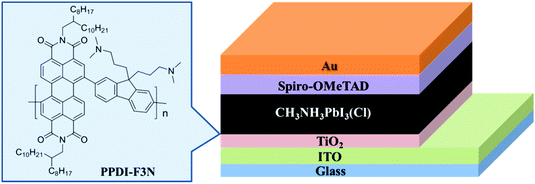
An amino-substituted perylene diimide polymer for conventional perovskite solar cells†
Mingyu
Zhang
a,
Tengfei
Li
a,
Guanhaojie
Zheng
a,
Liang
Li
a,
Meng
Qin
a,
Shiming
Zhang
b,
Huanping
Zhou
 *a and
Xiaowei
Zhan
*a and
Xiaowei
Zhan
 *a
*a
aDepartment of Materials Science and Engineering, College of Engineering, Peking University, Beijing 100871, P. R. China. E-mail: xwzhan@pku.edu.cn; happy_zhou@pku.edu.cn
bKey Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing 211816, China
First published on 26th June 2017
Abstract
We design and synthesize an amino-functionalized conjugated polymer (PPDI-F3N) based on perylene diimide and use it as a multifunctional interfacial layer of TiO2/perovskite in conventional planar perovskite solar cells. The work function of TiO2 is modulated by PPDI-F3N to better align with the conduction band of the perovskite, leading to efficient charge extraction. PPDI-F3N can passivate the TiO2 surface to reduce the severe recombination loss and rapid degradation caused by oxygen vacancies on the UV-sensitive TiO2 surface. Moreover, modulated polarity of PPDI-F3N is beneficial to optimal perovskite crystallization and morphology. All these features contribute to a higher efficiency (18.3%) of the PSCs with the PPDI-F3N interlayer relative to the control devices without the interlayer (16.7%) as well as improved stability and a reduced hysteresis effect.
Introduction
Organic–inorganic lead halide perovskite solar cells (PSCs) have attracted growing attention lately, since their power conversion efficiency (PCE) has rapidly increased up to 22%.1–8 High-performance PSCs are mainly fabricated with two dominant device structures, namely, mesostructured and planar heterojunction (PHJ) PSCs.9 Compared to the mesostructured PSCs,10 PHJ PSCs are currently becoming popular due to their low temperature processing and simple device architecture.3,5,11–16TiO2 has been regarded as one of the promising electron extraction and transport materials for n–i–p PSCs, due to its proper conduction band compatible with that of hybrid perovskites. Meanwhile, its deep valence band is able to block the holes. In particular, a low temperature processed TiO2 layer is suitable for PHJ PSCs.17 However, there are two drawbacks for low temperature processed TiO2. First, the low electron mobility results in charge accumulation in the TiO2 layer.18,19 Second, exposure of TiO2 to UV light further induces formation of oxygen vacancies, which are energetically deep trap levels responsible for severe recombination loss.20,21 Moreover, the strong oxidative photon-generated holes in TiO2 can react with I ions from bulk perovskite, resulting in the degradation of perovskite films.22 As a result, the TiO2/perovskite interface leads to reduced efficiency, anomalous hysteresis behavior and severe instability for the corresponding devices.
Since the TiO2/perovskite interface is critically important,23,24 considerable efforts have focused on the interfacial modification between TiO2 and perovskite to improve the device efficiency and stability and suppress the hysteresis behavior. For example, researchers used inorganic materials like CsBr and Sb2S3,21,22 and organic materials like small molecule fullerene derivatives,25–35 as an interlayer of TiO2/perovskite.
