A new approach to switchable photochromic materials by combining photochromism and piezochromism together in an AIE-active molecule†
Tao
Yu
a,
Depei
Ou
a,
Leyu
Wang
a,
Shizhao
Zheng
a,
Zhiyong
Yang
a,
Yi
Zhang
 *a,
Zhenguo
Chi
*a,
Zhenguo
Chi
 *a,
Siwei
Liu
a,
Jiarui
Xu
a and
Matthew P.
Aldred
b
*a,
Siwei
Liu
a,
Jiarui
Xu
a and
Matthew P.
Aldred
b
aPCFM Lab, GD HPPC Lab, Guangdong Engineering Technology Research Center for High-Performance Organic and Polymer Photoelectric Functional Films, State Key Laboratory of Optoelectronic Material and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou, 510275, P. R. China. E-mail: ceszy@mail.sysu.edu.cn; chizhg@mail.sysu.edu.cn
bDepartment of Chemistry, Durham University, DH1 3LE, UK
First published on 2nd June 2017
Abstract
A carbazole containing triphenylethylene derivative (TrPEBCar) has been designed and synthesized, which combines aggregation-induced emission (AIE), piezochromism and photochromism together. In the crystalline state, an intensive emission band at 448 nm was detected. After pressing the crystalline solid, a [crystalline–amorphous] morphological transition takes place, in which the resulting amorphous state of TrPEBCar exhibits a different color and a red-shifted emission band at 470 nm. Photochromic behavior of TrPEBCar is achieved by utilizing a stilbene-type intramolecular photocyclization in the crystalline state. In addition, we report for the first time morphology-dependent photochromism, in which the photochromic properties of TrPEBCar are only observed in the crystalline state but not in the amorphous state. Therefore, the methods of pressing/fuming and heating/fuming act as gates to switch the photochromic properties by changing the aggregation states between the crystalline and amorphous states. By combining the piezochromic properties with the photochromic properties, the ON/OFF states of TrPEBCar can easily be observed by the different colors and emission properties. The triphenylethylene derivative with simple molecular structure provides a new approach for gated photochromic materials with both switch and indicator.
Introduction
Photochromic materials have been widely investigated for their emerging potential applications in security markings, optical shutters, photo-switchable molecular devices, topographical change materials and optical memory storage systems.1 Combining gated properties to photochromic materials is considered as an emerging development in nondestructive readout and multi-responsive photochromic materials.2–7 Several physical or chemical methods such as temperature,2 acidity,3 hydrogen bonding,4 ion-binding,5 chemical reactions6 and oxidation/reduction7 have been investigated as gates to switch or control the photochromic properties. However, the development of gated photochromic materials has been restricted due to a limited number of suitable materials and the complex synthetic methods employed to make them. In addition, the difficulty in realizing real-time monitoring techniques to investigate the ON/OFF states has also become an important issue to be solved.2–7In recent years, there has been a growing interest regarding AIE-active materials, which can overcome the shortcomings of materials that exhibit significant fluorescence loss due to aggregation-caused quenching (ACQ).8 For AIE-active materials, emission intensities are significantly enhanced in the solid-state where the restriction of intramolecular rotation can efficiently occur. Several AIE-active molecular systems have been designed and developed, such as silole derivatives,9 tetraphenylethene derivatives,10 triphenylethylene derivatives11 and others.12 In addition, some interesting properties of AIE-active materials, such as self-assembling properties,13 piezochromic properties14 and very recently photochromic properties15 have also been discovered and investigated. For these piezochromic materials, the different aggregation states (crystalline or amorphous) can easily be altered and converted by pressing processes that is mainly attributed to the twisted packing structures of the AIE-active molecules. Subsequently, the colors and emission bands can be drastically altered.
