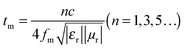Reduced graphene oxide modified mesoporous FeNi alloy/carbon microspheres for enhanced broadband electromagnetic wave absorbers†
Juan
Li
a,
Lei
Wang
 b,
Dong
Zhang
c,
Yue
Qu
a,
Guangming
Wang
a,
Ge
Tian
a,
Anhua
Liu
ad,
Huijuan
Yue
b,
Dong
Zhang
c,
Yue
Qu
a,
Guangming
Wang
a,
Ge
Tian
a,
Anhua
Liu
ad,
Huijuan
Yue
 *ad and
Shouhua
Feng
*ad and
Shouhua
Feng
 a
a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: huijuan@jlu.edu.cn
bKey Laboratory of Eco-chemical Engineering, Ministry of Education, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
cKey Laboratory of Physics and Technology for Advanced Batteries, Ministry of Education, College of Physics, Jilin University, Changchun 130012, P. R. China
dKey Laboratory of High Performance Ceramic Fibers of Ministry of Education, College of Materials, Xiamen University, Xiamen 361005, China
First published on 27th April 2017
Abstract
Absorbers have been investigated widely so as to eliminate or at least significantly attenuate the hazards of electromagnetic radiation. A porous structure is believed to be beneficial for the high-performance of microwave absorption. Here, an embedded magnetic mesoporous composite (FeNi alloyed porous carbon microspheres, FeNi/CS) is for the first time evaluated as a microwave absorbing material. Upon combining reduced graphene oxide (rGO) with the FeNi/CS composite, a multiple-component absorber of FeNi/CS/rGO is synthesized via hydrothermal and freeze-drying processes. Compared to unmodified FeNi/CS, the FeNi/CS/rGO composite provides an effective component and a more specific structure, which is favorable for translating microwave into thermal energy or other forms of energy. The minimum reflection loss (RL) value of the FeNi/CS/rGO composite reaches −45.2 dB at a thickness of 1.5 mm, and the maximum effective microwave absorption bandwidth (RL < −10 dB) is up to 5.0 GHz at d = 1.5 mm. In virtue of the dielectric loss, magnetic loss, unique heterostructure of the absorber, and impedance matching, the FeNi/CS/rGO composite exhibits overwhelming advantages of low density, small thickness, broad bandwidth, strong absorption and high anti-oxidation capability.
1. Introduction
Electromagnetic (EM) wave pollution escaping from expanded electronic equipment and communication facilities has gradually become one of the main pollution problems associated with human health and the living environment.1–5 By converting the incident EM wave energy into thermal energy or other forms of energy or dissipating them through interference, microwave absorbers have been recognized as an effective solution to solve such issues.6,7 An ideal microwave absorbing material is now required to have the features of lightweight, small thickness, wide absorption bandwidth, strong absorption and antioxidant capability.8,9 According to the basic principle of microwave absorption, the loss mechanism of a microwave absorber consists of magnetic loss and dielectric loss.3,10,11 Therefore, plenty of materials are researched as microwave absorbers, such as those with a high dielectric constant (carbon materials,12–15 zinc oxide,9,16–18 silicon carbide,19etc.) or magnetic permeability2,3,20–25(Fe, Co, Ni, or ferrite, etc.).Graphene, carbon nanotubes, carbon foams, and carbon fibers, etc. have been comprehensively researched as microwave absorbing materials with dielectric loss because of their excellent physical and chemical properties, such as lightweight, thermal stability and high dielectric constant.15,26–32 Nevertheless, pure carbon-based materials suffer from the limited loss mechanism caused by impedance mismatching, thus highly limiting their practical applications.21 One effective strategy, which is the incorporation of magnetic permeability into carbon-based materials, has been widely studied to solve this problem (e.g. Ni/carbon foam,25 Fe3O4/C core–shell nanorings,33 and Fe3O4–Fe/G34 and MoS2–Ni–CNTs composites35). On the other hand, previous work has already proved that the EM absorption performance depends strongly on the microstructure besides composition.6,36,37 It was reported that materials with novel microstructures, including yolk–shell, core–shell, hollow, etc., may not only contribute considerably to microwave absorption due to their interfacial polarization, confinement effect, and synergistic effect, but can also create highly chemical homogeneity for good functional reproducibility, for example, hollow carbon nanospheres,6 yolk–shell C@C microspheres,7 Ni–CuO core–shell composites,38 Ni–TiO2 core–shell microspheres,39 hollow polydopamine@α-MnO2 microspindles,40 core–shell PPy@PANI composites,41 and yolk–shell ellipsoidal Fe3O4@CuSilicate.42 The absorption properties of materials can be improved with porous structures by strong reflections and scattering of electromagnetic waves. In this regard, a lot of effort has been devoted to the preparation of absorbers with porous structures.11,20,43 Yuan et al. synthesized three-dimensionally ordered arrays of core–shell Fe3O4@mesoporous carbon materials.11 The synthesized magnetic porous material displayed excellent microwave absorption performance due to the existence of a mesoporous and specifically ordered core–shell structure. Chen et al. fabricated porous Fe3O4/carbon core/shell nanorods, and the absorption bandwidth with the reflection loss value below −18 dB was up to 10.5 GHz for the absorber with a thickness of 2–5 mm.20 Despite high-performance microwave absorption being obtained, the synthetic process of these materials is relatively complex.
As is known, the FeNi alloy is one of the most widely used magnetic materials in EM wave absorber application due to its high saturation magnetization, low coercivity and high Snoek's limit at high-frequency bands, which is beneficial to the impedance matching behavior and permeability values including the real part (μ′) and the imaginary part (μ′′).44–48 In our previous work, FeNi alloyed porous carbon microspheres (FeNi/CS) have been greenly and facilely synthesized. Tests disclosed that the morphology of the composite is mesoporous carbon microspheres and the FeNi alloy nanoparticles are embedded in the carbon matrix uniformly.49 The presence of numerous interfaces in this structure can lead to strong interface polarization, and it benefits the conversion of the incidence electromagnetic wave into thermal energy. Carbonaceous spheres as templates with nanometer-to-micrometer dimensions are utilized for designing multifarious morphologies with many valuable applications.50–54 However, there have been few reports on the EM wave absorption properties of this sort of embedded magnetic mesoporous composites, compared to the other porous absorbers.
Herein, we investigated the performance of FeNi alloyed porous carbon microspheres (FeNi/CS) as a microwave absorbing material in detail. Furthermore, graphene was introduced by a hydrothermal and freeze-drying process, which modified the material to form a unique embedded structure of the FeNi/CS/rGO composite with much enhanced EM wave absorption performance. The minimum reflection loss (RL) value of the FeNi/CS/rGO composite reaches −45.2 dB at an extremely small thickness of 1.5 mm. The corresponding absorption mechanism of the obtained composite is discussed in detail.