In this work, we demonstrate the first example of a nonfullerene polymer interlayer of TiO2/perovskite in n–i–p PHJ PSCs. We design and synthesize a new conjugated copolymer of perylene diimide and fluorene with amino side chains (PPDI-F3N, Fig. 1). Our molecular design rationale is as follows. First, perylene diimide has a LUMO energy level of ca. −3.9 eV, which well aligns with the conduction band of the perovskite (−3.9 eV) and TiO2 (−4.0 eV).36 Moreover, perylene diimide small molecules and polymers are classical n-type organic semiconductors and exhibit high electron mobility.36,37 Second, fluorene incorporation endows the polymer with a deep HOMO energy level and good hole-blocking property.38 Third, the terminal amino groups in 3-(dimethylamino)propyl side chains can adjust the wetting capability of the TiO2 surface and passivate traps of perovskite films, leading to a high-quality perovskite film.38–41 Finally, polymer materials exhibit better solution-processing film-forming ability and mechanical flexibility and stability relative to their small molecule counterpart like conventional fullerene derivatives.42 Indeed, the polymer, PPDI-F3N, is readily soluble in nonpolar solvents like chlorobenzene (CB) instead of polar solvents like DMF to achieve multilayer coating capability via solution processing. The modification of TiO2 with PPDI-F3N can enhance electron extraction due to the compatible energy alignment with CH3NH3PbI3(Cl). Furthermore, the PPDI-F3N polymer can passivate the TiO2 surface to avoid severe recombination loss and the rapid degradation caused by the oxygen vacancies. The deposition of PPDI-F3N results in an improved morphology of the crystalline perovskite film. All these features contribute to a higher PCE (18.3%) and better stability of the PSCs with the PPDI-F3N interlayer relative to the control devices without the interlayer (16.7%).
Results and discussion
Synthesis and characterization
The new amino-functionalized n-type polymer, PPDI-F3N, was synthesized by palladium-catalyzed Suzuki coupling copolymerization of perylene diimide dibromide and fluorene diboronic ester (Scheme S1, ESI†). The polymer is readily soluble in common organic solvents, such as chlorobenzene and chloroform at room temperature. PPDI-F3N has a weight-average molecular weight (Mw) of 3.7 × 104 with a polydispersity index (Mw/Mn) of 6.5 measured by gel permeation chromatography (GPC). The polymer exhibits good thermal stability with a decomposition temperature (Td) of 328 °C, measured by thermogravimetric analysis (TGA) in a nitrogen atmosphere (Fig. S1a, ESI†). Normalized UV-vis absorption spectra of PPDI-F3N in chloroform solution and thin films are shown in Fig. S1b (ESI†). The polymer in solution exhibits two absorption peaks around 490 and 528 nm; its thin film shows a red-shifted and broader absorption spectrum relative to its solution. When we deposited the polymer thin film onto an ITO/TiO2 substrate, the optical transmittance of the bare TiO2 film did not change (Fig. 2a).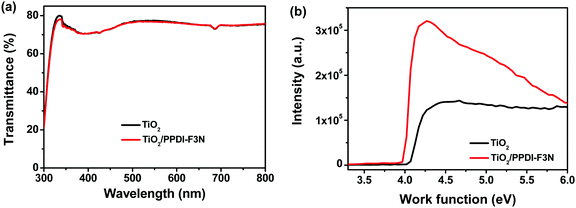 | ||
| Fig. 2 (a) Transmittance spectra of bare TiO2 and TiO2/PPDI-F3N; (b) UPS spectra of bare TiO2 and TiO2/PPDI-F3N. | ||
Cyclic voltammetry (CV) measurement was performed to estimate the HOMO and LUMO energy levels of the polymer as shown in Fig. S1c (ESI†). The LUMO of PPDI-F3N is located at −3.73 eV, while the HOMO is at −5.73 eV, which is significantly deeper than the valence band of the perovskite film (−5.4 eV), and therefore this polymer has a good hole blocking property. The ultraviolet photoelectron spectroscopy (UPS) measurement (Fig. 2b) indicates that the work function of TiO2 is appreciably lowered from 4.05 to 3.95 eV with deposition of PPDI-F3N, which better matches with the conduction band (−3.9 eV) of the perovskite film and is favorable for electron extraction to PPDI-F3N.43
