal reaction conditions, a variety of 3,3′-pyrrolidinyl spirooxindoles were synthesized and the results are summarized in Table 2. Isocyanoacetates bearing different ester groups were all suitable substrates, producing the corresponding products 5a–d in uniformly high yields with excellent diastereoselectivities and enantioselectivities. Different substitution patterns on the aryl ring of methyleneindolinones were well tolerated (5e–i). As expected, these substrates underwent smooth cycloaddition reactions to give the spirooxindoles, whereas both diastereoselectivity and enantioselectivity showed some dependence on the electronic feature of the substituents. The substrates with electron-withdrawing substituents (Cl, Br) afforded the products in excellent yields, dr and ee values (5e, 5f). When Bz was introduced, the yield and diastereoselectivity were still very high, albeit the ee value decreased (5g). In the methyleneindolinones bearing electron-donating substituents (Me, MeO), the diastereo- and enantioselectivities were slightly decreased without eroding the yield (5h, 5i). When the two methyl groups of methyleneindolinone were replaced by two ethyl groups, the reaction proceeded smoothly, thus providing product 5j with good stereocontrol and in excellent yield. It is worth noting that all the reactions were carried out under an ambient atmosphere, and exclusion of air or moisture was not required.
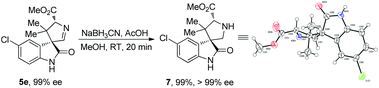
Notably, the formed cycloaddition products are highly functionalized, along with an imine moiety which is easy for further structural modifications. As shown in Scheme 4, when product 5e was treated with NaBH3CN, it was readily transformed into 3,3′-pyrrolidinyl spirooxindole 7 in nearly quantitative yield without loss of diastereo- and enantioselectivities, and its X-ray crystallographic analysis11 confirmed the relative and absolute configurations of the cycloaddition products 5a–j.
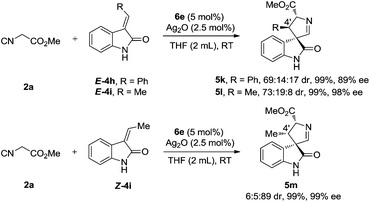
The formal [3 + 2] cycloaddition of isocyanoacetates is not limited to the coupling with disubstituted 3-methyleneindolinones. Mono-substituted (phenyl, methyl) 3-methyleneindolinones are also suitable partners, and provided the corresponding cycloadducts in excellent yields and with good to excellent diastereo- and enantioselectivities (Scheme 5). More interestingly, although we noticed that a similarly excellent yield, enantioselectivity and switched diastereoselectivity were obtained when the reactions were conducted with E-4i and Z-4i, and a reversal of the absolute configuration of the 4′-position was observed (5l, 5m). These results suggest that these two substrates have the same mode of enantiofacial selection and that the olefin geometry does not play a major role in determining the facial selectivity of the cyclization. The relative configurations of the products were assigned by the NOE interactions of the two isomers (see the ESI† for details).
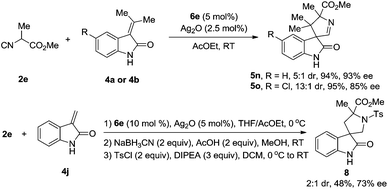
We have explored the possibility that the isocyanoacetate can bear α-alkyl substituents, e.g., α-methyl. To the best of our knowledge, α-alkyl substituted isocyanoacetates were inactive in the reported transformations of this type, due to the decreased acidity of the α-hydrogen atoms in these isocyanoacetates.5a In fact, the use of α-methyl isocyanoacetate 2e could also lead to a smooth formal [3 + 2] cycloaddition with methyleneindolinone in excellent yield, good diastereo- and enantioselectivities (Scheme 6). Most specifically, the unsubstituted 3-methyleneindolinone 4j could also be used as a reaction partner with 2e providing the spiro compound, which is too unstable to be purified. The crude product was then reduced with NaBH3CN and the resulting amine was protected with the p-toluenesulfonyl (Ts) group to generate 3,3′-pyrrolidinyl spirooxindole 8 in 48% yield over three steps with 73% ee and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity. Unfortunately, despite vigorous efforts, a qualified single crystal of 5o or 8 for X-ray crystallographic analysis could not be obtained. Thus, the absolute configuration of these products has not been assigned. Systematic optimization of the formal [3 + 2] cycloaddition reaction of α-alkyl isocyanoacetates with methyleneindolinones is ongoing, and the results will be reported in due course.