2. Materials and methods
2.1 Materials
All the chemicals were of analytical grade and used without further purification. GO used in this work was prepared from natural graphite powder (282863, Sigma-Aldrich). Iron(III) nitrate nonahydrate, nickel(II) nitrate hexahydrate, and D-glucose were purchased from Sinopharm Chemical Reagent Co. Ltd, China. Alcohol (C2H5OH) was purchased from Tianjin Tiantai Fine Chemical Reagent Co. Ltd, China. Deionized water was employed to prepare the solutions in our experiments.2.2 Preparation of graphene oxide (GO)
Graphene oxide (GO) was synthesized by using natural graphite according to a modified Hummer's method. The detailed process is shown in the ESI.†2.3 Preparation of FeNi alloy/carbon spheres (FeNi/CS)
The FeNi/CS composite was synthesized using an in situ method by glucose-induced carbonization and self-reduction, according to the published literature by our group earlier.49 First, 0.36 mmol of iron(III) nitrate nonahydrate, 2.5 mmol of nickel(II) nitrate hexahydrate, and 7.6 mmol of D-glucose were completely dissolved in 10 mL of deionized water; then, the mixed solution was diverted into a 30 mL Teflon-lined stainless-steel autoclave and kept at 180 °C for 22 h in an electric oven. Subsequently, the black precipitate was washed with ethanol/water several times and dried at 80 °C in the oven overnight. Lastly, the dried sample was carbonized at 700 °C for 2 h under the protection of an inert argon flow in a tube furnace.2.4 Preparation of rGO-modified FeNi alloy/carbon spheres (FeNi/CS/rGO)
To prepare FeNi/CS/rGO, 30 mg of GO was dispersed in 10 mL of deionized water by means of ultrasonication for 30 min. Then, 0.1 g of FeNi/CS composites were added into the above solution under sonication. Finally, the resultant solution was transferred into a 30 mL Teflon-lined stainless autoclave and kept in an oven at 180 °C for 22 h. After cooling to room temperature, the resultant sample was washed with ethanol/water and then freeze-dried overnight.2.5 Characterization
The crystal structure and phase composition of the obtained samples were analyzed by powder X-ray diffraction (XRD) on a Rigaku D/MAX 2550 V/PC diffractometer with Cu Kα radiation operating at 50 kV and 200 mA in an ambient environment. The morphology and energy dispersive X-ray spectroscopy (EDS) analysis were characterized by scanning electron microscopy (SEM, JSM-6700F) and transmission electron microscopy (TEM, Tecnai G2 S-Twin F20). X-ray Photoelectron Spectroscopy (XPS) measurements were performed using an ESCALAB250 photoelectron spectrometer equipped with a charge neutralizer and an Mg Kα X-ray source. The elemental composition was characterized by CHNS elemental analysis (Elementer Vario Micro). The magnetic properties were tested by a magnetic property measurement system (SQUID-VSM) at 300 K. The Raman spectrum was measured using a Micro-Raman system (Renishaw, model INVIA), equipped with an Ar laser (514.5 nm). The N2 adsorption–desorption isotherms for determining the BET-specific surface area and pore volume are evaluated using an ASAP 2420 system (Mckesson Corporation, USA).2.6 Property measurement
The electromagnetic wave absorption properties of the obtained samples were studied using a vector network analyzer (Agilent, N5222A). The tested samples were prepared by uniformly mixing the as-prepared samples with paraffin in a mass fraction of 50 wt%, and the sample-to-paraffin mixture was pressed into a toroidal shape with an outer diameter of 7.0 mm and an inner diameter of 3.04 mm. The electromagnetic parameters such as relative complex permittivity (ε′ and ε′′) and relative complex permeability (μ′ and μ′′) were measured between 2 GHz and 18 GHz, and the reflection loss (RL) values at different thicknesses and frequencies were calculated according to the transmission line theory.3. Results and discussion
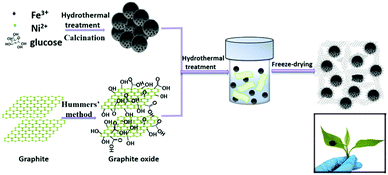
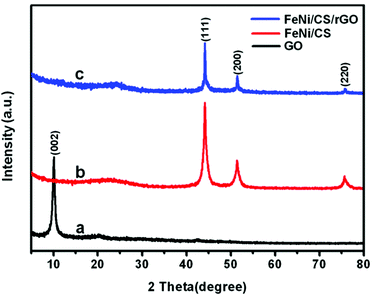
The formation mechanism of the FeNi/CS/rGO composite is schematically presented in Fig. 1. First, FeNi/CS was synthesized via a hydrothermal method/calcination process. In the meantime, graphene oxide (GO) was prepared from purified commercial graphite by using modified Hummers' method. Second, a homogeneous GO suspension was mixed with a certain amount of FeNi/CS, which was then facilely hydrothermally treated to form a cylindrical solid in the solvent. Finally, the FeNi/CS/rGO composite was achieved by a freeze-drying process. The representative photo of the obtained composite in our work demonstrated its intuitively lightweight character, which can be held up by one tree leaf.The crystalline structure and phase purity are analyzed by X-ray diffraction (XRD). Fig. 2 displays the X-ray diffraction patterns of GO, FeNi/CS and FeNi/CS/rGO composites. From Fig. 2a, it can be observed that the diffraction peak appears at 10.2° corresponding to the (002) plane of graphite oxide, indicating an increase in the interlayer distance due to the formation of the hydroxyl, carboxyl and epoxy groups between the layers.55 For FeNi/CS and FeNi/CS/rGO composites, it is clear that the diffraction peaks are located at 2θ = 44.2°, 51.4° and 75.7°, which can be well indexed to the (111), (200) and (220) crystal planes of FeNi with a cubic structure (a = 0.3552 nm, JCPDS No. 65-3244, and space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).49 The XRD result is further checked using a comparative diagram of FeNi/CS composites, FeNi/CS/rGO composites, FeO, Fe2O3, Fe3O4 and NiO (Fig. S1, ESI†). The addition of graphene oxide did not change the phase of the FeNi alloy in the final product. A weak and broad peak appears at around 24.2° which is well assigned to the (002) plane of graphitic carbon and no obvious graphene oxide peaks (10.2°) appear in the XRD patterns, indicating that all of the graphene oxide has been transformed to reduced graphene oxide in the hydrothermal process with significantly less functional groups.8,55 Above all, FeNi/CS and FeNi/CS/rGO are composite materials containing the FeNi alloy and carbon-based materials.
m).49 The XRD result is further checked using a comparative diagram of FeNi/CS composites, FeNi/CS/rGO composites, FeO, Fe2O3, Fe3O4 and NiO (Fig. S1, ESI†). The addition of graphene oxide did not change the phase of the FeNi alloy in the final product. A weak and broad peak appears at around 24.2° which is well assigned to the (002) plane of graphitic carbon and no obvious graphene oxide peaks (10.2°) appear in the XRD patterns, indicating that all of the graphene oxide has been transformed to reduced graphene oxide in the hydrothermal process with significantly less functional groups.8,55 Above all, FeNi/CS and FeNi/CS/rGO are composite materials containing the FeNi alloy and carbon-based materials.