Surface morphology and electron extraction
X-ray diffraction (XRD) was conducted to characterize the perovskite films grown on TiO2 with and without PPDI-F3N. In Fig. 3a, both samples show similar crystal structures with distinctive (110) and (220) diffraction peaks at 14.2° and 28.3°, respectively, in accordance with the previous work.44 Similar to the film grown on the TiO2 surface, we do not see other peaks or a significant change in the diffraction peak ratio, indicating a pure perovskite phase with the same crystal orientation on both surfaces. The width at half maximum of the diffraction peak (110) is the same for perovskite films on TiO2 and TiO2/PPDI-F3N (0.11°). However, all these perovskite diffraction peaks are significantly enhanced with the modification of PPDI-F3N, indicating the improvement in the crystallinity of the perovskite film.45 A high-quality perovskite film with larger crystalline domains is formed on the surface of TiO2/PPDI-F3N (Fig. 3b and c). We studied the wettability of the TiO2 and TiO2/PPDI-F3N surfaces shown in Fig. S2 (ESI†). The water contact angles of ITO/TiO2, ITO/TiO2/CB and ITO/TiO2/PPDI-F3N are 43.3 ± 1.4°, 48.9 ± 1.9° and 79.6 ± 0.4°, respectively, indicating that the substrate after PPDI-F3N modification yields a more incompatible wetting surface. Meanwhile, after washing with DMF, the water contact angle of ITO/TiO2/PPDI-F3N slightly decreases from 79.6 ± 0.4° to 71.4 ± 0.4°, indicating that the PPDI-F3N film has good resistance against DMF washing. The incompatible wetting surface of substrates with the perovskite will provide a weaker surface tension and dragging force for the perovskite precursor solution to form larger perovskite grains.46 The perovskite film with higher crystallinity and larger crystalline domains is essentially important for the device performance.3,47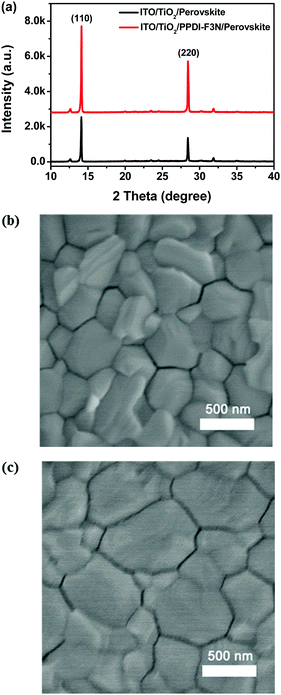 | ||
| Fig. 3 (a) XRD pattern of perovskite films on ITO/TiO2 and ITO/TiO2/PPDI-F3N substrates; top-view SEM images of perovskite films grown on (b) TiO2 and (c) TiO2/PPDI-F3N. | ||
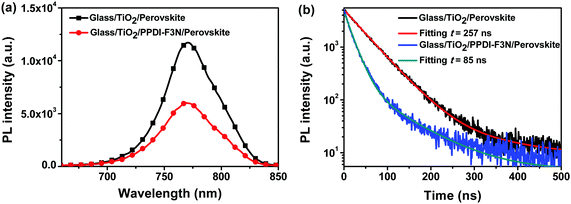
To investigate charge extraction, we measured the steady-state and time-resolved photoluminescence (PL) spectra of glass/TiO2/CH3NH3PbI3(Cl) and glass/TiO2/PPDI-F3N/CH3NH3PbI3(Cl) (Fig. 4). The PPDI-F3N layer leads to 50% PL quenching, indicating efficient charge transfer at the interface. The time-resolved PL decay was measured with peak emission at 780 nm (Fig. 4b). Fitting the data with two-component exponential decay (here, the longer lifetime was used for comparison) yields the lifetime of carriers. The PL decay of the glass/TiO2/CH3NH3PbI3(Cl) film deduces a PL lifetime of 257 ns, while the PL lifetime is substantially shortened to 85 ns when TiO2 is coated with the PPDI-F3N layer, indicating efficient charge extraction at the perovskite/PPDI-F3N interface. Alternatively, it’s also possible that PPDI-F3N reduces the trap state density of TiO2via passivation, since the trap sites will prolong the charge carrier life.30,45,48
Device performance