Triphenylethylene derivatives have been investigated, separately, for their piezochromic and photochromic properties.14,15 Furthermore, the photochromic process of some previously reported triphenylethylene derivatives are associated with their aggregation states (crystalline or amorphous).15b In this regard, we envisaged that piezochromism and photochromism might be realized together in a triphenylethylene derivative with rational molecular design, and external stimuli could play as a switch to control the photochromic properties by changing the aggregation state. As previous literature reported, an electron withdrawing group in triphenylethylene derivatives could promote the photochromic process.1b,15 In addition, rigid and bulky groups might lead to the twisted packing mode in the solid-state and bring about the piezochromic phenomenon. Herein, we report two boronic acid pinacol ester containing AIE-active photochromic compounds TrPEB and TrPEBCar, as shown in Fig. 1. It was discovered that TrPEBCar exhibits multi-responsive properties (both photochromism and piezochromism) and AIE properties. Furthermore, the photochromic properties can be easily tuned by controlling the aggregation state (crystalline or amorphous). Thus, pressing/fuming and heating/fuming processes can influence the aggregation state and, consequently, act as gates to switch the photochromic properties. In addition, different colors and emission bands are observed in the crystalline and amorphous aggregation states. These features are able to promote real-time monitoring of the gated photochromic ON/OFF states.
Results and discussion
Synthesis and characterization
The synthetic details for TrPEB and TrPEBCar are shown in the ESI† following literature procedures for previously reported triphenylethylene-based systems.11a The chemical structures of TrPEB and TrPEBCar were characterized by 1H-NMR spectroscopy, EI mass spectrometry and the purity was evaluated by elemental analysis (see ESI†).AIE and piezochormism studies
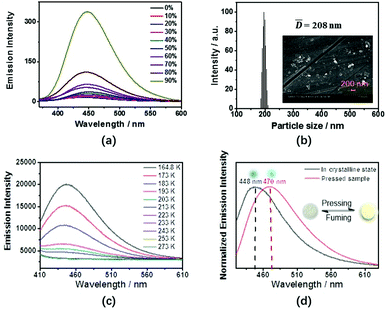
Triphenylethylene is a typical π-conjugated unit that can be used for the construction of AIE-active molecules.11 Indeed, TrPEB and TrPEBCar both display AIE properties. The emission spectra of compound TrPEBCar in a THF/water mixed solvent system with varying amounts of water at room temperature is shown in Fig. 2(a). The emission band of TrPEBCar in dilute THF is not detectable, which is attributed to the intramolecular rotations.8 However, the emission intensity is slowly enhanced with the increment of the water fraction. When the water fraction reaches 90% (v/v), the PL intensity is boosted with an emission peak at ca. 448 nm. This fluorescence enhancement indicates that the aggregates are formed and that intramolecular rotation is restricted, as is typically observed in AIE-active molecules. Dynamic light scattering (DLS) studies were performed on the solution of TrPEBCar in 90% water/THF (v/v) to investigate the particle size distribution. As shown in Fig. 2(b), particle size analyses reveal the existence of particles with average size of![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) = 208 nm. To further confirm the size of the nanoparticles, scanning electron microscopy (SEM) studies were performed with the same solution and is shown in the inset picture of Fig. 2(b). From the SEM results, the size of the nanoparticles were in accordance with the DLS data. To demonstrate the rotational properties of TrPEBCar in the dilute solution state and its effects on fluorescence properties, temperature-dependent emission studies (from 164.8 K to 273 K) were carried out for TrPEBCar in THF solution (Fig. 2(c)). At 164.8 K, a strong emission band can be detected, wherein the emission intensity decreases with increasing temperature. When the temperature increases to 273 K, negligible fluorescence is observed and the once prominent emission band has decreased so much to become indiscernible. These results are ascribed to the restriction of the intramolecular rotations and vibrations of TrPEBCar at low temperature and the blocking of the non-radiative decay processes. Similar results were also reported for other AIE-active molecules in the previous literature.9b In addition, the emission band mainly originates from the π–π* transition of TrPEBCar according to the previous reports.11a