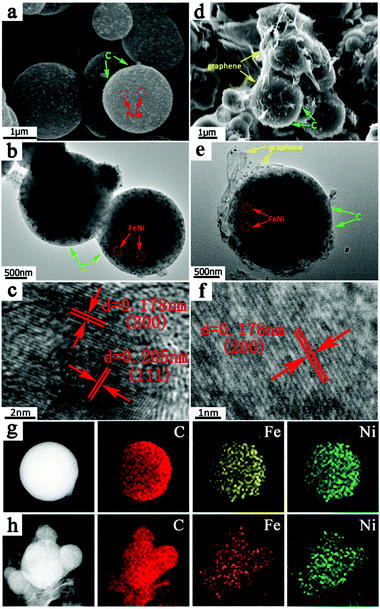
The morphology characteristics and microstructure of the obtained samples are investigated by using SEM and TEM, as shown in Fig. 3. Fig. 3a and b display the spherical shapes of the FeNi/CS composite with diameters between 1.5 and 2.5 μm, and its microstructure contains multiple small FeNi cores encapsulated uniformly by a carbon matrix, as indicated by the corresponding arrows. In the composite of FeNi/CS/rGO, there is an ultrathin rGO layer (identified by an arrow in Fig. 3d) on the surfaces of all FeNi/CS microspheres (Fig. 3d), implying the strong interaction between FeNi/CS and rGO layers. In addition, it can be seen that the adjacent microspheres are connected through crumpled rGO sheets. A closer observation is made by TEM as shown in Fig. 3e, which provides further evidence that the porous magnetic carbon microspheres are successfully encapsulated in the rGO sheets. The HRTEM images (Fig. 3c and f) well certify the crystallinity of the as-prepared FeNi/CS and FeNi/CS/rGO composites with a clearly resolved lattice spacing of around 0.178 nm and 0.205 nm, corresponding to that of (200) and (111) of the crystal structure of the FeNi alloy, respectively. No other metal oxide was detected, which is in accordance with the XRD result. This further confirms that the majority composition of the FeNi alloy still remains after a hydrothermal/freeze-drying process. Moreover, the EDS mapping images of two composites are investigated using transmission electron microscopy as shown in Fig. 3g and h. The elemental maps of Fe, Ni and C demonstrate an extremely homogeneous distribution throughout the as-prepared composites, which implies that the samples contain the FeNi alloy rather than a random mixture of Fe and Ni nanoparticles. The region of rGO in the FeNi/CS/rGO composite can be distinctly observed. This again verifies that a desirable core–shell microstructure has been successfully built through coupling FeNi/CS with rGO nanosheets. Therefore, the FeNi/CS/rGO composite can be visibly described as a rGO-wrapped core–shell structure. This unique heterostructure can take great responsibility due to its superior electromagnetic wave absorbing properties, which will be discussed later.
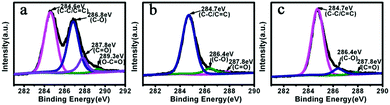
The surface composition of the samples and chemical states of the elements are analyzed by X-ray photoelectron spectroscopy (XPS). Fig. 4 shows the C 1s spectra of GO, FeNi/CS and FeNi/CS/rGO composites. As shown in Fig. 4a, the C 1s photoelectron spectra of GO consist of four common signals. The binding energy of 284.6 eV is attributed to the aliphatic carbon groups (C1 C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), that of 286.8 eV is ascribed to the epoxy groups or alkoxy (C2 C–O), and the ones of 287.8 eV and 289.3 eV are indexed to the carbonyl group (C3 C
C), that of 286.8 eV is ascribed to the epoxy groups or alkoxy (C2 C–O), and the ones of 287.8 eV and 289.3 eV are indexed to the carbonyl group (C3 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and carboxyl group (C4 O–C
O) and carboxyl group (C4 O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively, indicating that GO is highly oxidized.8 In the spectra of FeNi/CS and FeNi/CS/rGO, the peak intensities of C2 peaks and C3 peaks become distinctly weakened and C4 peaks are hardly found compared with the C 1s spectrum of GO, suggesting the strong reduction of GO by the hydrothermal process, which corresponds with the XRD result.56 The deoxygenation reduction of GO can cause good electrical conductivity and is beneficial to microwave absorption properties. The Fe 2p and Ni 2p spectra of FeNi/CS and FeNi/CS/rGO are shown in Fig. S2 (ESI†). There are peaks related to Fe0 2p3/2 (binding energies at 707.4 eV and 707.5 eV in Fig. S2(a and c), ESI†) and Ni0 2p3/2 (binding energies at 852.9 eV and 852.8 eV in Fig. S2(b and d), ESI†), which demonstrates the formation of the zero-valence state of Fe and Ni in both samples. This result is consistent with the diffraction peaks of the FeNi alloy as shown in the XRD patterns. The peaks at 712.4 eV and 712.2 eV can be assigned to oxidized Fe3+ 2p3/2. Meanwhile, the binding energies of 855.9 eV (861.1 eV, the satellite peak) and 856.0 eV (860.3 eV, the satellite peak) can be indexed to divalent Ni2+ 2p3/2 of these two composites. The appearances of Fe3+ and Ni2+ stem from the surface oxidation of the exterior FeNi alloy exposed to air because of its large surface energy.44,57–59 Taking account of the XRD patterns and after-mentioned HRTEM results, the surface composition has not affected the one of the bulk phase. Consequently, the principal composition is held to be the FeNi alloy.