We fabricated planar heterojunction perovskite solar cells with a structure of glass/ITO/TiO2 (or TiO2/PPDI-F3N)/CH3NH3PbI3(Cl)/spiro-OMeTAD/Au (Fig. 1), and Fig. S3 (ESI†) illustrates the cross-sectional structure of PSCs observed by scanning electron microscopy (SEM). J–V curves of the PSCs with or without the PPDI-F3N modification layer were measured under simulated solar illumination of AM1.5G 100 mW cm−2. The photovoltaic parameters of the modified devices with the PPDI-F3N interfacial layer at different concentrations are summarized in Table S1 (ESI†). The optimal PPDI-F3N concentration is 0.1 mg mL−1. As shown in Fig. 5a, the TiO2/PPDI-F3N based devices exhibit the best PCE of 18.3% with a short circuit current density (JSC) of 22.8 mA cm−2, open circuit voltage (VOC) of 1.09 V and fill factor (FF) of 73.7% from reverse scans, while the control devices without PPDI-F3N exhibit a maximum PCE of 16.7%, with a JSC of 22.3 mA cm−2, VOC of 1.08 V and FF of 69.1% (Table 1). The higher VOC could be attributed to the lower work function of TiO2/PPDI-F3N (3.95 eV) than that of TiO2 (4.05 eV). The steady-state current density and power output measured at the maximum power point are shown in Fig. 5b. The device with PPDI-F3N exhibits a stabilized efficiency of 17.5%, superior to that of the control device without PPDI-F3N (15.8%). Both of the stabilized efficiencies match well with those from J–V measurements.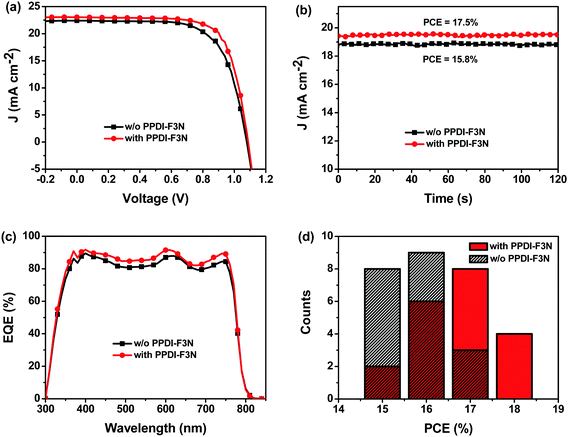 | ||
| Fig. 5 (a) J–V curves; (b) steady-state measurement of current and PCE; (c) EQE spectra of PSCs without and with PPDI-F3N; (d) PCE histogram of 20 devices. | ||
| Device | J SC (mA cm−2) | Calculated JSC (mA cm−2) | V OC (V) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|---|
| Best | Average | |||||
| W/o PPDI-F3N | 22.3 | 21.4 | 1.08 | 69.1 | 16.7 | 15.8 |
| With PPDI-F3N | 22.8 | 22.4 | 1.09 | 73.7 | 18.3 | 16.7 |
External quantum efficiency (EQE) spectra of the PSCs are shown in Fig. 5c. The PSCs with the PPDI-F3N interlayer show a stronger photocurrent response from 300 to 800 nm than the control devices without PPDI-F3N, and the calculated JSC values from the EQE spectra match very well with those obtained from J–V measurements with an error of <4% (Table 1). The PCE histogram of 20 devices (Fig. 5d) demonstrates that the reliability and repeatability of the device performance are improved by the PPDI-F3N modification. Fig. S4 (ESI†) shows the hysteresis curves of PSCs with and without the PPDI-F3N interfacial layer. Obviously, PPDI-F3N modified devices show reduced hysteresis in the J–V curve compared with the control device without PPDI-F3N, which benefits from the suppression of the charge recombination by the passivation of the trap states on the TiO2 surface efficiently.49,50
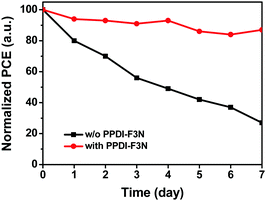
Stability tests of PSCs with and without PPDI-F3N were performed under ambient conditions (humidity of 30% and temperature of 25 °C) with 10 full devices without encapsulation, and the average data are shown in Fig. 6. The PCEs of the PSCs without PPDI-F3N decay dramatically to 27% of their initial values over 7 days, while the devices with the PPDI-F3N interlayer retain about 85% of their initial PCEs during the same test period. From the XRD patterns and top-view SEM images of perovskite films grown on different substrates, we can find that a perovskite film with higher crystallinity and larger crystalline domains is formed on the PPDI-F3N modified TiO2 substrate. The high-quality and compact perovskite film can efficiently prevent moisture from penetrating into the interior of the perovskite layer, resulting in slower degradation of the perovskite material and better device stability.