= 208 nm. To further confirm the size of the nanoparticles, scanning electron microscopy (SEM) studies were performed with the same solution and is shown in the inset picture of Fig. 2(b). From the SEM results, the size of the nanoparticles were in accordance with the DLS data. To demonstrate the rotational properties of TrPEBCar in the dilute solution state and its effects on fluorescence properties, temperature-dependent emission studies (from 164.8 K to 273 K) were carried out for TrPEBCar in THF solution (Fig. 2(c)). At 164.8 K, a strong emission band can be detected, wherein the emission intensity decreases with increasing temperature. When the temperature increases to 273 K, negligible fluorescence is observed and the once prominent emission band has decreased so much to become indiscernible. These results are ascribed to the restriction of the intramolecular rotations and vibrations of TrPEBCar at low temperature and the blocking of the non-radiative decay processes. Similar results were also reported for other AIE-active molecules in the previous literature.9b In addition, the emission band mainly originates from the π–π* transition of TrPEBCar according to the previous reports.11a
To investigate the piezochromic properties of compounds TrPEB and TrPEBCar, emission spectra of non-pressed crystalline and pressed samples were recorded. For compound TrPEBCar piezochromism is observed. The emission maxima of TrPEBCar is red-shifted from 448 nm to 470 nm after pressing (Fig. 2(d)) and the color of the sample also changes from white to yellow, as shown in the inset pictures of Fig. 2(d). Consistent with the previous literature regarding AIE piezochromic materials, the piezochromic properties of TrPEBCar can be mainly attributed to its twisted molecular structure in the crystalline state.14a After pressing the sample, in which the crystalline state is converted to the amorphous state, the molecular configuration becomes more planar, which leads to a narrower π–π* energy gap.14 Differential scanning calorimetry (DSC) studies of TrPEBCar in the crystalline and amorphous states were carried out and the DSC scans are shown in Fig. S3 (ESI†). For the pressed sample a glass transition temperature (Tg) is detected at ca. 146 °C but not in the non-pressed crystalline sample, which reveals the aggregation state of TrPEBCar changes from a crystalline morphology to the amorphous state after pressing. The changes in morphology after pressing were further investigated by powder X-ray diffraction (pXRD) studies (vide infra). However, for compound TrPEB, no significant changes in the emission properties are observed after pressing. By comparing the structures of TrPEB and TrPEBCar, it can be concluded that the presence of the two bulky and rigid carbazole units bestows its piezochromic properties.
Switchable photochromism studies
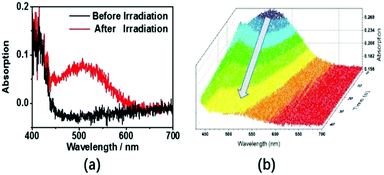
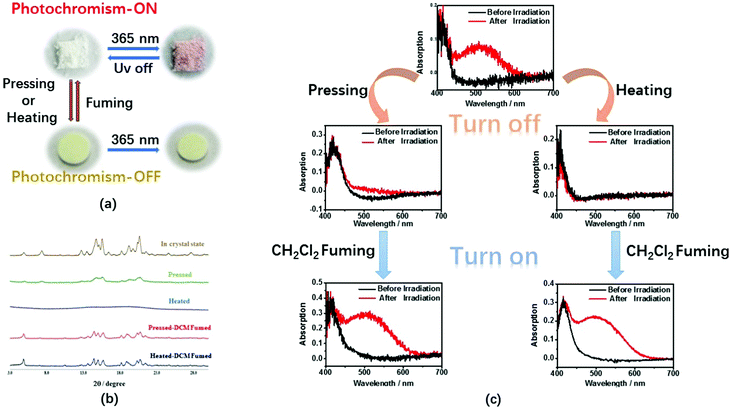
Photochromism of triphenylethylene derivatives and other AIE-active systems has been reported previously and is attributed to stilbene-type 6-π electron ring closures.15 TrPEB and TrPEBCar also exhibit similar photochromic properties in the crystalline state. The color of crystalline TrPEBCar changes from white to red within 30 s under UV-light irradiation (365 nm), and the blue emission decreases during the photochromic process. When the UV-light irradiation ceases, within 1 minute the red crystals revert back into white crystals, and under UV-light the blue emission is able to be detected again. UV-vis reflectance spectroscopy studies were carried out for the crystals of the two compounds to further investigate their photochromic properties. The spectra for TrPEBCar are shown in Fig. 3. As shown in Fig. 3(a), a low-energy absorption band at around 510 nm is observed after irradiation, which suggests an increase in conjugation within the molecule. Time dependent UV-vis reflectance spectra for the reverse ring-opening process of an irradiated TrPEBCar sample is shown in Fig. 3(b). The absorption band at 510 nm starts to decrease as soon as the irradiation stops and disappears after 45 s (ring-opening time). Similar measurements have also been carried out for compound TrPEB and are shown in Fig. S4 and S5 (ESI†). The ring-closing reaction of TrPEBCar and the mechanism of the photochromic process, which is similar to previous literature reports, are shown in Fig. S8 (ESI†).15b During the photochromic processes, no photo-oxidation reaction is detected,16 which is ascribed to the weak electron-withdrawing nature of the boronic acid pinacol ester group and/or due to the fact that the photochemistry is taking place in the solid-state and not in the solution-state.The ring-closing reaction of triphenylethylene