O), respectively, indicating that GO is highly oxidized.8 In the spectra of FeNi/CS and FeNi/CS/rGO, the peak intensities of C2 peaks and C3 peaks become distinctly weakened and C4 peaks are hardly found compared with the C 1s spectrum of GO, suggesting the strong reduction of GO by the hydrothermal process, which corresponds with the XRD result.56 The deoxygenation reduction of GO can cause good electrical conductivity and is beneficial to microwave absorption properties. The Fe 2p and Ni 2p spectra of FeNi/CS and FeNi/CS/rGO are shown in Fig. S2 (ESI†). There are peaks related to Fe0 2p3/2 (binding energies at 707.4 eV and 707.5 eV in Fig. S2(a and c), ESI†) and Ni0 2p3/2 (binding energies at 852.9 eV and 852.8 eV in Fig. S2(b and d), ESI†), which demonstrates the formation of the zero-valence state of Fe and Ni in both samples. This result is consistent with the diffraction peaks of the FeNi alloy as shown in the XRD patterns. The peaks at 712.4 eV and 712.2 eV can be assigned to oxidized Fe3+ 2p3/2. Meanwhile, the binding energies of 855.9 eV (861.1 eV, the satellite peak) and 856.0 eV (860.3 eV, the satellite peak) can be indexed to divalent Ni2+ 2p3/2 of these two composites. The appearances of Fe3+ and Ni2+ stem from the surface oxidation of the exterior FeNi alloy exposed to air because of its large surface energy.44,57–59 Taking account of the XRD patterns and after-mentioned HRTEM results, the surface composition has not affected the one of the bulk phase. Consequently, the principal composition is held to be the FeNi alloy.
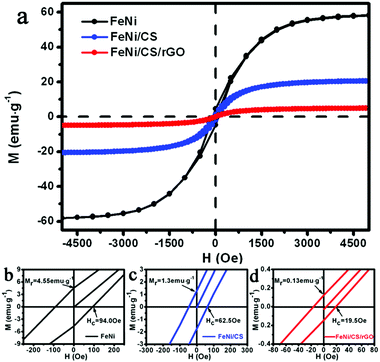
To investigate the composition-dependent magnetic properties of the as-synthesized composites, magnetic hysteresis loops at 300 K are shown in Fig. 5, in comparison to the pure FeNi alloy which was synthesized using the procedure shown in the ESI.† The M–H curves exhibit a similar S-type shape, indicating the classic ferromagnetic characteristics. The measured saturation magnetization (Ms), remanent magnetization (Mr) and coercivity (Hc) of the pure FeNi alloy are 59.6 emu g−1, 4.55 emu g−1 and 94 Oe, respectively. The CHNS elemental analysis reveals that the content of C-based materials for FeNi/CS and FeNi/CS/rGO is 46.56 wt% and 56.59 wt%, respectively. The amount of rGO in the FeNi/CS/rGO composite can be calculated to be 18.77 wt%. The various weight fractions in the composites are listed in Table S1 (ESI†). With increasing weight fraction of nonmagnetic carbon materials and relatively reduced amount of FeNi nanoparticles, the values of Ms decrease to 20.6 emu g−1 for FeNi/CS and 5.0 emu g−1 for FeNi/CS/rGO, respectively, which is consistent with the literature reports.33,60 The values of Hc and Mr drop at the same time, which can be explained by the variation in the anisotropy, defects, size effect, or heterostructures due to the introduction of carbon materials into FeNi/CS and FeNi/CS/rGO composites.61 The corresponding Ms, Mr and Hc values are plotted as a histogram in Fig. S3a (ESI†). Notably, in the case of the FeNi/CS/rGO composite, rGO is deposited on the surface of the FeNi/CS and crystallite boundaries, which covers surface defects, such as pores and cracks, thus resulting in a dramatic decrease in the downtrend of Ms, Mr and Hc values (see Fig. S3b, ESI†).62 Broadly speaking, the existence of rGO has a crucial influence on magnetic properties.
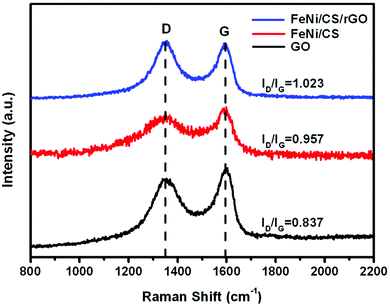
Raman spectroscopy is used to investigate the existence and graphitization degree of carbon, as shown in Fig. 6. Two characteristic peaks can be clearly observed at 1349 cm−1 (D band) and 1593 cm−1 (G band), which correspond with the disordered or defective graphitic structures and graphitic carbon, respectively. The intensity ratio (ID/IG) of GO, FeNi/CS and FeNi/CS/rGO is 0.837, 0.957 and 1.023, respectively. As is known, the ID/IG ratio is sensitive to the disordered composite, and its increasing value manifests the formation of defects.63,64 The value of the FeNi/CS/rGO composite is higher than those of GO and FeNi/CS, which can be attributed to the reduction of graphene oxide. It is well-known that proper defects and graphitization degree in carbon-based materials are beneficial for the loss of EM waves on account of improving the matched characteristic impedance and prompting energy transition from contiguous states to the Fermi level and introducing defect polarization relaxation and dipole relaxation.65
On the basis of the above characterization, it is concluded that the FeNi/CS/rGO composite has been successfully synthesized. This rGO-modified porous FeNi alloy/carbon sphere exhibits some interesting features: (I) there is a porous carbon matrix (the porous feature has also been studied and is displayed in Fig. S4, ESI†); (II) reduced graphene oxide has unusual characteristics with better conductivity, defects and residual functional groups, which are beneficial to the polarization loss and conductivity loss of the composite;36,55,66 (III) numerous interfaces exist among the core FeNi alloy, shell carbon matrix, and graphene. As a consequence, FeNi/CS/rGO can be expected to exhibit outstanding microwave absorption performance.
It is well-known that the electromagnetic wave absorption properties are generally determined by the relative complex permittivity (εr = ε′ + iε′′) and relative complex permeability (μr = μ′ + iμ′′).67 Based on previous relevant studies, the real parts (ε′, μ′) are associated with the storage capability of electric and magnetic energy, and the imaginary parts (ε′′, μ′′) represent the loss capability of electric and magnetic energy, respectively.68,69 In order to investigate the microwave absorption properties of the FeNi/CS and FeNi/CS/rGO samples, the electromagnetic parameters (ε′, ε′′, μ′, μ′′) of paraffin composites containing 50 wt% of composites are measured in the frequency region of 2–18 GHz.