Conclusions
We synthesized an amino-substituted conjugated polymer (PPDI-F3N) based on perylene diimide and used it as a multifunctional interfacial layer of TiO2/perovskite in the conventional planar perovskite solar cells. UPS measurement indicates that PPDI-F3N can modulate the work function of TiO2 to match with the conduction band of the perovskite. Steady-state and time-resolved PL spectra reveal that PPDI-F3N is beneficial to efficient electron extraction from the perovskite film and transfer to TiO2. SEM and XRD measurements validate that PPDI-F3N is beneficial to optimal perovskite crystallization. Benefited from the efficient electron extraction, TiO2 surface passivation and high-quality perovskite film, the PSCs with the PPDI-F3N interfacial layer exhibit a maximum PCE of 18.3%, superior to those of the control devices without PPDI-F3N (16.7%), and the stability and hysteresis in J–V curves are also improved. This work demonstrates that nonfullerene polymers are promising interfacial materials for stable, high-efficiency PSCs with a reduced hysteresis effect.Experimental
Materials
Unless stated otherwise, all reagents were obtained commercially and were used without further purification. Monomer 1 and monomer 2 were purchased from Suna Tech Inc. PbI2 (99.999%) was purchased from Sigma-Aldrich. Aminomethane was purchased from Beijing Chemical Works. Hydrogen iodide (57%) was purchased from Alfa Aesar. Spiro-OMeTAD was purchased from Luminescence Technology Corp.Device fabrication
PSCs were fabricated with a structure of ITO/TiO2 (or TiO2/PPDI-F3N)/CH3NH3PbI3(Cl)/spiro-OMeTAD/Au. Indium tin oxide (ITO) glass was pre-cleaned in an ultrasonic bath of detergent water, ultrapure water, acetone and isopropanol. A 40 nm thick layer of TiO2 nanoparticles in ethanol (5.3 mg mL−1) with an appropriate amount of titanium diisopropoxide bis(acetylacetonate) was spin-coated onto an ITO glass substrate and annealed at 150 °C for 30 min in air. Next, the substrates were transferred into a nitrogen-filled glove box. A chlorobenzene (CB) solution of PPDI-F3N (0.1 mg mL−1) was spin coated on the TiO2 film at 3000 rpm for 30 s. PbI2 solution (dissolved in DMF, 450 mg mL−1) was then spin-coated on the TiO2 (or TiO2/PPDI-F3N) substrate at 3000 rpm for 30 s and annealed at 70 °C for 20 min. A mixture of CH3NH3I (50 mg mL−1) and CH3NH3Cl (5 mg mL−1) dissolved in isopropanol was spin-coated onto the PbI2 layer at 3000 rpm for 30 s. Then, the obtained films were annealed at 150 °C for 15 min in air. Spiro-OMeTAD was spin-coated at 3000 rpm for 30 s at a concentration of 80 mg mL−1 in CB with the addition of 35 μL Li-TFSI/acetonitrile (260 mg mL−1) and 28 μL 4-tert-butylpyridine. Finally, an 80 nm gold layer was deposited on top of the hole transporting material through shadow masks via thermal evaporation under high vacuum (∼2 × 10−6 Torr).Measurements
1H NMR spectra were measured on a Bruker AVANCE 400 MHz spectrometer. The molecular weights and dispersity were measured by gel permeation chromatography (GPC) on an Agilent PL-GPC220 at 150 °C using trichlorobenzene (TCB) as an eluent and polystyrene as the standard. TGA measurements were performed on a Shimadzu thermogravimetric analyzer (model DTG-60) under a nitrogen flow at a heating rate of 10 °C min−1. UV-vis absorption spectra and transmittance spectra of solution (chloroform, 10−6 M) or thin films (on quartz substrates) were recorded on a JASCO V-570 spectrometer. CV measurements were carried out under nitrogen in a deoxygenated solution of tetra-n-butylammonium hexafluorophosphate (0.1 M) in acetonitrile (CH3CN) using a computer-controlled CHI660C electrochemical workstation, a glassy-carbon working electrode coated with samples, a platinum-wire auxiliary electrode, and Ag/AgCl as a reference electrode. Potentials were referenced to the ferrocenium/ferrocene (FeCp20/+) couple by using ferrocene as an internal standard. UPS spectra were obtained from AXIS ULTRA DLD (Kratos) with He I (21.22 eV) excitation lines and a sample bias of 9 V in a vacuum of 3.0 × 10−8 Torr. The current density–voltage (J–V) characteristics of the photovoltaic devices were obtained using a Keithley 2400 source-measure unit under simulated sunlight from an Oriel 300 solar simulator, and the light intensity was calibrated using a KG-5 Si diode. The effective area of each cell was 0.102 cm2 defined by masks for all the photovoltaic devices discussed in this work. The EQE spectrum was measured using a Solar Cell Spectral Response Measurement System QE-R3011 (Enlitech Co., Ltd). The light intensity at each wavelength was calibrated using a standard single crystal Si photovoltaic cell. The XRD spectra were obtained using a D/MAX 2400 diffractometer with Cu Kα radiation (Rigaku). The top-view and cross-sectional SEM images were measured using a scanning electron microscope (Hitachi S4800). Steady-state PL and time-resolved transient-state PL were measured using a FLS980 (Edinburgh Instruments Ltd) under 470 nm excitation. Static contact angles were measured on a Dataphysics OCA20 contact-angle system at ambient temperature (the test liquid is water). All the measurements of the solar cells were performed under ambient atmosphere at room temperature without encapsulation.Acknowledgements
This work was supported by NSFC (21673011, 51672008).Notes and references
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395 CrossRef CAS PubMed.
- J.-W. Lee, D.-J. Seol, A.-N. Cho and N.-G. Park, Adv. Mater., 2014, 26, 4991 CrossRef CAS PubMed.
- H. Zhou, Q. Chen, G. Li, S. Luo, T.-b. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234 CrossRef CAS PubMed.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J.-P. C. Baena, J.-D. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Gratzel and A. Hagfeldt, Sci. Adv., 2016, 2, e1501170 Search PubMed.
- M. Saliba, S. Orlandi, T. Matsui, S. Aghazada, M. Cavazzini, J.-P. Correa-Baena, P. Gao, R. Scopelliti, E. Mosconi, K.-H. Dahmen, F. De Angelis, A. Abate, A. Hagfeldt, G. Pozzi, M. Graetzel and M. K. Nazeeruddin, Nat. Energy, 2016, 1, 15017 CrossRef CAS.
- J. Wang, K. Liu, L. Ma and X. Zhan, Chem. Rev., 2016, 116, 14675 CrossRef CAS PubMed.
- K. Liu, Y. Yao, J. Wang, L. Zhu, M. Sun, B. Ren, L. Xie, Y. Luo, Q. Meng and X. Zhan, Mater. Chem. Front., 2017, 1, 100 RSC.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982 CAS.
- S. D. Stranks and H. J. Snaith, Nat. Nano, 2015, 10, 391 CrossRef CAS PubMed.
- T.-B. Song, Q. Chen, H. Zhou, C. Jiang, H.-H. Wang, Y. Yang, Y. Liu, J. You and Y. Yang, J. Mater. Chem. A, 2015, 3, 9032 CAS.
- M. Zhang, J. Wang, L. Li, G. Zheng, K. Liu, M. Qin, H. Zhou and X. Zhan, Adv. Sci., 2017 DOI:10.1002/advs.201700025.
- Y. Lin, L. Shen, J. Dai, Y. Deng, Y. Wu, Y. Bai, X. Zheng, J. Wang, Y. Fang, H. Wei, W. Ma, X. C. Zeng, X. Zhan and J. Huang, Adv. Mater., 2017, 29, 1604545 CrossRef PubMed.
- F. Meng, K. Liu, S. Dai, J. Shi, H. Zhang, X. Xu, D. Li and X. Zhan, Mater. Chem. Front., 2017, 1, 1079 RSC.
- K. Wojciechowski, M. Saliba, T. Leijtens, A. Abate and H. J. Snaith, Energy Environ. Sci., 2014, 7, 1142 CAS.
- H.-S. Kim, I. Mora-Sero, V. Gonzalez-Pedro, F. Fabregat-Santiago, E. J. Juarez-Perez, N.-G. Park and J. Bisquert, Nat. Commun., 2013, 4, 2242 Search PubMed.
- H. J. Snaith, A. Abate, J. M. Ball, G. E. Eperon, T. Leijtens, N. K. Noel, S. D. Stranks, J. T.-W. Wang, K. Wojciechowski and W. Zhang, J. Phys. Chem. Lett., 2014, 5, 1511 CrossRef CAS PubMed.
- T. Leijtens, G. E. Eperon, S. Pathak, A. Abate, M. M. Lee and H. J. Snaith, Nat. Commun., 2013, 4, 2885 Search PubMed.
- S. Ito, S. Tanaka, K. Manabe and H. Nishino, J. Phys. Chem. C, 2014, 118, 16995 CAS.
- W. Li, W. Zhang, S. Van Reenen, R. J. Sutton, J. Fan, A. A. Haghighirad, M. B. Johnston, L. Wang and H. J. Snaith, Energy Environ. Sci., 2016, 9, 490 CAS.
- J. Shi, X. Xu, D. Li and Q. Meng, Small, 2015, 11, 2472 CrossRef CAS PubMed.
- C.-C. Chueh, C.-Z. Li and A. K. Y. Jen, Energy Environ. Sci., 2015, 8, 1160 CAS.
- A. Abrusci, S. D. Stranks, P. Docampo, H.-L. Yip, A. K. Y. Jen and H. J. Snaith, Nano Lett., 2013, 13, 3124 CrossRef CAS PubMed.
- K. Wojciechowski, S. D. Stranks, A. Abate, G. Sadoughi, A. Sadhanala, N. Kopidakis, G. Rumbles, C.-Z. Li, R. H. Friend, A. K. Y. Jen and H. J. Snaith, ACS Nano, 2014, 8, 12701 CrossRef CAS PubMed.
- C. Tao, S. Neutzner, L. Colella, S. Marras, A. R. S. Kandada, M. Gandini, M. De Bastiani, G. Pace, L. Manna, M. Caironi, C. Bertarelli and A. Petrozza, Energy Environ. Sci., 2015, 8, 2365 CAS.
- C. Liu, K. Wang, P. Du, T. Meng, X. Yu, S. Z. D. Cheng and X. Gong, ACS Appl. Mater. Interfaces, 2015, 7, 1153 CAS.
- Y. Dong, W. Li, X. Zhang, Q. Xu, Q. Liu, C. Li and Z. Bo, Small, 2016, 12, 1098 CrossRef CAS PubMed.
- Y. Li, Y. Zhao, Q. Chen, Y. Yang, Y. Liu, Z. Hong, Z. Liu, Y.-T. Hsieh, L. Meng, Y. Li and Y. Yang, J. Am. Chem. Soc., 2015, 137, 15540 CrossRef CAS PubMed.
- T. Cao, Z. Wang, Y. Xia, B. Song, Y. Zhou, N. Chen and Y. Li, ACS Appl. Mater. Interfaces, 2016, 8, 18284 CAS.
- Y. Zhang, P. Wang, X. Yu, J. Xie, X. Sun, H. Wang, J. Huang, L. Xu, C. Cui, M. Lei and D. Yang, J. Mater. Chem. A, 2016, 4, 18509 CAS.
- C. Tao, J. Van Der Velden, L. Cabau, N. F. Montcada, S. Neutzner, A. R. Srimath Kandada, S. Marras, L. Brambilla, M. Tommasini, W. Xu, R. Sorrentino, A. Perinot, M. Caironi, C. Bertarelli, E. Palomares and A. Petrozza, Adv. Mater., 2017, 29, 1604493 CrossRef PubMed.
- W. Zhou, J. Zhen, Q. Liu, Z. Fang, D. Li, P. Zhou, T. Chen and S. Yang, J. Mater. Chem. A, 2017, 5, 1724 CAS.
- F. Cai, L. Yang, Y. Yan, J. Zhang, F. Qin, D. Liu, Y.-b. Cheng, Y. Zhou and T. Wang, J. Mater. Chem. A, 2017, 5, 9402 CAS.
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed.
- X. Zhan, Z. a. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS PubMed.
- C. Sun, Z. Wu, H.-L. Yip, H. Zhang, X.-F. Jiang, Q. Xue, Z. Hu, Z. Hu, Y. Shen, M. Wang, F. Huang and Y. Cao, Adv. Energy Mater., 2016, 6, 1501534 CrossRef.
- Z. Zhu, J.-Q. Xu, C.-C. Chueh, H. Liu, Z. a. Li, X. Li, H. Chen and A. K. Y. Jen, Adv. Mater., 2016, 28, 10786 CrossRef CAS PubMed.
- H. Zhang, L. Xue, J. Han, Y. Q. Fu, Y. Shen, Z. Zhang, Y. Li and M. Wang, J. Mater. Chem. A, 2016, 4, 8724 CAS.
- D. Li, C. Sun, H. Li, H. Shi, X. Shai, Q. Sun, J. Han, Y. Shen, H.-L. Yip, F. Huang and M. Wang, Chem. Sci., 2017, 8, 4587 RSC.
- X. Zhao and X. Zhan, Chem. Soc. Rev., 2011, 40, 3728 RSC.
- Z. Zhu, J. Ma, Z. Wang, C. Mu, Z. Fan, L. Du, Y. Bai, L. Fan, H. Yan, D. L. Phillips and S. Yang, J. Am. Chem. Soc., 2014, 136, 3760 CrossRef CAS PubMed.
- Q. Chen, H. Zhou, Y. Fang, A. Z. Stieg, T.-B. Song, H.-H. Wang, X. Xu, Y. Liu, S. Lu, J. You, P. Sun, J. McKay, M. S. Goorsky and Y. Yang, Nat. Commun., 2015, 6, 7269 CrossRef CAS PubMed.
- P.-W. Liang, C.-Y. Liao, C.-C. Chueh, F. Zuo, S. T. Williams, X.-K. Xin, J. Lin and A. K. Y. Jen, Adv. Mater., 2014, 26, 3748 CrossRef CAS PubMed.
- C. Bi, Q. Wang, Y. Shao, Y. Yuan, Z. Xiao and J. Huang, Nat. Commun., 2015, 6, 7747 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897 CrossRef CAS PubMed.
- L. Zuo, Z. Gu, T. Ye, W. Fu, G. Wu, H. Li and H. Chen, J. Am. Chem. Soc., 2015, 137, 2674 CrossRef CAS PubMed.
- A. Abate, M. Saliba, D. J. Hollman, S. D. Stranks, K. Wojciechowski, R. Avolio, G. Grancini, A. Petrozza and H. J. Snaith, Nano Lett., 2014, 14, 3247 CrossRef CAS PubMed.
- D. Credgington and J. R. Durrant, J. Phys. Chem. Lett., 2012, 3, 1465 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Absorption spectra, CV diagrams, TGA curves, cross-sectional SEM images, J–V hysteresis curves, and photovoltaic parameters of the devices with PPDI-F3N at different concentrations. See DOI: 10.1039/c7qm00221a |
| This journal is © the Partner Organisations 2017 |