derivatives are related to the aggregation state (crystalline or amorphous).15b For the bulky carbazole containing molecule TrPEBCar, the photochromism is quite different in the crystalline state compared to the amorphous state. We investigated the relationship between the photochromic properties and the morphology, in which photochromic studies of TrPEB and TrPEBCar were carried out with the samples in both crystalline and amorphous states and the UV-vis reflectance spectra recorded (Fig. S4–S7, ESI†). These results reveal that the photochromic process of TrPEB can be observed in both the crystalline state and amorphous state and is independent of morphology. However, on the contrary the photochromism of TrPEBCar just occurs in the crystalline state, not in the amorphous state and is, therefore, dependent on morphology. Although the amorphous state should be more disordered than the crystalline state, the molecules in the amorphous phase can be rigidly bound. There is evidence in the previous literature that planarization in triarylethylene-based materials takes place in the amorphous state compared to the crystalline state. This planarized conformation could enhance the packing and molecular rigidity,14 and consequently the restricted molecular conformations might prevent the necessary chemistry for ring closure taking place. This results in a loss of photochromism for TrPEBCar in the amorphous state. Combining this with the aforementioned piezochromic studies, pressing/fuming, heating/fuming are possible gates for the photochromic properties of TrPEBCar by switching the aggregation state between crystalline and amorphous. Therefore, these external stimuli could act as switches to turn off/turn on the photochromic properties of TrPEBCar. Fig. 4(a) demonstrates the process of turning off/turning on the photochromism using pressing/fuming as a switch. For the samples in the crystalline state, the color can be changed into red after irradiation, which is named photochromism ON-state. After pressing, no obvious color change is detected after irradiation indicating that the photochromism is in the OFF-state. In addition, the ON/OFF state can be easily distinguished by the color and emission properties of sample, as shown in Fig. 4(a). TrPEBCar in the OFF-state (pressed sample) is turned yellow and displays a red-shifted emission band, which is in accordance with the piezochromic properties. To further investigate the photochromic properties, temperature-dependent photochromic studies were performed for TrPEBCar both in the crystalline state and in the amorphous state (Fig. S9, ESI†). By gradually increasing the temperature, the photochromism of TrPECar in the crystalline state becomes weaker and finally undetectable. These results are mainly attributed to the unstable structure of the ring-closed form of TrPEBCar at elevated temperatures, according to the previous literatures.1b,2a For TrPEBCar in the amorphous state, no photochromism is observed at all the different temperatures. To further prove the photochromic properties are dependent on the aggregation state (crystalline or amorphous), UV-vis reflectance studies and XRD measurements were carried out for the TrPEBCar samples in different states: (1) sample in the crystalline state; (2) pressed sample; (3) sample in the crystalline state heated to 240 °C for 3 minutes; (4) pressed sample fumed with CH2Cl2 for 10 minutes; (5) sample in crystalline state heated to 240 °C for 3 minutes and fumed with CH2Cl2 for 10 minutes. The XRD spectra and UV-vis reflectance spectra of these samples are shown in Fig. 4(b and c), respectively. After pressing or heating, the peaks in the XRD pattern of TrPEBCar becomes more diffuse and less defined compared to the crystalline state. This indicates that most of the sample has been converted to the amorphous state from the crystalline state.17 Concurrently, negligible photochromic properties are observed in the amorphous state (OFF-state), which is shown in both the spectra and pictures in Fig. 4(a and c). For both the pressed sample and the heated sample, the XRD spectra becomes sharper and more defined after solvent fuming with dichloromethane, which subsequently turns on the photochromism (ON-state), as shown in Fig. 4(b and c). In addition, with regards to TrPEBCar the absorption and emission bands are quite different between the ON and OFF states of the gated photochromic material. Therefore, it is straightforward to conduct real-time monitoring of the ON/OFF states by distinguishing the color and emission properties.