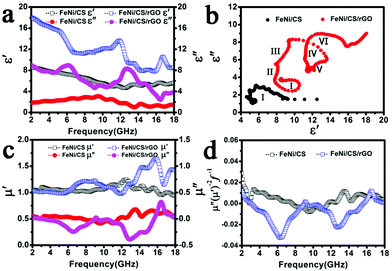
Fig. 7a shows the real part (ε′) and the imaginary part (ε′′) of the complex permittivity of the two samples. The FeNi/CS/rGO composite displays relatively higher values of ε′ and ε′′ than those of the bare FeNi/CS sample in the whole frequency range. According to the information that we are aware of, the dielectric loss values are determined by conductivity loss and polarization loss. The polarization loss commonly depends on the ionic polarization, electron polarization, electric dipolar polarization and interfacial polarization occurring in the materials. Now that the frequency of the electron and ion polarizations occurring is higher than 18 GHz (103–106 GHz), conductivity loss, interfacial polarization and dipolar polarization are responsible for the dielectric loss mechanism.6,41,70 For the FeNi/CS composite, the appearance of the relaxation peaks indicates that the polarization effects work. First, when the free charge accumulates on the multiple interfaces between the FeNi alloy, carbon matrix, paraffin and air cavities, interfacial polarization occurs in the material.40 Second, the small size of the FeNi alloy (shown in Fig. 3(b and e)) can also increase dipolar polarization.34 Third, the special structure with the FeNi alloy evenly embedded in the carbon matrix could not only generate interfacial polarization but also increase the conductivity of the sample to optimize the absorption performance. Besides the above several factors, reduced graphene oxide in the FeNi/CS/rGO composite can highly affect the dielectric parameters. The defects and residual functional groups in the rGO sheets can act as polarized centers to enhance microwave attenuation by defect polarization relaxation and groups' electronic dipolar polarization. Moreover, the introduction of rGO ameliorates the impedance matching characteristic to some degree so as to modify the EM absorption performance. Furthermore, the coating of reduced graphene oxide on the FeNi/CS composite will generate more interfaces, resulting in improved interfacial polarization.63
Conventionally, Debye dipolar relaxation can be considered as a significant mechanism to account for the dielectric loss, which can be expressed by the following equation:
| (ε′ − (εs + ε∞)/2)2 + (ε′′)2 = ((εs − ε∞)/2)2 |
According to the formula, the curve of ε′ versus ε′′ is a single semicircle denoted as the Cole–Cole semicircle and each semicircle represents one Debye relation process.10,21,39Fig. 7b shows the curve of ε′ versus ε′′ of FeNi/CS and FeNi/CS/rGO. It can be clearly seen that six Cole–Cole semicircles in FeNi/CS/rGO are displayed, while one semicircle can be found in the FeNi/CS composite, manifesting that the FeNi/CS/rGO composite has multi-dielectric relaxation processes and the extra Debye dipolar relaxation effort is caused due to the introduction of the rGO sheets.
Fig. 7c shows the real part (μ′) and the imaginary part (μ′′) of the complex permeability of the FeNi/CS and FeNi/CS/rGO composites in the 2-18 GHz frequency range. The values of μ′ appear in the range of 0.94–1.24 and 0.97–1.65, respectively and those of μ′′ are in the range of −0.09 to 0.18 and −0.40 to 0.32, respectively. The fluctuations of curves are observed in the whole frequency range. There is an obvious negative region of μ′′ values, especially for the FeNi/CS/rGO composite. According to the Maxwell equations, a magnetic field can be induced by an electric field and can be radiated out. Here, the negative value of μ′′ indicated that the magnetic energy is radiated out from the composites due to the motion of charges and transferred to electric energy.25,36 Generally, magnetic loss of magnetic materials can be explained by the magnetic hysteresis, domain-wall effect, eddy current loss, exchange resonance and natural resonance.68 On account of magnetic hysteresis occurring only in a strong applied field, it is usually ignorable in a weak applied field. The domain-wall effect occurs in a much lower frequency range in multi-domain materials, which can be usually negligible. That is to say, the samples may be governed by the eddy current loss, exchange resonance and natural resonance.18,47 Usually, natural resonance plays a dominant role at low frequency, and exchange resonance will be regarded as the dominant contributor at high frequency.18,33 The equation μ′′(μ′)−2f−1 = 2πμ0d2σ is often applied to evaluate the eddy current loss, where μ0 is the vacuum permeability, σ is the electric conductivity, and d is the diameter of the nanoparticle. According to the skin-effect criterion, if the magnetic loss only stems from the eddy current loss, the values of μ′′(μ′)−2f−1 should remain constant with varying frequency.69,71 As presented in Fig. 7d, the values of μ′′(μ′)−2f−1 for FeNi/CS reveal a trend of fluctuations in a frequency range of around 2.0–14.5 GHz and basically remain constant in the 14.5–18.0 GHz frequency range. Therefore, the magnetic loss observed is chiefly caused by natural resonance and by the eddy current loss. Nevertheless, the FeNi/CS/rGO composite exhibits two strong fluctuating peaks at 9.1 GHz and 16.5 GHz, indicating that the magnetic loss in the present material is mainly caused from the consequence of natural resonance and exchange resonance.
Fig. 8 displays the frequency dependence of the dielectric loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε = ε′′/ε′) and magnetic loss tangent (tan
δε = ε′′/ε′) and magnetic loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ = μ′′/μ′) of two samples, which represent the materials' conversion of microwave radiation into other energy forms. The higher the dielectric and magnetic loss tangents, the more the EM wave energy that gets absorbed.22 It can be found that, for FeNi/CS and FeNi/CS/rGO composites, both values of tan
δμ = μ′′/μ′) of two samples, which represent the materials' conversion of microwave radiation into other energy forms. The higher the dielectric and magnetic loss tangents, the more the EM wave energy that gets absorbed.22 It can be found that, for FeNi/CS and FeNi/CS/rGO composites, both values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε are higher than the values of tan
δε are higher than the values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ in the 2–18 GHz frequency range, indicating that the dielectric loss plays a dominant role in their microwave absorption performances. The tan
δμ in the 2–18 GHz frequency range, indicating that the dielectric loss plays a dominant role in their microwave absorption performances. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε curve of FeNi/CS exhibits fluctuation behavior as approximately a broad peak. Unlikely, this curve of FeNi/CS/rGO displays three peaks at 6.5 GHz, 12.9 GHz and 14.5 GHz, respectively, which can be interpreted as the relaxation processes. In the frequency regions of 2.0–8.7 GHz and 11.4–18.0 GHz, the values of tan
δε curve of FeNi/CS exhibits fluctuation behavior as approximately a broad peak. Unlikely, this curve of FeNi/CS/rGO displays three peaks at 6.5 GHz, 12.9 GHz and 14.5 GHz, respectively, which can be interpreted as the relaxation processes. In the frequency regions of 2.0–8.7 GHz and 11.4–18.0 GHz, the values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε of the FeNi/CS/rGO composite are higher than those of the FeNi/CS composite, stating clearly the superior dielectric loss factor in the corresponding frequency range. Apparently, the introduction of rGO into the FeNi/CS/rGO composite plays an essential role. Both of the FeNi/CS and FeNi/CS/rGO composites have relatively weak magnetic loss due to the high proportion of dielectric materials. In general, complementarity between magnetic loss and dielectric loss has a strong impact on the EM wave absorption performance. In comparison, FeNi/CS/rGO possesses more effective complementarity as a result of the contribution of reduced graphene oxide layers. It is expected that the FeNi/CS/rGO composite exhibits excellent microwave absorption performance.