1 diastereoselectivity. Unfortunately, despite vigorous efforts, a qualified single crystal of 5o or 8 for X-ray crystallographic analysis could not be obtained. Thus, the absolute configuration of these products has not been assigned. Systematic optimization of the formal [3 + 2] cycloaddition reaction of α-alkyl isocyanoacetates with methyleneindolinones is ongoing, and the results will be reported in due course.
In summary, we have developed the first enantioselective formal [3 + 2] cycloaddition reactions of α-unsubstituted isocyanoacetates with the less active protecting-group-free methyleneindolinones using mild reaction conditions. A variety of optically enriched 3,3′-pyrrolidinyl spirooxindoles were obtained in excellent yields, diastereo- and enantioselectivities (up to 99% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 99% ee). The high efficiency of this process, coupled with operational simplicity, makes it an attractive method for spirooxindole synthesis. Further studies on expanding the application of this approach to synthesize more promising candidates for drug discovery as well as the biological evaluation are in progress. The asymmetric formal [4 + 3] cycloaddition of isocyanoacetates with methyleneindolinones is underway in our lab.
1 dr, 99% ee). The high efficiency of this process, coupled with operational simplicity, makes it an attractive method for spirooxindole synthesis. Further studies on expanding the application of this approach to synthesize more promising candidates for drug discovery as well as the biological evaluation are in progress. The asymmetric formal [4 + 3] cycloaddition of isocyanoacetates with methyleneindolinones is underway in our lab.
The work described here was supported by the National Natural Science Foundation of China (21372267, 21402150 and 21572027) and the Singapore National Research Foundation (NRF Fellowship).
Notes and references
- (a) P.-L. Shao, X.-Y. Chen and S. Ye, Angew. Chem., Int. Ed., 2010, 49, 8412 CrossRef CAS PubMed; (b) P.-L. Shao, X.-Y. Chen, L.-H. Sun and S. Ye, Tetrahedron Lett., 2010, 51, 2316 CrossRef CAS; (c) P. Shao, L. Shen and S. Ye, Chin. J. Chem., 2012, 30, 2688 CAS; (d) P.-L. Shao, J.-Y. Liao, Y. A. Ho and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 5435 CrossRef CAS PubMed; (e) J.-Y. Liao, P.-L. Shao and Y. Zhao, J. Am. Chem. Soc., 2015, 137, 628 CrossRef CAS PubMed.
- (a) Y.-R. Zhang, L. He, X. Wu, P.-L. Shao and S. Ye, Org. Lett., 2008, 10, 277 CrossRef CAS PubMed; (b) N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith, Org. Biomol. Chem., 2008, 6, 1108 RSC; (c) X.-L. Huang, L. He, P.-L. Shao and S. Ye, Angew. Chem., Int. Ed., 2009, 48, 192 CrossRef CAS PubMed; (d) X.-N. Wang, P.-L. Shao, H. Lv and S. Ye, Org. Lett., 2009, 11, 4029 CrossRef CAS PubMed; (e) L.-T. Shen, P.-L. Shao and S. Ye, Adv. Synth. Catal., 2011, 353, 1943 CrossRef CAS; (f) T.-Y. Jian, L. He, C. Tang and S. Ye, Angew. Chem., Int. Ed., 2011, 50, 9104 CrossRef CAS PubMed.