Conclusions
In summary, a carbazole containing triphenylethylene derivative TrPEBCar has been designed and successfully synthesized to act as a switchable photochromic material. We have demonstrated that TrPEBCar exhibits AIE, piezochromic and photochromic properties. In relation to the piezochromic properties, colors and emission bands of TrPEBCar are drastically changed by switching the aggregation state between the crystalline state and the amorphous state. UV-vis reflectance spectroscopy studies and XRD studies have been performed to demonstrate that the photochromism of TrPEBCar is very much related to the aggregation state (crystalline or amorphous). Although TrPEBCar exhibits photochromism in the crystalline state, no photochromism is observed in the amorphous state. Therefore, the photochromic properties are easily manipulated by changing the aggregation state. Pressing/fuming or heating/fuming can play the role as switches to turn off/turn on the photochromic properties. It provides a rare example of a gate photochromic material whose photochromism can be switched by pressing/fuming and heating/fuming processes. When we combine this with the piezochromic properties, the ON/OFF states of the photochromism can be easily monitored by the absorption or emission properties/spectra. Therefore, the multifunctional triphenylethylene derivative presents a new strategy for designing switchable photochromic materials with real-time monitoring properties.Acknowledgements
The authors gratefully acknowledge the financial support from the NSF of China (51473185, 51603232, 21672267), 863 Program (SS2015AA031701), the Fundamental Research Funds for the Central Universities, and Guangdong Science and Technology Plan (2015B090913003, 2015B090915003).Notes and references
- (a) R. M. Kellogg, M. B. Groen and H. Wynberg, J. Org. Chem., 1967, 32, 3093 CrossRef CAS; (b) M. Irie, Chem. Rev., 2000, 100, 1685 CrossRef CAS PubMed; (c) H. Tian and S. Yang, Chem. Soc. Rev., 2004, 33, 85 RSC; (d) F. M. Raymo and M. Tomasulo, Chem. Soc. Rev., 2005, 34, 327 RSC; (e) J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378 CrossRef CAS PubMed; (f) G. M. Tsivgoulis and J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1995, 34, 1119 CrossRef CAS; (g) S. Nakamura, S. Yokojima, K. Uchida and T. Tsujioka, J. Photochem. Photobiol., C, 2011, 12, 138 CrossRef CAS; (h) K. Uchida, N. Nishikawa, N. Izumi, S. Yamazoe, H. Mayama, Y. Kojima, S. Yokojima, S. Nakamura, K. Tsujii and M. Irie, Angew. Chem., Int. Ed., 2010, 49, 5942 CrossRef CAS PubMed; (i) D. Kitagawa, I. Yamashita and S. Kobatake, Chem. Commun., 2010, 46, 3723 RSC.
- (a) D. Dulić, T. Kudernac, A. Pužys, B. L. Feringa and B. J. Wees, Adv. Mater., 2007, 19, 2898 CrossRef; (b) A. Julià-López, J. Hernando, D. Ruiz-Molina, P. González-Monje, J. Sedó and C. Roscini, Angew. Chem., Int. Ed., 2016, 55, 1 CrossRef PubMed.
- (a) K. Yumoto, M. Irie and K. Matsuda, Org. Lett., 2008, 10, 2051 CrossRef CAS PubMed; (b) S. H. Kawai, S. L. Gilat and J. M. Lehn, Eur. J. Org. Chem., 1999, 2359 CrossRef CAS.
- M. Irie, O. Miyatake and K. Uchida, J. Am. Chem. Soc., 1992, 114, 8715 CrossRef CAS.
- (a) M. Takeshita, C. F. Soong and M. Irie, Tetrahedron Lett., 1998, 39, 7717 CrossRef CAS; (b) C. T. Poon, W. H. Lam and V. W. W. Yam, J. Am. Chem. Soc., 2011, 133, 19622 CrossRef CAS PubMed.
- (a) V. Lemieux and N. R. Branda, Org. Lett., 2005, 7, 2969 CrossRef CAS PubMed; (b) V. Lemieux, S. Gauthier and N. R. Branda, Angew. Chem., Int. Ed., 2006, 45, 6820 CrossRef CAS PubMed.
- (a) M. Irie, O. Miyatake, K. Uchida and T. Eriguchi, J. Am. Chem. Soc., 1994, 116, 9894 CrossRef CAS; (b) X. Li, Y. Ma, B. Wang and G. Li, Org. Lett., 2008, 10, 3639 CrossRef CAS PubMed; (c) J. C.-H. Chan, W. H. Lam, H.-L. Wong, W. T. Wong and V. W.-W. Yam, Angew. Chem., Int. Ed., 2013, 52, 11504 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC.