δε of the FeNi/CS/rGO composite are higher than those of the FeNi/CS composite, stating clearly the superior dielectric loss factor in the corresponding frequency range. Apparently, the introduction of rGO into the FeNi/CS/rGO composite plays an essential role. Both of the FeNi/CS and FeNi/CS/rGO composites have relatively weak magnetic loss due to the high proportion of dielectric materials. In general, complementarity between magnetic loss and dielectric loss has a strong impact on the EM wave absorption performance. In comparison, FeNi/CS/rGO possesses more effective complementarity as a result of the contribution of reduced graphene oxide layers. It is expected that the FeNi/CS/rGO composite exhibits excellent microwave absorption performance.
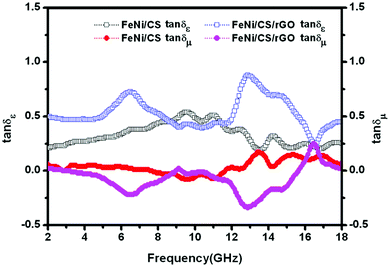 | ||
Fig. 8 Frequency dependence of the dielectric loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε) and magnetic loss tangent (tan δε) and magnetic loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ) of FeNi/CS and FeNi/CS/rGO composites. δμ) of FeNi/CS and FeNi/CS/rGO composites. | ||
The reflection loss values of the as-designed samples are calculated using the relative complex permittivity and permeability at a given frequency and absorber thickness according to the transmission line theory, which is summarized as the following equations:
Zin = Z0(μr/εr)1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tanh[j(2πfd/c)(μrεr)1/2] tanh[j(2πfd/c)(μrεr)1/2] |
RL (dB) = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log|(Zin − Z0)/(Zin + Z0)| log|(Zin − Z0)/(Zin + Z0)| |
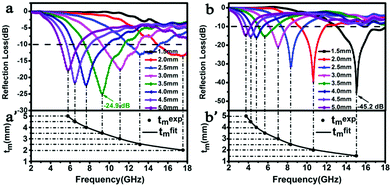
The reflection loss is measured to determine the electromagnetic wave absorption for the prepared samples, and the results are given in Fig. 9. Both of them reveal that the peaks shift to high frequency with a decrease in the thickness of the absorber. As shown in Fig. 9a, the minimum reflection loss value of the FeNi/CS composite is up to −24.9 dB at 9.2 GHz, and the effective absorption bandwidth with RL exceeding −10 dB can reach 3.9 GHz with an absorber thickness of 3.5 mm. On the basis of the above analysis, the microwave absorption ability is attributed to the weak dielectric loss, porous structure and embedded structure of FeNi alloy particles. Fig. 9b shows the reflection loss values of FeNi/CS/rGO in a thickness range of 1.5–5.0 mm. The minimum RL value is −45.2 dB and the effective absorption bandwidth of 5.0 GHz is achieved at an absorber thickness of 1.5 mm. We can easily find that the modification of FeNi/CS with rGO effectively expands the absorption bandwidth and improves the reflection loss with a smaller thickness. Hence, the addition of rGO enhances the electromagnetic (EM) performance on the whole.
To further understand the loss mechanism of the composites, the quarter-wavelength matching model will explain the electromagnetic absorption properties. From the equation:
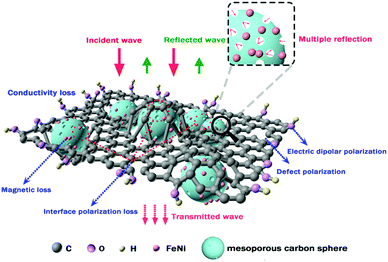
The possible EM absorption mechanism of the FeNi/CS/rGO composite is presented in Fig. 10. When microwaves irradiate on the material's surface, only a small part of the electromagnetic waves is reflected on the surface, while the vast majority can enter the interior and then are transformed into thermal energy or other forms of energy. In this work, the FeNi/CS/rGO composite is provided with an embedded and porous structure with rGO as a modifier. First, rGO sheets provide defects and residual functional groups that could cause defect polarization relaxation and electronic dipole polarization. Second, the desired features of the rGO sheets increase the conductivity loss, multiple scattering and reflection. Third, the existence of FeNi nanoparticles could generate magnetic loss. Fourth, the interfaces brought in by the FeNi nanoparticles, carbon matrix and rGO sheets have a dominant role in enhancing the dielectric performance and can also cause multiple reflections, further consuming the EM energy. However, for the FeNi/CS absorber without electric dipoles and interfaces provided by rGO, the enhancement of the interaction of microwaves does not occur. Finally, the pores residing in the magnetic carbon microspheres can not only form more defects and dangling bonded atoms, but also restrict the microwaves in the materials to produce multiple reflections and scattering, so that it is difficult for microwaves to escape from the composite until being dissipated and absorbed.11,15,25,43 Consequently, the FeNi/CS/rGO composite exhibits superior microwave absorption performance in virtue of the dielectric loss, magnetic loss, unique structure of the absorber, and better impedance matching.
4. Conclusions
In summary, an embedded magnetic mesoporous composite (FeNi/CS) was evaluated in detail as a microwave absorbing material. After modifying with rGO, the FeNi/CS/rGO composite was synthesized as a unique structure consisting of an rGO shell and an embedded FeNi/CS core. The minimum reflection loss value can reach −45.2 dB at 15.04 GHz and the effective absorption bandwidth (RL less than −10 dB) is up to 5.0 GHz with an ultra-small thickness of 1.5 mm. This composite exhibits efficient wideband microwave absorption properties, which meet the standards of ideal electromagnetic wave absorbers. The EM absorption mechanism of the composite has been explored and may be extended to fabricate other kinds of strong electromagnetic wave absorption materials.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 21401070 and 21201073), the S&T Development Program of Jilin Province (No. 20160101320JC and 20150204030GX) and the Open Fund of the Key Laboratory of High Performance Ceramic Fibers (Xiamen University), Ministry of Education (No. 201602).References
- J. Y. Shen, Y. T. Yao, Y. J. Liu and J. S. Leng, J. Mater. Chem. C, 2016, 4, 7614–7621 RSC.
- R. C. Che, L. M. Peng, X. F. Duan, Q. Chen and X. L. Liang, Adv. Mater., 2004, 16, 401–405 CrossRef CAS.
- J. J. Jiang, D. Li, D. Y. Geng, J. An, J. He, W. Liu and Z. D. Zhang, Nanoscale, 2014, 6, 3967–3971 RSC.
- M. S. Cao, W. L. Song, Z. L. Hou, B. Wen and J. Yuan, Carbon, 2010, 48, 788–796 CrossRef CAS.
- A. Ohlan, K. Singh, A. Chandra and S. K. Dhawan, ACS Appl. Mater. Interfaces, 2010, 2, 927–933 CAS.
- C. Zhou, S. Geng, X. W. Xu, T. H. Wang, L. Q. Zhang, X. J. Tian, F. Yang, H. T. Yang and Y. F. Li, Carbon, 2016, 108, 234–241 CrossRef CAS.
- R. Qiang, Y. C. Du, Y. Wang, N. Wang, C. H. Tian, J. Ma, P. Xu and X. J. Han, Carbon, 2016, 98, 599–606 CrossRef CAS.
- L. Wang, Y. Huang, X. Sun, H. J. Huang, P. B. Liu, M. Zong and Y. Wang, Nanoscale, 2014, 6, 3157–3164 RSC.
- F. Wu, Y. L. Xia, Y. Wang and M. Y. Wang, J. Mater. Chem. A, 2014, 2, 20307–20315 CAS.
- H. Wang, Y. Y. Dai, W. J. Gong, D. Y. Geng, S. Ma, D. Li, W. Liu and Z. D. Zhang, Appl. Phys. Lett., 2013, 102, 223113 CrossRef.
- K. P. Yuan, R. C. Che, Q. Cao, Z. K. Sun, Q. Yue and Y. H. Deng, ACS Appl. Mater. Interfaces, 2015, 7, 5312–5319 CAS.
- M. Mishra, A. P. Singh, V. Gupta, A. Chandra and S. K. Dhawan, J. Alloys Compd., 2016, 688, 399–403 CrossRef CAS.
- X. Bai, Y. H. Zhai and Y. Zhang, J. Phys. Chem. C, 2011, 115, 11673–11677 CAS.
- C. C. Wan and J. Li, Carbohydr. Polym., 2016, 150, 172–179 CrossRef CAS PubMed.
- G. Li, T. S. Xie, S. L. Yang, J. H. Jin and J. M. Jiang, J. Phys. Chem. C, 2012, 116, 9196–9201 CAS.
- G. S. Wang, Y. Y. Wu, X. J. Zhang, Y. Li, L. Guo and M. S. Cao, J. Mater. Chem. A, 2014, 2, 8644–8651 CAS.
- D. P. Sun, Q. Zou, Y. P. Wang, Y. J. Wang, W. Jiang and F. S. Li, Nanoscale, 2014, 6, 6557–6562 RSC.
- G. Z. Wang, X. G. Peng, L. Yu, G. P. Wan, S. W. Lin and Y. Qin, J. Mater. Chem. A, 2015, 3, 2734–2740 CAS.
- H. J. Yang, W. Q. Cao, D. Q. Zhang, T. J. Su, H. L. Shi, W. Z. Wang, J. Yuan and M. S. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 7073–7077 CAS.
- Y. J. Chen, G. Xiao, T. S. Wang, Q. Y. Ouyang, L. H. Qi, Y. Ma, P. Gao, C. L. Zhu, M. S. Cao and H. B. Jin, J. Phys. Chem. C, 2011, 115, 13603–13608 CAS.
- X. Jian, B. Wu, Y. F. Wei, S. X. Dou, X. L. Wang, W. D. He and N. Mahmood, ACS Appl. Mater. Interfaces, 2016, 8, 6101–6109 CAS.
- X. F. Zhang, Y. X. Li, R. G. Liu, Y. Rao, H. W. Rong and G. W. Qin, ACS Appl. Mater. Interfaces, 2016, 8, 3494–3498 CAS.
- Q. H. Liu, Q. Cao, H. Bi, C. Y. Liang, K. P. Yuan, W. She, Y. J. Yang and R. C. Che, Adv. Mater., 2016, 28, 486–490 CrossRef CAS PubMed.
- D. P. Sun, Q. Zou, G. Q. Qian, C. Sun, W. Jiang and F. S. Li, Acta Mater., 2013, 61, 5829–5834 CrossRef CAS.
- H. B. Zhao, Z. B. Fu, H. B. Chen, M. L. Zhong and C. Y. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 1468–1477 CAS.
- X. H. Li, J. Feng, Y. P. Du, J. T. Bai, H. M. Fan, H. L. Zhang, Y. Peng and F. S. Li, J. Mater. Chem. A, 2015, 3, 5535–5546 CAS.
- L. Wang, Y. Huang, C. Li, J. J. Chen and X. Sun, Phys. Chem. Chem. Phys., 2015, 17, 2228–2234 RSC.
- Y. L. Yang and M. C. Gupta, Nano Lett., 2005, 5, 2131–2134 CrossRef CAS PubMed.
- X. S. Qi, J. L. Xu, Q. Hu, Y. Deng, R. Xie, Y. Jiang, W. Zhong and Y. W. Du, Sci. Rep., 2016, 6, 28310 CrossRef CAS PubMed.
- Z. P. Chen, C. Xu, C. Q. Ma, W. C. Ren and H. M. Cheng, Adv. Mater., 2013, 25, 1296–1300 CrossRef CAS PubMed.
- W. L. Song, X. T. Guan, L. Z. Fan, W. Q. Cao, C. Y. Wang and M. S. Cao, Carbon, 2015, 93, 151–160 CrossRef CAS.
- R. B. Wu, Z. H. Yang, M. S. Fu and K. Zhou, J. Alloys Compd., 2016, 687, 833–838 CrossRef CAS.
- T. Wu, Y. Liu, X. Zeng, T. T. Cui, Y. T. Zhao, Y. N. Li and G. X. Tong, ACS Appl. Mater. Interfaces, 2016, 8, 7370–7380 CAS.
- B. Qu, C. L. Zhu, C. Y. Li, X. T. Zhang and Y. J. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 3730–3735 CAS.
- Y. Sun, J. L. Xu, W. Qiao, X. B. Xu, W. L. Zhang, K. Y. Zhang, X. Zhang, X. Chen, W. Zhong and Y. W. Du, ACS Appl. Mater. Interfaces, 2016, 8, 31878–31886 CAS.
- P. B. Liu, Y. Huang, J. Yan, Y. W. Yang and Y. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 5536–5546 CAS.
- X. J. Zhang, S. Li, S. W. Wang, Z. J. Yin, J. Q. Zhu, A. P. Guo, G. S. Wang, P. G. Yin and L. Guo, J. Phys. Chem. C, 2016, 120, 22019–22027 CAS.
- B. Zhao, G. Shao, B. B. Fan, W. Y. Zhao and R. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 6044–6052 RSC.
- B. Zhao, G. Shao, B. B. Fan, W. Y. Zhao, Y. J. Xie and R. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 8802–8810 RSC.
- W. She, H. Bi, Z. W. Wen, Q. H. Liu, X. B. Zhao, J. Zhang and R. C. Che, ACS Appl. Mater. Interfaces, 2016, 8, 9782–9789 CAS.
- C. H. Tian, Y. C. Du, P. Xu, R. Qiang, Y. Wang, D. Ding, J. L. Xue, J. Ma, H. T. Zhao and X. J. Han, ACS Appl. Mater. Interfaces, 2015, 7, 20090–20099 CAS.
- J. J. Xu, J. W. Liu, R. C. Che, C. Y. Liang, M. S. Cao, Y. Li and Z. W. Liu, Nanoscale, 2014, 6, 5782–5790 RSC.
- M. T. Qiao, X. F. Lei, Y. Ma, L. D. Tian, W. B. Wang, K. H. Su and Q. Y. Zhang, J. Alloys Compd., 2017, 693, 432–439 CrossRef CAS.
- T. Ma, M. W. Yuan, S. M. Islam, H. F. Li, S. L. Ma, G. B. Sun and X. J. Yang, J. Alloys Compd., 2016, 678, 468–477 CrossRef CAS.
- X. Ding, Y. Huang and M. Zong, Mater. Lett., 2015, 157, 285–289 CrossRef CAS.
- X. Ding, Y. Huang, J. G. Wang, H. W. Wu and P. B. Liu, Appl. Surf. Sci., 2015, 357(part A), 908–914 CrossRef CAS.
- X. Ding, Y. Huang and J. G. Wang, RSC Adv., 2015, 5, 64878–64885 RSC.
- X. G. Liu, B. Li, D. Y. Geng, W. B. Cui, F. Yang, Z. G. Xie, D. J. Kang and Z. D. Zhang, Carbon, 2009, 47, 470–474 CrossRef CAS.
- X. X. Liu, D. Zhang, B. Guo, Y. Qu, G. Tian, H. J. Yue and S. H. Feng, RSC Adv., 2015, 5, 93491–93498 RSC.
- S. M. Xu, C. M. Hessel, H. Ren, R. B. Yu, Q. Jin, M. Yang, H. J. Zhao and D. Wang, Energy Environ. Sci., 2014, 7, 632–637 CAS.
- J. Y. Wang, H. J. Tang, L. J. Zhang, H. Ren, R. B. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. R. Liu, Y. Zhang, Y. R. Wen, L. Gu, G. H. Ma, Z. G. Su, Z. Y. Tang, H. J. Zhao and D. Wang, Nat. Energy, 2016, 1, 16050 CrossRef CAS.
- Z. M. Li, X. Y. Lai, H. Wang, D. Mao, C. J. Xing and D. Wang, J. Phys. Chem. C, 2009, 113, 2792–2797 CAS.
- X. Y. Lai, J. Li, B. A. Korgel, Z. H. Dong, Z. M. Li, F. B. Su, J. Du and D. Wang, Angew. Chem., 2011, 123, 2790–2793 CrossRef.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 CAS.
- B. Wen, M. S. Cao, M. M. Lu, W. Q. Cao, H. L. Shi, J. Liu, X. X. Wang, H. B. Jin, X. Y. Fang, W. Z. Wang and J. Yuan, Adv. Mater., 2014, 26, 3484–3489 CrossRef CAS PubMed.
- M. Fu, Q. Z. Jiao and Y. Zhao, J. Mater. Chem. A, 2013, 1, 5577–5586 CAS.
- G. Abellán, H. Prima-García and E. Coronado, J. Mater. Chem. C, 2016, 4, 2252–2258 RSC.
- L. Ding, M. Zhang, Y. W. Zhang, J. B. Yang, J. Zheng and J. L. Xu, Green Chem., 2016, 18, 6282–6290 RSC.
- N. Li, C. W. Hu and M. H. Cao, Phys. Chem. Chem. Phys., 2013, 15, 7685–7689 RSC.
- J. Feng, F. Z. Pu, Z. X. Li, X. H. Li, X. Y. Hu and J. T. Bai, Carbon, 2016, 104, 214–225 CrossRef CAS.
- R. B. Yang, P. M. Reddy, C. J. Chang, P. A. Chen, J. K. Chen and C. C. Chang, Chem. Eng. J., 2016, 285, 497–507 CrossRef CAS.
- C. Y. Zhao, M. Y. Shen, Z. X. Li, R. Sun, A. L. Xia and X. G. Liu, J. Alloys Compd., 2016, 689, 1037–1043 CrossRef CAS.
- W. Feng, Y. M. Wang, J. C. Chen, L. Wang, L. X. Guo, J. H. Ouyang, D. C. Jia and Y. Zhou, Carbon, 2016, 108, 52–60 CrossRef CAS.
- H. X. Pan, X. W. Yin, J. M. Xue, L. F. Cheng and L. T. Zhang, Carbon, 2016, 107, 36–45 CrossRef CAS.
- Y. Wang, Y. C. Du, R. Qiang, C. H. Tian, P. Xu and X. J. Han, Adv. Mater. Interfaces, 2016, 3, 1500684 CrossRef.
- Y. Ding, L. Zhang, Q. L. Liao, G. J. Zhang, S. Liu and Y. Zhang, Nano Res., 2016, 9, 2018–2025 CrossRef CAS.
- L. H. Tian, X. D. Yan, J. L. Xu, P. Wallenmeyer, J. Murowchick, L. Liu and X. B. Chen, J. Mater. Chem. A, 2015, 3, 12550–12556 CAS.
- Y. C. Yin, M. Zeng, J. Liu, W. K. Tang, H. R. Dong, R. Z. Xia and R. H. Yu, Sci. Rep., 2016, 6, 25075 CrossRef CAS PubMed.
- X. J. Zhang, G. S. Wang, W. Q. Cao, Y. Z. Wei, J. F. Liang, L. Guo and M. S. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 7471–7478 CAS.
- Y. P. Wang, Z. Peng and W. Jiang, Ceram. Int., 2016, 42, 10682–10689 CrossRef CAS.
- X. X. Wang, B. Q. Zhang, M. X. Yu and J. Q. Liu, RSC Adv., 2016, 6, 36484–36490 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qm00067g |
| This journal is © the Partner Organisations 2017 |