A bifunctional covalent organic framework as an efficient platform for cascade catalysis†
Qi
Sun
,
Briana
Aguila
and
Shengqian
Ma
 *
*
Department of Chemistry, University of South Florida, 4202 E. Fowler Avenue, Tampa, FL 33620, USA. E-mail: sqma@usf.edu
First published on 23rd January 2017
Abstract
A bifunctional covalent organic framework (COF) has been illustrated in the context of partial metalation of a highly porous and chemically robust pyridine containing COF (COF-TpPa-Py) with Pd species and by taking advantage of the base catalytic behavior of pyridine. The resultant bifunctionalized COF exhibits excellent performance in catalyzing one-pot cascade aerobic oxidation–Knoevenagel condensation reactions, outperforming the corresponding homogeneous and porous organic polymer based catalytic systems, thereby opening a new avenue for multifunctional COFs as a promising platform for heterogeneous cascade catalysis.
Introduction
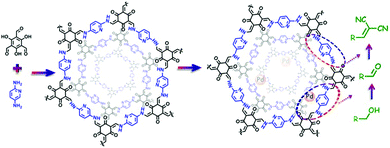
The development of cascade reaction processes that incorporate several reactions to give the final product in one operation has recently emerged as a promising field for advancing modern and sustainable chemistry. Given that the consecutive reaction steps are performed in one pot, costly intermediate separations and purification processes will be avoided, thus offering enormous economic advantages.1 In most cases, a multistep chemical process involves several active components such as a metal, ligand, base, and other cofactors.2 Efforts are thus being made to integrate different catalytically active sites within a single and recyclable material to facilitate one-pot cascade catalysis.3,4 It has been well-documented that careful positioning of these individual functions on a solid support provides a promising strategy for improving catalytic performance by tailoring synergistic interactions, in a way reminiscent of enzymatic catalysis.5 In this context, a material which enables precise control over chemical functionality, density, and spatial arrangement of the active sites is highly desirable.Covalent organic frameworks (COFs)6 as a new class of crystalline materials, with highly ordered organic building blocks and discrete nanopores, have spurred great interest due to their potential applications pertaining to environmental remediation,7 catalysis,8 proton conduction,9 and many more.10 A distinct feature of COFs is that total control over their structure and properties can be achieved through careful selection of the building units prior to synthesis. This unique feature is very attractive to design multifunctional catalysts, considering that the functionality, density, and spatial arrangement of the active sites can be precisely managed. In particular, two dimensional (2D) COFs exhibit unique architectures wherein the monomers that make up their 2D layers stack almost perfectly into infinite 1D columns that are ideal for docking catalysts through the modification of the building blocks, due to the potentially improved accessibility of active sites and mass transport. COFs are thus expected to serve as an auspicious platform for integrating multiple components to bring into effect cascade catalysis.
Considering the attractive catalytically related functionality of pyridine, which can act as a base catalyst and a nitrogen donor to bind metal species, in this contribution, a chemically stable COF bearing pyridine (COF-TpPa-Py) was chosen to demonstrate a proof-of-concept for the utilization of COFs as a promising platform for cascade catalysis. Representatively, after partial metalation of the pyridine groups in COF-TpPa-Py with Pd(OAc)2, the resultant catalyst (Pd/COF-TpPa-Py) was found to be highly active, selective, and recyclable for the one-pot cascade synthesis involving Pd-catalyzed aerobic oxidation of alcohols to aldehydes and base-catalyzed Knoevenagel condensation with the remaining free pyridine groups (Scheme 1). Notably, the COF catalyst outperforms the corresponding homogeneous analogues and the porous organic polymer based catalytic systems in terms of turnover frequency. The site-isolation manner in conjunction with the accessibility of the active sites in Pd/COF-TpPa-Py accounts for the observed excellent performance. Our work thereby sheds light on the great potential of COFs for the design of heterogeneous cascade catalysis.
Experimental
Materials
Solvents were purified according to standard laboratory methods. Other commercially available reagents were purchased in high purity and used without further purification.Catalyst synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/v
1/v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v solution of 1,4-dioxane
v solution of 1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mesitylene
mesitylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 M aqueous acetic acid. The tube was flash frozen at 77 K (liquid N2 bath), evacuated and flame sealed. Upon sealing the length of the tube was reduced to ca. 15 cm. The reaction mixture was heated at 120 °C for 3 days to afford a reddish brown precipitate which was isolated by filtration and washed with anhydrous THF using soxhlet extraction for 3 days. The product was dried under vacuum to afford COF-TpPa-Py (27.9 mg, 75%).
6 M aqueous acetic acid. The tube was flash frozen at 77 K (liquid N2 bath), evacuated and flame sealed. Upon sealing the length of the tube was reduced to ca. 15 cm. The reaction mixture was heated at 120 °C for 3 days to afford a reddish brown precipitate which was isolated by filtration and washed with anhydrous THF using soxhlet extraction for 3 days. The product was dried under vacuum to afford COF-TpPa-Py (27.9 mg, 75%).