- (a) G. Yu, S. Yin, Y. Liu, J. Chen, X. Xu, X. Sun, D. Ma, X. Zhan, Q. Peng, Z. Shuai, B. Tang, D. Zhu, W. Fang and Y. Luo, J. Am. Chem. Soc., 2005, 127, 6335 CrossRef CAS PubMed; (b) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC.
- (a) Y. Dong, J. W. Y. Lam, A. Qin, J. Liu, Z. Li, B. Z. Tang, J. Sun and H. S. Kwok, Appl. Phys. Lett., 2007, 91, 011111 CrossRef; (b) C. Y. K. Chan, Z. Zhao, J. W. Y. Lam, J. Liu, S. Chen, P. Lu, M. Faisal, X. Chen, H. H. Y. Sung, H. S. Kwok, Y. Ma, I. D. Williams, K. S. Wong and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 378 CrossRef CAS; (c) L. Chen, G. Lin, H. Peng, S. Ding, W. Luo, R. Hu, S. Chen, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, Mater. Chem. Front., 2017, 1, 176 RSC; (d) J. Yang, L. Li, Y. Yu, Z. Ren, Q. Peng, S. Ye, Q. Li and Z. Li, Mater. Chem. Front., 2017, 1, 91 RSC.
- (a) Z. Yang, Z. Chi, T. Yu, X. Zhang, M. Chen, B. Xu, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2009, 19, 5541 RSC; (b) H. Li, Z. Chi, B. Xu, X. Zhang, Z. Yang, X. Li, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2010, 20, 6103 RSC; (c) W. Qin, Z. Yang, Y. Jiang, J. W. Y. Lam, G. Liang, H. S. Kwok and B. Z. Tang, Chem. Mater., 2015, 27, 3892 CrossRef CAS.
- (a) H. C. Su, O. Fadhel, C. J. Yang, T. Y. Cho, C. Fave, M. Hissler, C. C. Wu and R. Reau, J. Am. Chem. Soc., 2006, 128, 983 CrossRef CAS PubMed; (b) Y. S. Zheng and Y. J. Hu, J. Org. Chem., 2009, 74, 5660 CrossRef CAS PubMed; (c) K. Shiraishi, T. Kashiwabara, T. Sanji and M. Tanaka, New J. Chem., 2009, 33, 1680 RSC; (d) K. Kokado and Y. Chujo, Macromolecules, 2009, 42, 1418 CrossRef CAS.
- (a) X. Yan, M. Wang, T. R. Cook, M. Zhang, M. L. Saha, Z. Zhou, X. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2016, 138, 4580 CrossRef CAS PubMed; (b) C. Ge, Y. Liu, X. Ye, X. Zheng, Q. Han, J. Liu and X. Tao, Mater. Chem. Front., 2017, 1, 530 RSC.
- (a) Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878 RSC; (b) Y. Dong, B. Xu, J. Zhang, X. Tan, L. Wang, J. Chen, H. Lv, S. Wen, B. Li, L. Ye, B. Zou and W. Tian, Angew. Chem., Int. Ed., 2012, 51, 10782 CrossRef CAS PubMed; (c) T. Xie, B. Zhang, X. Zhang and G. Zhang, Mater. Chem. Front., 2017, 1, 693 RSC.
- (a) Z. He, L. Shan, J. Mei, H. Wang, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, X. Gu, Q. Miao and B. Z. Tang, Chem. Sci., 2015, 6, 3538 RSC; (b) D. Ou, T. Yu, Z. Yang, T. Luan, Z. Mao, Y. Zhang, S. Liu, J. Xu, Z. Chi and M. R. Bryce, Chem. Sci., 2016, 7, 5302 RSC; (c) X. Gu, E. Zhao, T. Zhao, M. Kang, C. Gui, J. W. Y. Lam, S. Du, M. M. T. Loy and B. Z. Tang, Adv. Mater., 2016, 28, 5064 CrossRef CAS PubMed.
- M. P. Aldred, C. Li and M. Q. Zhu, Chem. – Eur. J., 2012, 18, 16037 CrossRef CAS PubMed.
- W. A. Morris, T. Butler, M. Kolpaczynska and C. L. Fraser, Mater. Chem. Front., 2017, 1, 158 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of TrPEB and TrPEBCar; physical measurements and instrumentations; DSC scans and UV-vis absorption/reflectance spectra. See DOI: 10.1039/c7qm00160f |
| This journal is © the Partner Organisations 2017 |