- (a) C. Zhu, X. Shen and S. G. Nelson, J. Am. Chem. Soc., 2004, 126, 5352 CrossRef CAS PubMed; (b) S. G. Nelson, C. Zhu and X. Shen, J. Am. Chem. Soc., 2004, 126, 14 CrossRef CAS PubMed; (c) X. Xu, K. Wang and S. G. Nelson, J. Am. Chem. Soc., 2007, 129, 11690 CrossRef CAS PubMed; (d) T. Bekele, M. H. Shah, J. Wolfer, C. J. Abraham, A. Weatherwax and T. Lectka, J. Am. Chem. Soc., 2006, 128, 1810 CrossRef CAS PubMed; (e) C. J. Abraham, D. H. Paull, M. T. Scerba, J. W. Grebinski and T. Lectka, J. Am. Chem. Soc., 2006, 128, 13370 CrossRef CAS PubMed; (f) J. Wolfer, T. Bekele, C. J. Abraham, C. Dogo-Isonagie and T. Lectka, Angew. Chem., Int. Ed., 2006, 45, 7398 CrossRef CAS PubMed.
- (a) L.-T. Shen, L.-H. Sun and S. Ye, J. Am. Chem. Soc., 2011, 133, 15894 CrossRef CAS PubMed; (b) L.-T. Shen, W.-Q. Jia and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 585 CrossRef CAS PubMed.
- (a) L.-L. Wang, J.-F. Bai, L. Peng, L.-W. Qi, L. N. Jia, Y.-L. Guo, X.-Y. Luo, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2012, 48, 5175 RSC; (b) B. Tan, X. Zhang and G. Zhong, ARKIVOC, 2014, iii, 124 Search PubMed; (c) W.-T. Wei, C.-X. Chen, R.-J. Lu, J.-J. Wang, X.-J. Zhang and M. Yan, Org. Biomol. Chem., 2012, 10, 5245 RSC; (d) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735 CrossRef CAS PubMed; (e) X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 13819 CrossRef CAS PubMed; (f) B. Tan, X. Zeng, W. W. Y. Leong, Z. Shi, C. F. Barbas III and G. Zhong, Chem. – Eur. J., 2012, 18, 63 CrossRef CAS PubMed; (g) H. Lv, X.-Y. Chen, L.-H. Sun and S. Ye, J. Org. Chem., 2010, 75, 6973 CrossRef CAS PubMed; (h) Y. Cao, X. Jiang, L. Liu, F. Shen, F. Zhang and R. Wang, Angew. Chem., Int. Ed., 2011, 50, 9124 CrossRef CAS PubMed; (i) X.-Y. Hao, L.-L. Lin, F. Tan, C.-K. Yin, X.-H. Liu and X.-M. Feng, ACS Catal., 2015, 5, 6052 CrossRef CAS; (j) C.-K. Yin, L.-L. Lin, D. Zhang, J.-H. Feng, X.-H. Liu and X.-M. Feng, J. Org. Chem., 2015, 80, 9691 CrossRef CAS PubMed; (k) L.-J. Huang, J. Weng, S. Wang and G. Lu, Adv. Synth. Catal., 2015, 357, 993 CrossRef CAS; (l) Y. Wang, M.-S. Tu, L. Yin, M. Sun and F. Shi, J. Org. Chem., 2015, 80, 3223 CrossRef CAS PubMed; (m) W. Dai, X.-L. Jiang, Q. Wu, F. Shi and S.-J. Tu, J. Org. Chem., 2015, 80, 5737 CrossRef CAS PubMed; (n) Y.-M. Wang, H.-H. Zhang, C. Li, T. Fan and F. Shi, Chem. Commun., 2016, 52, 1804 RSC; (o) T. Fan, H.-H. Zhang, C. Li, Y. Shen and F. Shi, Adv. Synth. Catal., 2016, 358, 2017 CrossRef CAS; (p) T. Arai, H. Ogawa, A. Awata, M. Sato, M. Watabe and M. Yamanaka, Angew. Chem., Int. Ed., 2015, 54, 1595 CrossRef CAS PubMed; (q) Z.-H. Zhang, W.-S. Sun, G.-M. Zhu, J.-X. Yang, M. Zhang, L. Hong and R. Wang, Chem. Commun., 2016, 52, 1377 RSC; (r) J.-X. Zhang, H.-Y. Wang, Q.-W. Jin, C.-W. Zheng, G. Zhao and Y.-J. Shang, Org. Lett., 2016, 18, 4774 CrossRef CAS PubMed.