Catalytic tests
The one-pot cascade aerobic oxidation–Knoevenagel condensation reactions were carried out in a 50 mL Schlenk flask with a magnetic stirrer. In a typical run, alcohol (1 mmol), the catalyst listed in Table 1, and toluene (10 mL) were transferred into the reactor. After being evacuated, the system was purged with O2 using a balloon. The tube was placed in a preheated oil bath and stirred for the desired time. After the reaction progressed for the desired period of time, the oxygen balloon was removed, and then malononitrile (1.05 mmol) was charged into the reaction system rapidly. The mixture was left at 80 °C for an additional time period. The conversion and the yield of the products were analyzed by 1H NMR.| Entry | Catalyst | Conv. (%) | Yield (%) | |
|---|---|---|---|---|
| A | B | C | ||
| a Reaction conditions: a mixture of benzyl alcohol (1 mmol), toluene (10 mL), and catalyst was stirred at 80 °C under O2 (1 atm) for 4 h and then malononitrile (1.05 mmol) was introduced and the reaction continued for another 1.5 h. b Pd/COF-TpPa-Py (65 mg containing 0.025 mmol Pd and 0.2 mmol pyridine moieties). c COF-TpPa-Py (65 mg). d Pd(OAc)2 (5.6 mg, 0.025 mmol Pd). e Pd(OAc)2 (5.6 mg) and pyridine (15.8 mg, 0.2 mmol). f Pd/POP-Py (68 mg containing 0.025 mmol Pd and 0.18 mmol pyridine moieties). | ||||
| 1b | Pd/COF-TpPa-Py | 98 | Trace | 98 |
| 2c | COF-TpPa-Py | Trace | Trace | Trace |
| 3d | Pd(OAc)2 | 5 | 5 | Trace |
| 4e | Pd(OAc)2 + pyridine | 79 | Trace | 79 |
| 5f | Pd/POP-Py | 72 | 4 | 67 |
For each catalyst recycling, the catalysts were separated by centrifugation under N2, washed with degassed toluene, and used directly for the next run.
Characterization
The gas adsorption isotherms were collected on an ASAP 2020 surface area analyzer. The N2 sorption isotherms were measured at 77 K using a liquid N2 bath. Powder X-ray diffraction (PXRD) data were collected on a Bruker AXS D8 Advance A25 Powder X-ray diffractometer (40 kV, 40 mA) using Cu Kα (λ = 1.5406 Å) radiation. Scanning electron microscopy (SEM) images were collected using a Hitachi SU 8010. IR spectra were recorded on a Nicolet Impact 410 FTIR spectrometer. ICP-OES was performed on a Perkin-Elmer Elan DRC II Quadrupole. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo ESCALAB 250 with Al Kα irradiation at θ = 90° for the X-ray source, and the binding energies were calibrated using the C1s peak at 284.9 eV. 1H NMR spectra were recorded on a Bruker Avance-400 (400 MHz) spectrometer. Chemical shifts are expressed in ppm downfield from tetramethylsilane (TMS) at δ = 0 ppm, and J values are given in Hz. The 13C (100.5 MHz) cross-polarization magic-angle spinning (CP-MAS) solid-state NMR experiment was recorded on a Varian infinity plus 400 spectrometer equipped with a magic-angle spin probe in a 4 mm ZrO2 rotor.Results and discussion
With the aim of optimizing the porosity and crystallinity of COF-TpPa-Py (Fig. 1a), we screened the synthetic conditions by varying the solvent combination/ratio and catalyst loading. Under optimal synthetic conditions, reacting 1,4-dioxane, mesitylene, and 6 M acetic acid in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) ratio at 120 °C for 3 days, we obtained COF-TpPa-Py as a reddish brown microcrystalline solid in a yield of about 75%. The Fourier transform infrared (FT-IR) spectrum of COF-TpPa-Py indicated the complete consumption of the reactants as evidenced by the disappearance of the characteristic N–H stretching bands (3286–3372 cm−1) of the free diamine and aldehydic –C
v) ratio at 120 °C for 3 days, we obtained COF-TpPa-Py as a reddish brown microcrystalline solid in a yield of about 75%. The Fourier transform infrared (FT-IR) spectrum of COF-TpPa-Py indicated the complete consumption of the reactants as evidenced by the disappearance of the characteristic N–H stretching bands (3286–3372 cm−1) of the free diamine and aldehydic –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1640 cm−1). Moreover, the absence of C
O (1640 cm−1). Moreover, the absence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching peaks at around 1620 cm−1 and the appearance of a new C
N stretching peaks at around 1620 cm−1 and the appearance of a new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak at 1570 cm−1 verified the transformation of the enol into a keto tautomer (Fig. S1, ESI†).12 Solid-state 13C NMR spectroscopy confirmed the existence of the keto tautomer supported by the presence of the peak at ∼183 ppm, which is attributed to the keto (–C
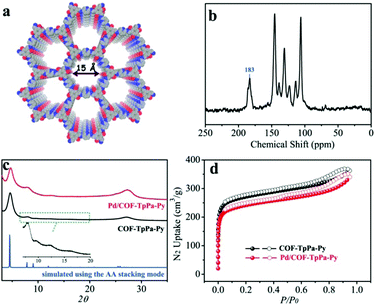
C peak at 1570 cm−1 verified the transformation of the enol into a keto tautomer (Fig. S1, ESI†).12 Solid-state 13C NMR spectroscopy confirmed the existence of the keto tautomer supported by the presence of the peak at ∼183 ppm, which is attributed to the keto (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group (Fig. 1b). The detailed assignment of these peaks is given in Fig. S2 (ESI†). The PXRD pattern of COF-TpPa-Py showed an intense reflection at 4.6° corresponding to the (100) plane (Fig. 1c). The reflections at 7.8°, 9.5°, 12.1°, and ∼27° are attributed to the (110), (200), (210), and (001) planes, respectively. This is in agreement with the theoretical prediction of an AA stacking arrangement. The permanent porosity of COF-TpPa-Py was determined by measuring nitrogen adsorption at 77 K. As shown in Fig. 1d, COF-TpPa-Py displayed a type I isotherm with a sharp uptake in the low relative pressure region (P/P0 < 0.1), which is characteristic of microporous materials. The Brunauer–Emmett–Teller surface area and the total pore volume of COF-TpPa-Py were calculated to be 1019 m2 g−1 and 0.56 cm3 g−1, respectively. DFT fitting of the adsorption branches showed pore size distributions mainly at 1.5 nm, in good agreement with that of the proposed model (Fig. S3, ESI†). The framework of COF-TpPa-Py is stable under a wide range of conditions as demonstrated by its well-retained crystallinity after soaking it in a variety of solvents as well as in both acid (2 M HCl) and base (2 M NaOH) aqueous solutions (Fig. S4, ESI†). In addition, thermogravimetric analysis (TGA) revealed that COF-TpPa-Py exhibited no weight loss under N2 up to 400 °C, indicating its excellent thermal stability (Fig. S5, ESI†).
O) group (Fig. 1b). The detailed assignment of these peaks is given in Fig. S2 (ESI†). The PXRD pattern of COF-TpPa-Py showed an intense reflection at 4.6° corresponding to the (100) plane (Fig. 1c). The reflections at 7.8°, 9.5°, 12.1°, and ∼27° are attributed to the (110), (200), (210), and (001) planes, respectively. This is in agreement with the theoretical prediction of an AA stacking arrangement. The permanent porosity of COF-TpPa-Py was determined by measuring nitrogen adsorption at 77 K. As shown in Fig. 1d, COF-TpPa-Py displayed a type I isotherm with a sharp uptake in the low relative pressure region (P/P0 < 0.1), which is characteristic of microporous materials. The Brunauer–Emmett–Teller surface area and the total pore volume of COF-TpPa-Py were calculated to be 1019 m2 g−1 and 0.56 cm3 g−1, respectively. DFT fitting of the adsorption branches showed pore size distributions mainly at 1.5 nm, in good agreement with that of the proposed model (Fig. S3, ESI†). The framework of COF-TpPa-Py is stable under a wide range of conditions as demonstrated by its well-retained crystallinity after soaking it in a variety of solvents as well as in both acid (2 M HCl) and base (2 M NaOH) aqueous solutions (Fig. S4, ESI†). In addition, thermogravimetric analysis (TGA) revealed that COF-TpPa-Py exhibited no weight loss under N2 up to 400 °C, indicating its excellent thermal stability (Fig. S5, ESI†).
With a porous and crystalline pyridine functionalized framework available, we proceeded to synthesize COF-TpPa-Py into heterogeneous multifunctional catalysts for cascade reactions. By virtue of the pyridine-related base catalytic behavior and its strong coordination ability to metal species, it is highly promising to develop a multifunctional catalyst to be used for a cascade process involving a redox step followed by base catalysis. Given that a Pd/pyridine catalytic system shows excellent activity for aerobic oxidation of alcohols,13 we envision that partially metalated COF-TpPa-Py with Pd species, the resultant Pd/COF-TpPa-Py catalyst bearing two types of active sites, holds great promise for application in one-pot cascade catalysis.
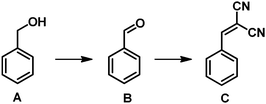
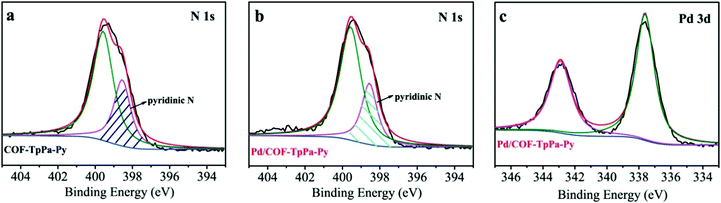
With these aspects in mind, we treated COF-TpPa-Py with Pd(OAc)2 to afford Pd/COF-TpPa-Py and investigated its catalytic performance in the synthesis of α,β-unsaturated dinitriles from alcohols, which are very important chemical intermediates in the pharmaceutical industry. Given that the catalytic component in the resultant catalysts changes along with the variation of Pd loading amounts, we adjusted the Pd amount on COF-TpPa-Py to achieve the optimal trade-off for the cascade reactions. Three catalysts with Pd loadings of 2.0 wt%, 4.1 wt%, and 7.5 wt%, as revealed by ICP-OES analysis, were synthesized, which were denoted as 2.0 wt% Pd/COF-TpPa-Py, 4.1 wt% Pd/COF-TpPa-Py, and 7.5 wt% Pd/COF-TpPa-Py, respectively. The 4.1 wt% Pd/COF-TpPa-Py (hereafter denoted as Pd/COF-TpPa-Py) was chosen as a representative sample for thorough investigation. The PXRD pattern of the obtained metalated material exhibited a diffraction pattern comparable to that of COF-TpPa-Py with an intensive reflection at 4.7° (Fig. 1c). Nitrogen sorption measurements were conducted to verify pore accessibility after partial metalation (Fig. 1d). The BET surface area of Pd/COF-TpPa-Py was determined to be 902 m2 g−1, thus indicating the retention of porosity. Correspondingly, the pore size distribution of Pd/COF-TpPa-Py concentrates at 13 Å and 15 Å (Fig. S6, ESI†). Scanning electron microscopy (SEM) images of Pd/COF-TpPa-Py showed a rough surface composed of thin rods. This is similar to the observed morphology of COF-TpPa-Py (Fig. S7, ESI†). Based on the above we can conclude that no distinct structural changes occurred during the introduction of Pd(OAc)2. To investigate the coordination of Pd species with COF-TpPa-Py, Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) were performed (Fig. 2). The appearance of a new peak at around 690 cm−1 in the IR spectrum of Pd/COF-TpPa-Py, which was also observed in the complex of Pd/pyridine (not present in the spectra of both Pd(OAc)2 and pyridine), indicates that complexes formed between Pd species and the pyridine moieties in COF-TpPa-Py (Fig. S8, ESI†). In addition, the XPS study of COF-TpPa-Py showed two different types of N species: one at 398.5 eV for the pyridinic (–C5H3N) nitrogen and the other at 400.2 eV, ascribed to the free secondary amine (–NH). The ratio of these N species is approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which is in good agreement with the theoretical composition. In contrast, the ratio of free pyridinic nitrogen in Pd/COF-TpPa-Py is obviously lower than COF-TpPa-Py as demonstrated by the decreased area ratio (0.5 to 0.36) of N species at around 398.5 eV, suggesting that part of the pyridinic nitrogens coordinate with the Pd species, thereby shifting to a higher binding energy.14 Meanwhile, Pd/COF-TpPa-Py demonstrated the binding energies of Pd 3d3/2 and Pd 3d5/2 at 342.9 and 337.6 eV, respectively, which are significantly lower than those of Pd(OAc)2 (343.8 and 338.6 eV, respectively).15 These results indicate that strong interactions exist between the Pd species and pyridine moieties in the Pd/COF-TpPa-Py. High angle annular dark field scanning transmission electron microscopy (HADDF-STEM) and EDX mapping results revealed that Pd species are homogeneously distributed throughout the material and no aggregated Pd nanoparticles are observed (Fig. S9, ESI†).
2, which is in good agreement with the theoretical composition. In contrast, the ratio of free pyridinic nitrogen in Pd/COF-TpPa-Py is obviously lower than COF-TpPa-Py as demonstrated by the decreased area ratio (0.5 to 0.36) of N species at around 398.5 eV, suggesting that part of the pyridinic nitrogens coordinate with the Pd species, thereby shifting to a higher binding energy.14 Meanwhile, Pd/COF-TpPa-Py demonstrated the binding energies of Pd 3d3/2 and Pd 3d5/2 at 342.9 and 337.6 eV, respectively, which are significantly lower than those of Pd(OAc)2 (343.8 and 338.6 eV, respectively).15 These results indicate that strong interactions exist between the Pd species and pyridine moieties in the Pd/COF-TpPa-Py. High angle annular dark field scanning transmission electron microscopy (HADDF-STEM) and EDX mapping results revealed that Pd species are homogeneously distributed throughout the material and no aggregated Pd nanoparticles are observed (Fig. S9, ESI†).
Given the accessibility together with the well-defined and arranged active sites, we set out to evaluate the performance of bifunctional COF materials as a bifunctional solid catalyst in the context of a metal-base catalyzed one pot cascade reaction involving the aerobic oxidation of alcohols and the subsequent Knoevenagel condensation. To achieve the optimal catalytic activities, we first investigated the relationship between the cascade catalytic performance and the ratio of the two types of active sites in the Pd metalated COF-TpPa-Py by comparison of the turnover frequency (TOF) values for each individual step. For the oxidation step, 2.0 wt% Pd/COF-TpPa-Py, Pd/COF-TpPa-Py, and 7.5 wt% Pd/COF-TpPa-Py gave TOF values of 16.1, 15.7, and 11.4 h−1, respectively, after 1 h. For ease of comparison, benzaldehyde was used as the reagent to test the efficiency of Knoevenagel condensation over various catalysts. The 2.0 wt% Pd/COF-TpPa-Py, Pd/COF-TpPa-Py, and 7.5 wt% Pd/COF-TpPa-Py afforded TOF values of 2.1, 2.5, and 2.6 h−1, respectively, after 30 min. In view of the TOF values for the two steps, Pd/COF-TpPa-Py was chosen for further detailed studies.
Next, to illustrate the advantages of the bifunctional COF catalytic systems in the one-pot synthesis, a set of control experiments including the homogeneous Pd(OAc)2/pyridine catalyst and a Pd(OAc)2 metalated pyridine-containing porous organic polymer (Pd/POP-py), which was synthesized by copolymerization of divinylbenzene and 4-vinylpyridine, were conducted. In a typical run, the reactions were carried out in a Schlenk tube purged with atmospheric O2 using benzyl alcohol and malononitrile as reactants and toluene as the solvent in the presence of catalysts at 80 °C, with the results listed in Table 1. Benzaldehyde was produced as an intermediate, and benzylidene malononitrile was the objective product in all cases. Remarkably, Pd/COF-TpPa-Py showed exceptional catalytic activity, giving rise to a benzylidene malononitrile yield as high as 98%, which outperformed all other catalytic systems tested under the same conditions. Considering that COF-TpPa-Py did not convert benzyl alcohol (Table 1, entry 2) and Pd(OAc)2 exhibited poor activity, affording benzaldehyde in a 5% yield with a negligible amount of benzylidene malononitrile, the excellent performance of Pd/COF-TpPa-Py thereby should be attributed to the combined contributions from the COF-TpPa-Py and Pd species. It is known that the presence of a base, such as pyridine, is beneficial to promote the Pd-catalyzed oxidation and additionally the base is the active species for the Knoevenagel condensation. Therefore, Pd/COF-TpPa-Py with these features can facilitate the cascade reaction. To further substantiate the cooperative effect, an excess of pyridine was introduced into the Pd(OAc)2 system. A greatly enhanced activity was observed, giving benzylidene malononitrile in a yield of 79% under identical reaction conditions; nonetheless, it is still lower than that obtained by Pd/COF-TpPa-Py (98%). Furthermore, Pd/POP-py afforded a product yield of 67%, which is also inferior to Pd/COF-TpPa-Py in terms of the reaction rate. Taking into account that these catalysts have similar amounts of catalytic components, the significant difference in the activities should be ascribed to different utilization efficiencies of these catalysts. In Pd/COF-TpPa-Py, the Pd metalated pyridine and free pyridine are site-isolated in the accessible ordered channel wall, thereby maximizing their utility, which leads to the high activity. In contrast, various catalytic related species dynamically exist in the homogeneous Pd(OAc)2/pyridine catalytic system, thus compromising their efficiency. With respect to Pd/POP-py, some active sites may be buried due to their irregular pore structures.
In order to further illustrate the advantages of COFs for the design of efficient catalysts for cascade reactions, we compared the activities of Pd/COF-TpPa-Py and Pd(OAc)2/pyridine at a higher substrate to catalyst ratio (the molar ratio of reagent to Pd, S/C = 250). To track the reaction pathway more directly, the one-pot cascade synthesis of benzylidene malononitrile from benzyl alcohol was performed for different reaction times. The difference in reactivity between the two catalytic systems was clearly manifested in the reaction kinetics. Time-dependent conversion curves indicated that Pd/COF-TpPa-Py was much faster than the homogeneous control in terms of turnover frequency. At 0.4 mol% catalyst loading (based on Pd species), the oxidation of benzyl alcohol was completed within 16 h for Pd/COF-TpPa-Py. In the case of Pd(OAc)2/pyridine, benzaldehyde was observed in a yield of 59% after 16 h and after prolonging the reaction time to 24 h, only a slightly increased conversion was obtained (67%). After introducing malononitrile into the reaction solution, the amount of intermediate benzaldehyde decreased rapidly to a low level, whereas the yield of benzylidene malononitrile increased sharply and leveled off at ca. 92% within 5 h for Pd/COF-TpPa-Py. In this step, Pd/COF-TpPa-Py is at least 2 times more active than the homogeneous control. These results revealed that Pd/COF-TpPa-Py exhibited superior performance in comparison with Pd(OAc)2/pyridine, thus underscoring the great potential of COFs for the deployment of multifunctional catalysts for cascade catalysis (Fig. 3).
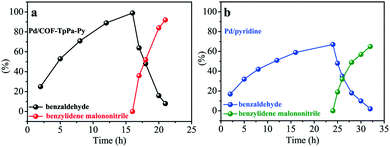 | ||
| Fig. 3 Yield versus reaction time of the (a) Pd/COF-TpPa-Py and (b) Pd/pyridine catalysts for the one-pot cascade oxidation–Knoevenagel condensation reaction. Reaction conditions are similar to those given in Table 1 except for the S/C ratio and reaction time. | ||
To identify if the catalysis occurs on the surface or within the pores, a relatively large size alcohol (cholesterol) was used as a reactant. Pd/COF-TpPa-Py afforded an 8% cholesterol conversion, while the homogeneous Pd/pyridine catalyst gave the corresponding oxide product in a yield as high as 87% under identical conditions, thus implying that cholesterol is too big to efficiently access the active sites anchored in the pores. These results reveal that most of the reactions occur in the pores (Table S1, ESI†).
Given that the recyclability of the catalyst is a crucial performance metric for cost-effective industrial processes, we investigated this property for Pd/COF-TpPa-Py. Analysis of the supernatant from the synthesis of benzylidene malononitrile by ICP-OES showed that the Pd species leaching was below the detection limit of 0.1 ppm. Moreover, it is observed that the catalyst could be recycled at least 6 times without a significant drop in the product yield (95% after 5 cycles), thus highlighting the heterogeneous nature of the catalytic process (Fig. 4). The robustness of the catalyst was further confirmed by the well-retained crystallinity and pore structure, and the chemical state of the Pd species in Pd/COF-TpPa-Py after the reaction as evidenced by XRD, N2 adsorption and XPS spectroscopy, respectively (Fig. S10, ESI†).
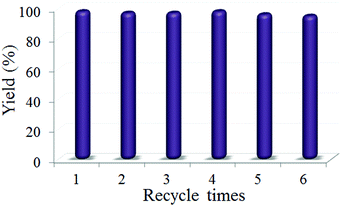 | ||
| Fig. 4 Recycling tests of the Pd/COF-TpPa-Py in the cascade synthesis of benzylidene malononitrile from benzyl alcohol. Reaction conditions are the same as those given in Table 1. | ||
Encouraged by the results described above, we extended the Pd/COF-TpPa-Py catalyzed one-pot cascade synthesis to other α,β-unsaturated dinitriles from alcohols, and the results are presented in Table 2. Primary benzylic alcohols were converted to the corresponding aldehydes in 92–96% yields (Table 2, entries 1–5) and then reacted with malononitrile to efficiently transform to the corresponding dinitriles. It is noteworthy that this catalytic system shows tolerance to a variety of substituents on aromatic nuclei. Aliphatic primary alcohols were oxidized to the aldehydes selectively and then to dinitriles, but they are relatively inert and thus required longer reaction times (Table 2, entries 6 and 7). To further extend the reaction scope, various methylene compounds instead of malononitrile were tested. When ethyl cyanoacetate was used, the cascade reaction can smoothly proceed, although more than threefold time is required to reach a similar final product yield (5 h, 97%) in comparison with employing malononitrile as a reagent in the Knoevenagel condensation step. However, when diethyl malonate was used, the second condensation step was suppressed greatly, which made the whole one-pot synthesis inefficient for production. Specifically, only 4% of benzaldehyde was observed to convert to diethyl 2-benzylidenemalonate after 8 h (Table S2, ESI†). Considering that the Knoevenagel condensation is a model reaction to evaluate the catalyst basicity, and Pd/COF-TpPa-Py can facilitate the Knoevenagel condensation between benzaldehyde and malononitrile or less active ethyl cyanoacetate, but not for relatively inert diethyl malonate, which is presumed to be catalyzed by strong base sites, therefore, the basicity of Pd/COF-TpPa-Py was evaluated to be moderately strong.
| Entry | R | Time (h) | Conv. (%) | Yield (%) | |
|---|---|---|---|---|---|
| [t1 + t2] | A | B | C | ||
| a Reaction conditions: a mixture of alcohol (1 mmol), toluene (10 mL), and Pd/COF-TpPa-Py was stirred at 80 °C under O2 (1 atm) for t1 h and then malononitrile (1.05 mmol) was introduced, and the reaction continued for t2 h. | |||||
| 1 | 4-CH3O–C6H5 | 4 + 1.5 | 99 | Trace | 99 |
| 2 | 4-CH3–C6H5 | 4 + 1.5 | 98 | Trace | 97 |
| 3 | 4-NO2–C6H5 | 7 + 2 | 92 | Trace | 91 |
| 4 | 4-Cl–C6H5 | 6 + 2 | 96 | Trace | 94 |
| 5 | 3-Cl–C6H5 | 6 + 2 | 93 | Trace | 93 |
| 6 | n-C6H13 | 12 + 5 | 91 | Trace | 91 |
| 7 | n-C8H17 | 12 + 5 | 94 | Trace | 93 |
Conclusions
In summary, we have demonstrated the deployment of a chemically stable pyridine-based COF in a bifunctional catalyst after introducing Pd species by virtue of the base catalytic behavior and as a nitrogen donor to bind metal species of pyridine moieties. The resultant catalyst demonstrates excellent performance in the cascade oxidation–Knoevenagel condensation reaction from alcohols to α,β-unsaturated dinitriles. Due to the combined contributions of the ordered porous structure, highly accessible active sites, and the site isolated manner of the two catalytic components, Pd/COF-TpPa-Py far outperforms the corresponding homogeneous catalyst (Pd/pyridine) in terms of the turnover frequency. Our work therefore not only advances COFs as a new type of host material for the deployment of multifunctional catalysts, but also provides a new perspective for the design of highly efficient and robust heterogeneous catalysts for cascade catalysis. Studies aimed at designing new COF-based catalysts for advanced cascade catalysis applications are currently underway in our laboratory.Acknowledgements
The authors acknowledge the University of South Florida the National Science Foundation (DMR-1352065) for financial support of this work.Notes and references
- (a) M. J. Climent, A. Corma, S. Iborra and M. J. Sabater, ACS Catal., 2014, 4, 870–891 CrossRef CAS; (b) M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072–1133 CrossRef CAS PubMed; (c) K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993–3009 RSC; (d) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167–178 CrossRef CAS PubMed; (e) T. L. Lohr and T. J. Marks, Nat. Chem., 2015, 7, 477–482 CrossRef CAS PubMed.
- (a) J. C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001–1020 CrossRef CAS PubMed; (b) A. E. Fernandes, O. Riant, K. F. Jensen and A. M. Jonas, Angew. Chem., Int. Ed., 2016, 55, 11044–11048 CrossRef CAS PubMed; (c) N. R. Shiju, A. H. Alberts, S. Khalid, D. R. Brown and G. Rothenberg, Angew. Chem., Int. Ed., 2011, 50, 9615–9619 CrossRef CAS PubMed.
- (a) A. Arnanz, M. Pintado-Sierra, A. Corma, M. Iglesias and F. Sánchez, Adv. Synth. Catal., 2012, 354, 1347–1355 CrossRef CAS; (b) Y. Zhang, B. Li and S. Ma, Chem. Commun., 2014, 50, 8507–8510 RSC; (c) E. Merino, E. Verde-Sesto, E. M. Maya, M. Iglesias, E. Sánchez and A. Corma, Chem. Mater., 2013, 25, 981–988 CrossRef CAS; (d) Y. Yang, X. Liu, X. Li, J. Zhao, S. Bai, J. Liu and Q. Yang, Angew. Chem., Int. Ed., 2012, 124, 9298–9302 CrossRef; (e) Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC; (f) Y. Huang, S. Xu and V. S.-Y. Lin, Angew. Chem., Int. Ed., 2011, 50, 661–664 CrossRef CAS PubMed; (g) K. K. Sharma and T. Asefa, Angew. Chem., Int. Ed., 2005, 44, 1826–1830 CrossRef PubMed.
- (a) D.-Y. Hong, Y. K. Hwang, C. Serre, G. Férey and J.-S. Chang, Adv. Funct. Mater., 2009, 19, 1537–1552 CrossRef CAS; (b) R. Srirambalaji, S. Hong, R. Natarajan, M. Yoon, R. Hota, Y. Kim, Y. H. Ko and K. Kim, Chem. Commun., 2012, 48, 11650–11652 RSC; (c) F. Vermoortele, R. Ameloot, A. Vimont, C. Serre and D. D. Vos, Chem. Commun., 2011, 47, 1521–1523 RSC; (d) Q. Han, B. Qi, W. Ren, C. He, J. Niu and C. Duan, Nat. Commun., 2015, 6, 10007 CrossRef PubMed; (e) T. Toyao, M. Fujiwaki, Y. Horiuchi and M. Matsuoka, RSC Adv., 2013, 3, 21582–21587 RSC; (f) A. Dhakshinamoorthy and H. Garcia, ChemSusChem, 2014, 7, 2392–2410 CrossRef CAS PubMed; (g) Y. Qi, Y. Luan, X. Peng, M. Yang, J. Hou and G. Wang, Eur. J. Inorg. Chem., 2015, 5099–5105 CrossRef CAS; (h) Y. Horiuchi, T. Toyao, M. Fujiwaki, S. Dorshi, T.-H. Kim and M. Matsuoka, RSC Adv., 2015, 5, 24687–24690 RSC; (i) Y.-Z. Chen, Y.-X. Zhou, H. Wang, J. Lu, T. Uchida, Q. Xu, S.-H. Yu and H.-L. Jiang, ACS Catal., 2015, 5, 2062–2069 CrossRef CAS; (j) Z. Miao, Y. Luan, C. Qi and D. Ramella, Dalton Trans., 2016, 45, 13917–13924 RSC.
- (a) K. M. Koeller and C. H. Wong, Chem. Rev., 2000, 100, 4465–4493 CrossRef CAS PubMed; (b) H. Seong, H.-T. Chen, J. W. Wiench, M. Pruski and V. S.-Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 1826–1830 CrossRef PubMed; (c) Á. G. Baribar, D. Reichert, C. Mügge, S. Seger, H. Gröger and R. Kourist, Angew. Chem., Int. Ed., 2016, 55, 14823–14827 CrossRef PubMed; (d) L. Chen, Y. Yang and D. Jiang, J. Am. Chem. Soc., 2010, 132, 9138–9143 CrossRef CAS PubMed; (e) L. Chen, Y. Honsho, S. Seki and D. Jiang, J. Am. Chem. Soc., 2010, 132, 6742–6748 CrossRef CAS PubMed.
- (a) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed; (b) X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC; (c) S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC; (d) L. Ascherl, T. Sick, J. T. Margraf, S. H. Lapidus, M. Calik, C. Hettstedt, K. Karaghiosoff, M. Döblinger, T. Clark, K. W. Chapman, F. Auras and T. Bein, Nat. Chem., 2016, 8, 310–316 CrossRef CAS; (e) D. N. Bunck and W. R. Dichtel, Angew. Chem., Int. Ed., 2012, 51, 1885–1889 CrossRef CAS PubMed; (f) Y. Zeng, R. Zou, Z. Luo, H. Zhang, X. Yao, X. Ma, R. Zou and Y. Zhao, J. Am. Chem. Soc., 2015, 137, 1020–1023 CrossRef CAS PubMed; (g) Z.-F. Pang, S.-Q. Xu, T.-Y. Zhou, R.-R. Liang, T.-G. Zhan and X. Zhao, J. Am. Chem. Soc., 2016, 138, 4710–4713 CrossRef CAS PubMed; (h) S. Chandra, S. Kandambeth, B. P. Biswal, B. Lukose, S. M. Kunjir, M. Chaudhary, R. Babarao, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 17853–17861 CrossRef CAS PubMed.
- (a) S.-Y. Ding, M. Dong, Y.-W. Wang, Y.-T. Chen, H.-Z. Wang, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed; (b) C. J. Doonan, D. J. Tranchemontagne, T. G. Glover, J. R. Hunt and O. M. Yaghi, Nat. Chem., 2010, 2, 235–238 CrossRef CAS PubMed; (c) Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- (a) H. Xu, J. Gao and D. Jiang, Nat. Chem., 2015, 7, 905–912 CrossRef CAS PubMed; (b) Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., Int. Ed., 2014, 53, 2878–2882 CrossRef CAS PubMed; (c) S.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed; (d) Y. Peng, Z. Hu, Y. Gao, D. Yuan, Z. Kang, Y. Qian, N. Yan and D. Zhao, ChemSusChem, 2015, 8, 3208–3212 CrossRef CAS PubMed; (e) H. B. Aiyappa, J. Thote, D. B. Shinde, R. Banerjee and S. Kurungot, Chem. Mater., 2016, 28, 4375–4379 CrossRef CAS; (f) V. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed; (g) S. Lin, C. S. Diercks, Y.-B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Science, 2015, 349, 1208–1213 CrossRef CAS PubMed; (h) X. Wang, X. Han, J. Zhang, X. Wu, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2016, 138, 12332–12335 CrossRef CAS PubMed; (i) Q. Sun, B. Aguila, J. A. Perman, N. Nguyen and S. Ma, J. Am. Chem. Soc., 2016, 138, 15790–15796 CrossRef CAS PubMed.
- (a) H. Ma, B. Liu, B. Li, L. Zhang, Y.-G. Li, H.-Q. Tan, H.-Y. Zang and G. Zhu, J. Am. Chem. Soc., 2016, 138, 5897–5903 CrossRef CAS PubMed; (b) H. Xu, S. Tao and D. Jiang, Nat. Mater., 2016 DOI:10.1038/nmat4611.
- (a) S. Mitra, S. Kandambeth, B. P. Biswal, M. A. Khayum, C. K. Choudhury, M. Mehta, G. Kaur, S. Banerjee, A. Prabhune, S. Verma, S. Roy, U. K. Kharul and R. Banerjee, J. Am. Chem. Soc., 2016, 138, 2823–2828 CrossRef CAS PubMed; (b) Y. Du, H. Yang, J. M. Whiteley, S. Wan, Y. Jin, S.-H. Lee and W. Zhang, Angew. Chem., Int. Ed., 2016, 55, 1737–1741 CrossRef CAS PubMed; (c) G. H. V. Bertrand, V. K. Michaelis, T.-C. Ong, R. G. Griffin and M. Dincă, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4923–4928 CrossRef CAS PubMed.
- Y.-L. Zhang, S. Liu, S. Liu, F. Liu, H. Zhang, Y. He and F.-S. Xiao, Catal. Commun., 2011, 12, 1212–1217 CrossRef CAS.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed.
- (a) T. Iwasawa, M. Tokunaga, Y. Obora and Y. Tsuji, J. Am. Chem. Soc., 2004, 126, 6554–6555 CrossRef CAS PubMed; (b) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 6750–6755 CrossRef CAS PubMed.
- S. Chandra, T. Kundu, K. Dey, M. Addicoat, T. Heine and R. Banerjee, Chem. Mater., 2016, 28, 1489–1494 CrossRef CAS.
- H. Yang, X. Han, G. Li and Y. Wang, Green Chem., 2009, 11, 1184–1193 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qm00363j |
| This journal is © the Partner Organisations 2017 |