- (a) L. Huang, L.-X. Dai and S.-L. You, J. Am. Chem. Soc., 2016, 138, 5793 CrossRef CAS PubMed; (b) Z. Shen, H. A. Khan and V. M. Dong, J. Am. Chem. Soc., 2008, 130, 2916 CrossRef CAS PubMed; (c) I. D. G. Watson, S. Ritter and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 2056 CrossRef CAS PubMed; (d) H. He, W.-B. Liu, L.-X. Dai and S.-L. You, Angew. Chem., Int. Ed., 2010, 49, 1496 CrossRef CAS PubMed; (e) R. Rohlmann, C.-G. Daniliuc and O. G. Mancheno, Chem. Commun., 2013, 49, 11665 RSC; (f) H. Xu, J.-L. Hu, L. Wang, S. Liao and Y. Tang, J. Am. Chem. Soc., 2015, 137, 8006 CrossRef CAS PubMed; (g) C.-J. Tan, Y.-T. Di, Y.-H. Wang, Y. Zhang, Y.-K. Si, Q. Zhang, S. Gao, X.-J. Hu, X. Fang, S.-F. Li and X.-J. Hao, Org. Lett., 2010, 12, 2370 CrossRef CAS PubMed.
- (a) K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps and S. Wang, J. Am. Chem. Soc., 2005, 127, 10130 CrossRef CAS PubMed; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (c) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS; (d) S. Shangary, D. Qin, D. McEachern, M. Liu, R. S. Miller, S. Qiu, Z. Nikolovska-Coleska, K. Ding, G. Wang, J. Chen, D. Bernard, J. Zhang, Y. Lu, Q. Gu, R. B. Shah, K. J. Pienta, X. Ling, S. Kang, M. Guo, Y. Sun, D. Yang and S. Wang, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3933 CrossRef CAS PubMed; (e) M. M. M. Santos, Tetrahedron, 2014, 70, 9735 CrossRef CAS; (f) B. M. Trost and M. K. Brennan, Synthesis, 2009, 3003 CrossRef CAS; (g) L.-L. Wang, L. Peng, J.-F. Bai, Q.-C. Huang, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2010, 46, 8064 RSC; (h) L.-L. Wang, L. Peng, J.-F. Bai, L.-N. Jia, X.-Y. Luo, Q.-C. Huang, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2011, 47, 5593 RSC.
- (a) F. Sladojevich, A. Trabocchi, A. Guarna and D. J. Dixon, J. Am. Chem. Soc., 2011, 133, 1710 CrossRef CAS PubMed; (b) I. Ortín and D. J. Dixon, Angew. Chem., Int. Ed., 2014, 53, 3462 CrossRef PubMed; (c) R. de la Campa, I. Ortín and D. J. Dixon, Angew. Chem., Int. Ed., 2015, 54, 4895 CrossRef CAS PubMed.
- D. C. Harrowven, K. J. Stenning, S. Whiting, T. Thompson and R. Walton, Org. Biomol. Chem., 2011, 9, 4882 CAS.
- J. Song, C. Guo, P.-H. Chen, J. Yu, S.-W. Luo and L.-Z. Gong, Chem. – Eur. J., 2011, 17, 7786 CrossRef CAS PubMed.
- CCDC 1496241 contains the supplementary crystallographic data for compound 7. Selected data are also included in the ESI.†.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data of new compounds, and crystallographic data. CCDC 1496241. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00555a |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |