New developments in non-fullerene small molecule acceptors for polymer solar cells
Ningning
Liang
ab,
Wei
Jiang
 *a,
Jianhui
Hou
*a,
Jianhui
Hou
 a and
Zhaohui
Wang
a
a and
Zhaohui
Wang
a
aKey Laboratory of Organic Solids, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: jiangwei@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 6th February 2017
Abstract
During the past two years, non-fullerene electron acceptors for organic solar cells have attracted considerable attention. Significant progress has been made in that the power conversion efficiency of polymer solar cells based on non-fullerene small molecule acceptors now exceeds 12%, exhibiting many advantages over their fullerene counterparts. One of the greatest strengths for NFAs is their tunability via chemical modulation to fine-tune their absorption, energy level and electronic mobility, which is difficult to achieve with fullerene derivatives. This review describes very recent developments of polymer donor:small molecular non-fullerene acceptors in several systems since 2015, including rylene imide, indacenodithiophene and diketopyrrolopyrrole-based small molecular acceptors. Molecular design considerations and structure–property relationships are also discussed.
1. Introduction
It is well known that the conversion of solar energy into electricity using solar cell devices is a major topic within academic and business communities. Recently, scientists from the Energy Department's National Renewable Energy Laboratory (NREL) and the Swiss Center for Electronics and Microtechnology (SCEM) have jointly set a new world record using a dual-junction III–V/Si solar cell with a newly certified record conversion efficiency of 29.8%, exceeding the theoretical limit of 29.4% for crystalline silicon solar cells.1,2Great improvements have also been achieved with solution processed bulk heterojunction (BHJ) organic solar cells (OSCs) consisting of electron donors and electron acceptors. Over the past few decades, these solar cells have also attracted attention from scientists worldwide due to the following important and distinct advantages: (1) low-cost, mechanical flexibility, light weight, and the ability to be produced using the roll-to-roll method, providing the possibility for large-area industrial production; (2) the donor and acceptor materials can be easily tuned via organic synthesis to fine-tune the energy level and the light capture capability.
The first bilayer OSC device was proposed by Tang in 1986 and the first BHJ OSCs based on polymer:fullerene were demonstrated by Heeger and coworkers in 1995.3,4 During the past few decades, fullerene derivatives as electron acceptors have played a dominant role in the field of OSCs. The power conversion efficiency (PCE) of single-junction polymer solar cells (PSCs) has exceeded 11.7% largely due to excellent morphology and high mobilities as well as the ultrafast photo-induced electron transfer between the fullerene acceptors and the polymer donors.5–8 However, fullerene derivatives do present some challenges in terms of chemical modification to tune the light absorption and the energy level. Thus, much attention had been paid to the non-fullerene acceptors (NFAs), for the judicious design and synthesis of novel chemical structures, the optimization of the device structure, and BHJ morphology. Efforts have been rewarded and some new NFAs have exhibited outstanding performance: PSCs with PCEs > 12% have recently been reported.9 Therefore, summarizing the critical achievements in NF-PSCs and performing a systematic and sensible analysis of these materials are considered to be essential to guide molecular design.
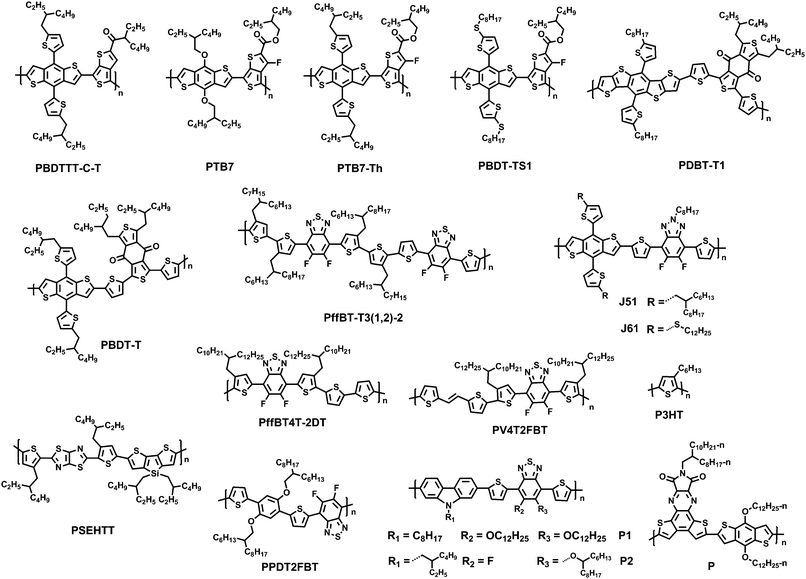
A detailed introduction and summary of the development of NFAs in polymeric donor-based solar cells (NF-PSCs) before 2015 have been provided in several reviews.10–13 In this review article, therefore, we only summarize recent progress, molecular design strategies and structure–property relationships in NF-PSCs since 2015. Fig. 2 presents the typical polymer donors adopted in NF-PSCs. According to the structural features, we classified non-fullerene electronic acceptors into three groups, including rylene imide dyes, and indacenodithiophene (IDT)-based and diketopyrrolopyrrole (DPP)-based small molecule acceptors (SMAs).
2. Rylene imide dyes
2.1 PDI based small molecule acceptors
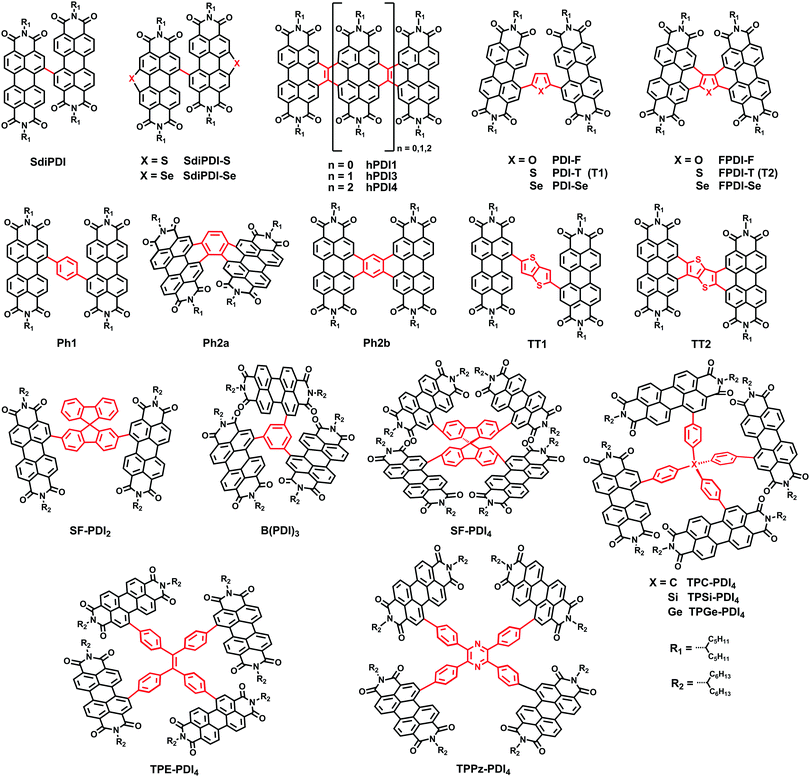
Among the non-fullerene electron acceptors, perylene diimide (PDI) derivatives have attracted considerable attention. They exhibit great potential due to their excellent electron-accepting ability, their high electron mobility, and especially their highly tunable electronic energy levels, achieved by chemical modification.14–16 However, PDI derivatives show strong self-aggregation to form overly large aggregates in BHJ active layers due to the large planar π-conjugated system. This prevents the formation of a sufficiently large donor–acceptor interfacial area for exciton diffusion/separation, thereby limiting the PCEs of such devices.17,18 Now, after several years of development, researchers have achieved great success in disrupting the aggregation tendency by means of chemical functionalization. Nevertheless, the highly twisted structures lead to low crystallinity and poor electron transport ability, which is a bottleneck for improvements in the short-circuit current (JSC), fill factor (FF), and PCE.19–25 It has therefore been challenging to design NFAs possessing both a suitable domain size in BHJs and high electron mobility. As is well known, an effective way to improve the carrier mobility is to expand ring functionalization. For PDI derivatives, two kinds of functionalization positions are termed as the imide position and lateral positions including bay- and nonbay-positions, respectively (Fig. 1).| Acceptor | Donor | Device structure | LUMO/HOMO (eV) | V OC (V) | J SC (mA cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from the onset oxidation and reduction potential. b Calculated from the absorption edge (λedge) with the equation of Eoptg = 1240/λedge (eV); the value of λedge is obtained from the absorption spectrum. | ||||||||
| SdiPDI | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.92/−5.99b | 0.79 | 12.86 | 0.54 | 5.56 | 28 |
| SdiPDI-S | PDBT-T1 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.85/−6.05b | 0.90 | 11.65 | 0.66 | 7.16 | 29 |
| SdiPDI-Se | PDBT-T1 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.87/−6.09b | 0.95 | 12.48 | 0.70 | 8.42 | 30 |
| PDI-T | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −4.02/−5.95a | 0.88 | 9.74 | 0.41 | 3.54 | 31 |
| FPDI-F | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.80/−6.01a | 0.94 | 11.76 | 0.51 | 5.69 | 31 |
| FPDI-T | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.77/−5.98a | 0.93 | 12.00 | 0.58 | 6.72 | 31 |
| FPDI-Se | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.76/−5.96a | 0.92 | 11.19 | 0.55 | 5.77 | 31 |
| Ph2 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −4.07/−6.22b | 0.91 | 5.50 | 0.41 | 2.19 | 32 |
| Ph2a | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −4.03/−6.36b | 0.93 | 7.68 | 0.54 | 3.89 | 32 |
| Ph2b | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −4.04/−6.18b | 0.89 | 1.51 | 0.29 | 0.23 | 32 |
| TT1 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −4.14/−6.16b | 0.87 | 5.55 | 0.42 | 2.02 | 32 |
| TT2 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.99/−6.20b | 0.98 | 3.95 | 0.39 | 1.50 | 32 |
| hPDI1 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −3.77/−6.04 | 0.80 | 13.50 | 0.55 | 5.94 | 33 |
| hPDI3 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −3.86/−6.23 | 0.81 | 14.50 | 0.67 | 7.9 | 34 |
| hPDI4 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −3.92/−6.26 | 0.80 | 15.10 | 0.68 | 8.27 | 34 |
| SF-PDI2 | PffBT4T-2DT | ITO/ZnO/BHJ/V2O5/Al | 3.71/5.71a | 0.98 | 10.74 | 0.57 | 6.30 | 36 |
| SF-PDI4 | P3TEA | ITO/ZnO/BHJ/V2O5/Al | — | 1.11 | 13.27 | 0.64 | 9.50 | 37 |
| B(PDI)3 | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.86/−6.00a | 0.83 | 13.12 | 0.52 | 5.65 | 39 |
| SF-PDI4 | P4T2FBT | ITO/ZnO/BHJ/V2O5/Ag | −3.78/−5.97a | 0.93 | 11.03 | 0.51 | 5.27 | 40 |
| SF-PDI4 | PV4T2FBT | ITO/ZnO/BHJ/V2O5/Ag | — | 0.90 | 12.02 | 0.54 | 5.98 | 40 |
| TPE-PDI4 | PTB7-Th | — | −3.72/−5.77b | 0.91 | 11.70 | 0.52 | 5.53 | 41 |
| TPC-PDI4 | PffBT4T-2DT | ITO/ZnO/BHJ/V2O5/Al | −3.75/−6.00b | 0.96 | 9.20 | 0.49 | 4.3 | 42 |
| TPSi-PDI4 | PffBT4T-2DT | ITO/ZnO/BHJ/V2O5/Al | −3.75/−6.01b | 0.94 | 8.5 | 0.53 | 4.2 | 42 |
| TPGe-PDI4 | PffBT4T-2DT | ITO/ZnO/BHJ/V2O5/Al | −3.68/−5.94b | 0.92 | 5.0 | 0.37 | 1.6 | 42 |
| TPC-PDI4 | PffBT-T3(1,2)-2 | ITO/ZnO/BHJ/MoO3/Al | — | 1.04 | 8.70 | 0.51 | 4.60 | 43 |
| TPE-PDI4 | PffBT-T3(1,2)-2 | ITO/ZnO/BHJ/MoO3/Al | — | 1.03 | 10.60 | 0.54 | 5.90 | 43 |
| TPPz-PDI4 | PffBT-T3(1,2)-2 | ITO/ZnO/BHJ/MoO3/Al | −3.76/−5.86b | 0.99 | 12.50 | 0.56 | 7.10 | 43 |
| αPPID | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −4.19/−5.66b | 0.77 | 10.15 | 0.44 | 3.49 | 44 |
| βPPID | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −4.21/−5.59b | 0.78 | 9.14 | 0.45 | 3.20 | 44 |
| αPBDT | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −4.13/−4.80b | 0.81 | 12.74 | 0.46 | 4.76 | 44 |
| βPBDT | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −4.13/−5.05b | 0.81 | 9.80 | 0.44 | 3.49 | 44 |
| TPB | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.81/−5.71a | 0.79 | 17.90 | 0.58 | 8.47 | 45 |
| H-di-PDI | PBDT-TS1 | ITO/PEDOT:PSS/BHJ/PFN/Al | −3.86/−5.90b | 0.82 | 12.85 | 0.53 | 5.58 | 47 |
| H-di-PDI | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | — | 0.79 | 13.12 | 0.60 | 6.41 | 28 |
| H-tri-PDI | PBDT-TS1 | ITO/ZnO/BHJ/MoO3/Al | −3.93/−6.01a | 0.73 | 16.52 | 0.60 | 7.25 | 48 |
| 2c-DACH-PPDIs | PTB7-Th | ITO/ZnO/BHJ/MoO3/Al | −3.88/−6.16b | 0.80 | 10.63 | 0.51 | 4.68 | 49 |
| Me-PDI4 | PBDTTT-C-T | ITO/ZnO/BHJ/PEDOT:PSS/Ag | −3.81/−5.96b | 0.77 | 7.83 | 0.45 | 2.73 | 50 |
Jen et al. developed a new family of fused derivatives with different chalcogen atoms from S to O to Se, namely FPDI-T, FPDI-F and FPDI-Se. Compared with the non-fused PDI-T, fused FPDI-T showed increased π-conjugation and delocalization of the LUMO over the whole molecule. As the atomic size increases from O to S to Se, the LUMO levels improved from −3.80, to −3.77 to −3.76 eV. As a result, PTB7-Th:FPDI-T based PSCs exhibited superior photovoltaic performance with a PCE of 6.72%.31 Thus, fused-ring functionalization on PDIs could reduce reorganization energy and extend effective π-conjugation, which is beneficial to facilitate exciton diffusion and charge transport. This result was confirmed by Marks and coworkers, who explored the effects of ring fusion in a series of PDI dimers bridged by thiophene (T1 (PDI-T) and T2 (FPDI-T)), phenylene (Ph1, Ph2a, and Ph2b), and thienothiophene (TT1, and TT2) linkers. The PSCs utilizing twisted dimers, T2 and Ph2a, blended with the donor PTB7-Th showed a significant increase in the performance compared to those fabricated with the non-fused dimers, T1 and Ph1, respectively, which was attributed to the decreased geminate recombination rates and increased electron mobility. Other fused dimers TT2 and Ph2b with more a planar conformation have decreased performances for PSCs due to increased charge recombination and excimer formation.32
A family of helical PDI molecules (hPDI1, hPDI3, hPDI4) has been reported by Nuckolls and coworkers. Those structures were prepared by fusing adjacent PDI unites with a two-carbon bridge. These small molecules have relatively high electron mobilities and LUMO energy levels (∼−4.0 eV), good absorption abilities from 350 to 600 nm (εmax of 1.1 × 105 M−1 cm−1, 1.5 × 105 M−1 cm−1 and 1.8 × 105 M−1 cm−1 for hPDI1, hPDI3 and hPDI4, respectively) and a slightly reduced aggregation tendency due to the twisted molecular conformation. Thus, stronger light absorption and lack of aggregation, together with the tendency of the isolated linear structure to form networks all contributed to ensuring that the PSCs based on PTB7:hPDI3 and PTB7-Th:hPDI4 show superior performances to those of PTB7-Th:hPDI1, with the maximum PCEs of 7.9% and 8.3%, respectively.33,34
Yan et al. adopted a difluorobenzothiadiazole (ffBT)-based polymer, named as PffBT4T-2DT, as an electronic donor material. When combined with two electronic acceptors, diPDI and SF-PDI2, as reported in a previous study,26,35 reasonably high PCEs of 5.4% and 6.3% were obtained, respectively.36 This group also reported PSCs based on novel polymers P3TEA and SF-PDI2, specifically possessing a low voltage loss of 0.61 eV, a high VOC of 1.11 V and an excellent PCE of 9.5%.37 Although the PSCs based on P3TEA:SF-PDI2 have a negligible driving force (Egap − ECT), the solar cells exhibit fast and efficient charge separation, creating a path towards highly efficient PSCs with low voltage loss.
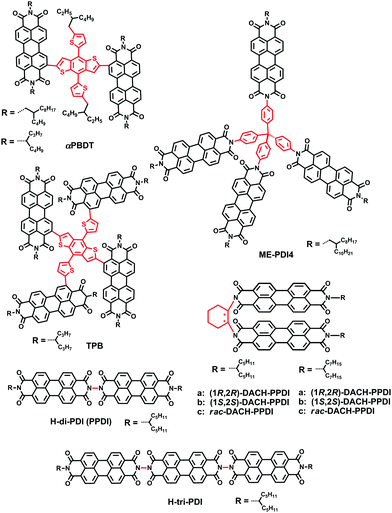
Recently, great achievements have been reported on the three-dimensional (3D) PDI SMAs linked with different core blocks; they have the advantages of high extinction coefficients and favorable BHJ morphology featuring a small domain size.38 Chen and coworkers reported a PDI derivative B(PDI)3, in which a central benzene unit is adopted to connect three PDI arms, leading to a twisted molecular geometry and suppressing the strong crystallization tendency of PDI chromophores. Therefore, the PSCs based on PTB7-Th:B(PDI)3 provided a good PCE of 5.65%.39 Cho et al. used a spiro-bifluorene (SF) core to connect four PDI building blocks and obtained a SF-PDI4 acceptor, with a highly twisted SF core that could suppress molecular aggregation and facilitate excitation energy transfer among PDI subunits. The devices combined SF-PDI4 with a difluorobenzo-thiadiazole (2FBT)-based donor polymer, PV4T2FBT, yielding a high JSC of 12.02 mA cm−2 and a VOC of 0.90 V, leading to a high PCE of 5.98%.40 Yan and coworkers reported a tetraphenylethylene (TPE) core-based 3D SMA, TPE-PDI4, enabling the NF-PSCs based on PTB7-Th to obtain a PCE of 5.53%.41 In 2015, this group reported a series of tetraphenyl carbon-group core based 3D PDI acceptors (tetraphenyl-methane (TPC-PDI4), tetraphenylsilane (TPSi-PDI4) and tetraphenylgermane (TPGe-PDI4)), enabling the PffBT4T-2DT based PSCs with PCEs of up to 4.3% and 4.2% for TPC and TPSi, respectively, which is higher than that for TPGe.42 So it appears that the extent of intramolecular twisting of adjoined PDI units is optimal to give the best performance. Rencently, Yan and coworkers carried out systematic research into this issue. Three types of 3D PDI dyes were prepared and compared. These dyes comprised four PDI units linked by three different cores, TPC, TPE, and tetraphenylpyrazine (TPPz), named as TPE-PDI4, TPC-PDI4 and TPPz-PDI4, respectively. In these cores, TPC has a sp3 carbon atom in the middle and TPE has a planar structure with a double bond at its core, leading to a TPE-shaped core. The extent of intramolecular twisting of TPPz-PDI4 is further reduced compared to TPE-PDI4 and TPC-PDI4. As the extent of intramolecular twisting decreases from TPC-PDI4, to TPE-PDI4 to TPPz-PDI4, the aggregation tendency and electron mobility of the SMAs increase. As a result, the optimized PffBT-T3(1,2)-2:TPPz-PDI4 based device exhibits a highest PCE of 7.1%, which is significantly higher than that of TPE-PDI4 (6.0%) and TPC-PDI4 (4.7%) based devices.43 Thus, it is also important to consider the intramolecular twisting of 3D molecular acceptors for improving the performance of non-fullerene OSCs.
Jen et al. then investigated the influence of molecular geometry of the donor polymers and the PDI dimers on the bulk heterojunction (BHJ) morphology with an inverted structure device. The results indicated that the pseudo 2D conjugated polymer PTB7-Th showed better miscibility with H-di-PDI (PPDI) compared to the 1D polymer PTB7, facilitating more efficient exciton dissociation in the BHJ films. In addition, the face-on oriented π–π stacking was only slightly disrupted by H-di-PDI with a more rigid structure providing suitable pathways for charge transport, compared with that of the bay-linked PDI dimer (SdiPDI). As a result, a very high PCE of 6.41% was achieved in the PTB7-Th:H-di-PDI device.28 Furthermore, the absorption intensity could be enhanced with the increasing number of n in the H-n-PDI molecules. Thus, H-tri-PDI consisting of three PDI blocks via the imide position with the εmax of 2.8 × 105 M−1 cm−1 and the LUMO level of −3.93 eV was synthesized. Benefiting from the appropriate energy level alignment and favorable morphology in the PBDT-TS1:H-tri-PDI active layer, the JSC of PSCs was as high as 16.5 mA cm−2 and the PCE reached 7.25%.48
Wang and coworkers then selected racemic 1,2-diaminocyclohexane (rac-DACH) and enantiomerically pure 1R,2R-DACH and 1S,2S-DACH as bridges to develop chiral bridged PPDI derivatives, namely DACH-PPDIs. The PSCs based on PTB7-Th:rac-DACH-PPDI achieved a PCE of 4.68%.49 Chen and coworkers reported a novel 3D PDI acceptor employing tetraphenyl methane with a tetrahedral architecture as the core connecting four PDI units via the imide position. The solution-processed BHJ OSCs based on PBDTTT-C-T:Me-PDI4 exhibited a PCE as high as 2.73%.50
Regarding concerted efforts to judiciously design new PDI acceptors, PSCs based on PDIs have attracted much attention and rapid developments have been made. On the basis of achieving effective disruption of excessive aggregation, attention was directed to the balance between the charge mobility and the twisted molecular configuration in PDI molecules. It is possible to find a PDI acceptor with high mobility that works well with a polymeric donor with high mobility. The non-fullerene solar cells based on PDI dyes will have further reduced recombination rates and higher FF values.
2.2 NI-based small molecule acceptors
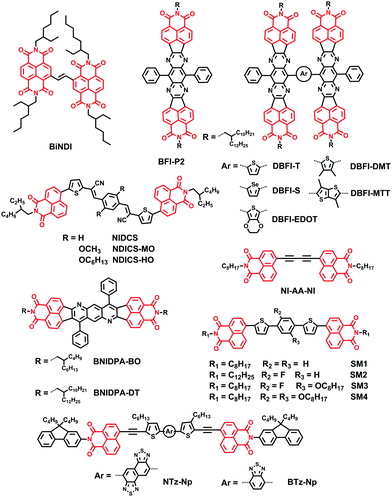
Naphthalene diimide (NDI) molecules possess a planar aromatic scaffold and two annulated electron-withdrawing imide groups; hence the aromatic naphthalene core possesses strong polarization of π-systems and low π-electron density.51–54 This particular structure means NDIs are among the most investigated classes of organic compounds, primarily due to the greatly modified optical and electronic properties via easy and effective modulation by appropriate simple functionalization. In addition, there are many literature reports on the NDIs possessing excellent field electronic transfer ability as air-stable n-type semiconductors due to the electron-deficient character. What's more, the broad absorption bands over the whole visible spectral range through the introduction of core substituents led to NDIs with interesting photosystems and photovoltaic applications.Core-functionalization of the NDIs has been considered as a general method to extend the planar, rigid core and prepare novel materials with new structures (Fig. 4).55–59 For example, in 2015, Russell and coworkers prepared a new small molecule, BiNDI, by linking two NDI monomers via a vinyl group, extending the π-conjugation length, planarizing the molecular backbone and enhancing the intermolecular π–π stacking. BiNDI exhibited excellent electron transport properties: the highest electron mobility for a neat film was 0.365 cm2 V−1 s−1 and the highest PCE for PSCs based on PTB7:BiNDI was 2.41% (Table 2).60
| Acceptor | Donor | Device structure | LUMO/HOMO (eV) | V OC (V) | J SC (mA cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from the onset oxidation and reduction potential. b Calculated from the absorption edge (λedge) with the equation of Eoptg = 1240/λedge (eV); the value of λedge is obtained from the absorption spectrum. c Calculated from the method of photoelectron spectroscopy in air (PESA). | ||||||||
| BiNDI | PTB7 | ITO/PEDOT:PSS/BHJ/Ca/Al | −4.35/−6.74 | 0.75 | 5.47 | 0.59 | 2.41 | 60 |
| NIDCS | PTB7 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.42/−5.90a | 0.73 | 8.04 | 0.46 | 2.71 | 61 |
| NIDCS-MO | PTB7 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.66/−5.75 | 0.54 | 6.23 | 0.43 | 1.45 | 61 |
| NIDCS-HO | PTB7 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.41/−5.79 | 0.67 | 8.15 | 0.47 | 2.55 | 61 |
| NIDCS-HO | PPDT2FBT | ITO/PEDOT:PSS/BHJ/Ca/Al | — | 1.03 | 11.88 | 0.63 | 7.64 | 62 |
| BNIDPA-BO | PSEHTT | ITO/ZnO/BHJ/MoO3/Ag | −3.60/−5.80 | 0.94 | 6.64 | 0.48 | 3.02 | 63 |
| BNIDPA-BO | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | — | 0.96 | 9.02 | 0.35 | 3.00 | 63 |
| BNIDPA-BO | PTB7 | ITO/ZnO/BHJ/MoO3/Ag | — | 0.98 | 8.13 | 0.39 | 3.08 | 63 |
| BNIDPA-DT | PSEHTT | ITO/ZnO/BHJ/MoO3/Ag | −3.60/−5.80 | 0.95 | 2.67 | 0.50 | 1.26 | 63 |
| BNIDPA-DT | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | — | 0.90 | 5.20 | 0.36 | 1.71 | 63 |
| BNIDPA-DT | PTB7 | ITO/ZnO/BHJ/MoO3/Ag | — | 0.95 | 4.12 | 0.36 | 1.42 | 63 |
| BFI-P2 | PSEHTT | ITO/ZnO/BHJ/MoO3/Al | −3.60/−5.80 | 0.94 | 3.16 | 0.49 | 1.44 | 64 |
| DFI-T | PSEHTT | ITO/ZnO/BHJ/MoO3/Al | −3.60/−5.80 | 0.86 | 10.14 | 0.58 | 5.04 | 64 |
| DBFI-S | PSEHTT | ITO/PEI/BHJ/MoO3/Ag | −3.70/−5.74 | 0.82 | 5.61 | 0.57 | 2.61 | 65 |
| DBFI-DMT | PSEHTT | ITO/PEI/BHJ/MoO3/Ag | −3.66/−5.82a | 0.92 | 12.56 | 0.55 | 6.37 | 65 |
| DBFI-MTT | PSEHTT | ITO/PEI/BHJ/MoO3/Ag | −3.67/−5.76a | 0.94 | 8.33 | 0.51 | 3.94 | 65 |
| DBFI-EDOT | PSEHTT | ITO/PEI/BHJ/MoO3/Ag | −3.65/−5.76a | 0.93 | 13.82 | 0.63 | 8.10 | 66 |
| SM1 | PCDTBT-C12 | ITO/PEDOT:PSS/BHJ/LiF/Al | −3.50/−5.93a | 1.04 | 4.79 | 0.56 | 2.78 | 67 |
| SM2 | PCDTBT-C12 | ITO/PEDOT:PSS/BHJ/LiF/Al | −3.62/−5.98a | 1.11 | 2.21 | 0.30 | 0.74 | 67 |
| SM3 | PCDTBT-C12 | ITO/PEDOT:PSS/BHJ/LiF/Al | −3.68/−5.95a | 1.09 | 1.71 | 0.30 | 0.56 | 67 |
| SM4 | PCDTBT-C12 | ITO/PEDOT:PSS/BHJ/LiF/Al | −3.65/−5.80a | 1.24 | 0.37 | 0.28 | 0.13 | 67 |
| NI-AA-NI | P1 | ITO/PEDOT:PSS/BHJ/LiF/Al | −3.57b/−6.00a | 1.06 | 4.10 | 0.43 | 1.87 | 68 |
| NI-AA-NI | P2 | ITO/PEDOT:PSS/BHJ/LiF/Al | — | 1.07 | 6.27 | 0.55 | 3.71 | 68 |
| NTz-Np | P3HT | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.60a/−6.01c | 0.90 | 5.18 | 0.60 | 2.81 | 69 |
| BTz-Np | P3HT | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.44a/−5.95c | 0.94 | 3.52 | 0.46 | 1.53 | 69 |
Furthermore, many research groups functionalized NDIs by inserting various electron-deficient chromophores, such as tetraazabenzodifluoranthene diimide (BFI) and diphenylanthrazoline (DPA), etc., between two naphthalimide (NI) units, to form a π-conjugated framework. Park and coworkers developed a class of novel NI-based NFAs (NIDCS, NIDCS-MO, and NIDCS-HO) by incorporating a NI moiety into the terminal position of dicyanodistyrylbenzene. These molecules consist of different substituents on the core phenyl unit, hydrogen, methoxy, and hexyloxy, respectively. Introduction of the cyano (CN) substituent as the electron-withdrawing moiety is beneficial for increasing the electron affinity; it promotes the formation of crystalline structures, and thus favors efficient charge transport. Thus, the PSCs based on P3HT:NIDCS-HO exhibited a maximum PCE of 2.71%.61 The PSCs based on PPDT2FBT:NIDCS-HO showed a maximum PCE of 7.6% with a remarkably high VOC of 1.03 V and also a reduced energy loss of 0.73 eV in the devices (Eoptg for PPDT2FBT is 1.76 eV).62
Jenekhe and coworkers reported a novel class of NI-based electron acceptors, 2,3,6,7-bis(naphthalene imide)-3,6-diphenyl-trans-anthrazolines by modulating the DPA unit via two terminated NI units. Photovoltaic devices composed of the BNIDPA-BO (2-butyloctyl) acceptor with PSEHTT, PTB7-Th, and PTB7 had higher PCEs of about 3.0% than those of BNIDPA-DT (2-decyltetradecyl)-based PSCs, emphasizing the critical role of the size of alkyl chains in NFAs.63
Jenekhe et al. also synthesized two related molecules, BFI-P2 and DBFI-T, and performed comparative studies of their charge photogeneration and photovoltaic properties by adopting a donor polymer, PSEHTT. Although BFI-P2 had a maximum organic field-effect transistor μe of 0.5 cm2 V−1 s−1, much higher than that of DBFI-T, i.e. 0.006 cm2 V−1 s−1, PSEHTT:DBFI-T blends showed sufficiently high and balanced electron and space-charge limited current (SCLC) hole mobilities (μe = 1.2 × 10−4 cm2 V−1 s−1, μh = 2.8 × 10−4 cm2 V−1 s−1) than those of PSEHTT:BFI-P2 blends (μe = 3.5 × 10−7 cm2 V−1 s−1, μh = 1.8 × 10−4 cm2 V−1 s−1). This may be caused by high anisotropy in charge transport of the highly crystalline molecule, BFI-P2. Thus, the PSCs based on DBFI-T with a nonplanar 3D architecture showed better performance (5.04% PCE) than PSCs based on BFI-P2.64 This research group then designed new acceptors (DBFI-T, DBFI-S, DBFI-DMT, BFI-MTT) by varying the arylene (Ar) linker (Ar = thiophene, selenophene, dimethylthiophene, dimethyl-thienothiophene) between two BFI building blocks. As a result, the interplanar angle between two BFI units in each molecule varied from 33° for DBFI-T, 40° for DBFI-S, and 53° for DBFI-MTT to 62° for DBFI-DMT. This played an important role in the absorption band, energy level, and electron transport properties, and thus the device performance. The best performing new electron acceptor material, DBFI-DMT, paired with PSEHTT, had a PCE of 6.4%, a VOC of 0.92 V and a maximum external quantum efficiency value of 80%.65 They also designed a very highly twisted 3D electron acceptor dimer, DBFI-EDOT, with a twisted angle of 76° between the two planar monomeric BFI units. A maximum PCE of 8.10% was achieved for the optimized DBFI-EDOT:PSEHTT devices and a PCE of 8.52% was achieved for DBFI-EDOT:PSEHTT:PBDTT-FTTE ternary blend solar cells.66 Thus, increasing the twisted angle between the large rigid π-conjugated building blocks in dimeric molecules can result in a large variation in the PCE of BHJ solar cells. This suggests that the twisted angle is a potential means to further improve the performance of NFAs in PSCs, since it improves molecular isotropy and symmetry, efficient molecular packing, isotropic carrier transport, and compatibility with a donor polymer in BHJ blends.
Bo and coworkers reported a series of NI based planar small molecules (SM1–4) comprising a central benzene core, two thiophene bridges and two NI terminal groups, attached with different substituents on the central benzene ring. With a decrease in the planarity of the small molecules, by attachment of different substituents on the central benzene ring, the twisted angles between the two NI end groups of SM1–4 gradually increased, and the PCEs based on these electron acceptors, when adopting PCDTBT-C12 as the electron donor, decreased from 2.78% to 0.13% due to the decreased electron mobility.67 Bo and coworkers also reported a new planar acceptor NI-AA-NI, with a diacetylene group as the bridge. This acceptor had a high crystallinity and a higher lying LUMO level of −3.57 eV. As a result, P2:NI-AA-NI based OPVs exhibited a PCE of 3.71% with a VOC of 1.07 V.68
Aso and coworkers developed new NI based electron-accepting compounds containing NTz and BTz as electron-deficient units, respectively. Conventional OPV devices fabricated from NTz-Np as an acceptor with P3HT as a donor showed superior performance with a PCE as high as 2.81% compared to the corresponding BTz-Np based devices.69
As a weaker electron withdrawing group, NI based small molecule acceptors have a high-lying LUMO level, which will reduce the energy loss and produce a high VOC. However, NI-based small molecule acceptors generally exhibit a low electron mobility, usually leading to unbalanced electron and hole mobility when blending with polymer donors. Therefore, strategies for designing NI based small molecule acceptors with planar configuration are urgently needed to facilitate electron transport in the active layer.
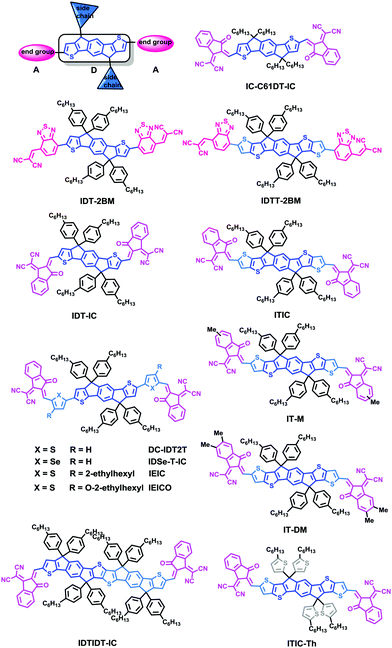
3. IDT-based small molecule acceptors
During the past two years, chemists have adopted rigid indacenodithiophene (IDT) or indacenodithieno[3,2-b]-thiophene (IDTT) as the core, substituted with strong electron-withdrawing groups, to provide electron acceptor materials, while also providing out-of-plane side chains for tuning processability and morphology/phase separation. Moreover, the rigid coplanar structure of the molecules can prevent rotational disorder and reduce reorganization energy, which may enhance charge carrier mobility. The IDT/IDTT based PSCs have exhibited great potential in achieving outstanding photovoltaic performance (Table 3).| Acceptor | Donor | Device structure | LUMO/HOMO (eV) | V OC (V) | J SC (mA cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from the onset oxidation and reduction potential. | ||||||||
| DC-IDT2T | PBDTTT-C-T | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.85/−5.43a | 0.90 | 8.33 | 0.52 | 3.93 | 70 |
| IDT-2BM | PTB7 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.80/−5.66a | 0.77 | 10.10 | 0.55 | 4.26 | 71 |
| IDTT-2BM | PTB7 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.80/−5.50a | 0.85 | 9.87 | 0.57 | 4.81 | 71 |
| IDT-IC | PTB7-Th | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.88/−5.61a | 0.83 | 9.53 | 0.40 | 3.16 | 72 |
| IDTIDT-IC | PTB7-Th | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.82/−5.42a | 0.94 | 14.5 | 0.48 | 6.48 | 72 |
| IEIC | PTB7-Th | ITO/PEDOT:PSS/BHJ/PDNI/Al | −3.82/−5.42a | 0.97 | 13.6 | 0.48 | 6.31 | 73 |
| IEIC | PffT2-FTAZ-2DT | ITO/ZnO/BHJ/V2O5/Al | — | 1.00 | 12.2 | 0.59 | 7.30 | 74 |
| IEICO | PTB7-Th | ITO/PEDOT:PSS/BHJ/PFN-Br/Al | −3.95/−5.32a | 0.82 | 17.7 | 0.58 | 8.40 | 75 |
| IDSe-T-IC | J51 | ITO/PEDOT:PSS/BHJ/PDNIO/Al | −3.79/−5.45a | 0.91 | 15.20 | 0.62 | 8.58 | 76 |
| ITIC | PTB7-Th | ITO/ZnO/BHJ/MoO3/Ag | −3.83/−5.48a | 0.90 | 5.20 | 0.36 | 1.71 | 77 |
| ITIC | PBDBT | ITO/ZnO/BHJ/MoO3/Al | — | 0.90 | 16.8 | 0.74 | 11.2 | 78 |
| ITIC | J51 | ITO/PEDOT:PSS/BHJ/PDNIO/Al | — | 0.82 | 16.5 | 0.69 | 9.26 | 79 |
| ITIC | J61 | ITO/PEDOT:PSS/BHJ/PDNIO/Al | — | 0.89 | 17.43 | 0.61 | 9.53 | 80 |
| IT-M | PBDBT | ITO/ZnO/BHJ/MoO3/Al | −3.98/−5.58a | 0.94 | 17.4 | 0.74 | 12.05 | 9 |
| IT-DM | PBDBT | ITO/ZnO/BHJ/MoO3/Al | −3.93/−5.56a | 0.97 | 16.5 | 0.71 | 11.29 | 9 |
| ITIC-Th | PDBT-T1 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.93/−5.66a | 0.88 | 16.2 | 0.67 | 9.60 | 81 |
| IC-C6IDT-IC | PDBT-T1 | ITO/PEDOT:PSS/BHJ/Ca/Al | −3.91/−5.69a | 0.89 | 15.1 | 0.65 | 8.71 | 82 |
In 2012, Zhan and coworkers for the first time reported a novel acceptor DC-IDT2T by applying the IDT unit as a central building block, 1,1-dicyanomethylene-3-indanone (DC) as electron-withdrawing end groups, and thiophene as π-bridges. Without any post-treatment, BHJ PSCs based on PBDTTT-C-T:DC-IDT2T blends yielded a PCE as high as 3.93%.70 In 2015, Zhan and coworkers explored two novel A–D–A type molecules, IDT-2BM and IDTT-2BM, with extended fused-ring IDT or IDTT units as cores and the strong electron-withdrawing unit 2-(benzo[c][1,2,5]thiadiazol-4-ylmethylene)-malononitrile (BM) as the end-capping group as electron acceptors in solution-processed PSCs. The PSCs based on PBDTTT-C-T:IDT-2BM and PBDTTT-C-T:IDTT-2BM blends exhibited promising PCEs of 4.26% and 4.81%, respectively; the BM unit held great potential for constructing efficient non-fullerene acceptors (Fig. 5).71
Liao and coworkers presented a non-fullerene electron acceptor bearing a fused 10-heterocyclic ring (indacenodithiopheno-indacenodithiophene), IDTIDT-IC. It showed broadened absorption (λedge = 810 nm) and a deep LUMO level due to the enhancement of effective conjugation length. In comparison with the device based on PTB7-Th:IDT-IC with a highest PCE value of 3.16% and a VOC of 0.83 V, the device based on IDTIDT-IC reached a maximum PCE value of 6.48% with the VOC of 0.94 V. More interestingly, when this molecule was paired with PTB7-Th, an Eloss as low as 0.59 eV was obtained.72
They then employed IDT as the core, end-capped with electron withdrawing 2-(3-oxo-2,3-dihydroinden-1-ylidene)malononitrile (INCN) groups, namely IEIC. The acceptor exhibited a strong and broad absorption spectrum in the 500–750 nm region, with an absorption edge of 790 nm. The optical bandgap was estimated to be 1.57 eV. The PSCs based on the blend of a narrow bandgap polymer donor PTB7-Th and IEIC exhibited PCEs of up to 6.31%.73 Nonetheless, it is observed that the IEIC's absorption range largely overlaps with the absorption spectrum of PTB7-Th. Yan and coworkers then presented a wide-bandgap (WBG) polymer named as PffT2-FTAZ-2DT with a complementary absorption to IEIC molecules from 450 to 800 nm, leading to a high PCE of 7.3% for the PffT2-FTAZ-2DT:IEIC-based PSCs.74 Therefore, developing and seeking a highly efficient donor material that matches well with NFAs is an important engineering tool. Based on IEIC, Hou replaced the alky groups in the IDT block with alkoxy ones, namely IEICO, possessing a narrow optical bandgap of 1.34 eV with a LUMO level of −3.95 eV. As a result, the PSC device based on PTB7-Th:IEICO showed a PCE of 8.4%. A PCE of 10.7% was recorded in a tandem PSC adopting PTB7-Th:IEICO as a rear cell.75 Liao coworkers presented an IDSe-T-IC acceptor bearing a fused five-membered heterocyclic ring containing selenium atoms, with an Eg of 1.52 eV, strong absorption in the 600–850 nm region, and a high LUMO level of −3.79 eV. The solar cell based on J51:IDSe-T-IC gave a PCE of 8.6%, much higher than that of J51:PC71BM-based PSCs under similar device fabrication conditions (PCE = 6.0%).76
Based on the acceptor IEIC, Zhan and coworkers designed ITIC by introducing rigid out-of-plane side chains onto the fused rings to facilitate the effective interchain π–π overlaps and enhance intermolecular charge transport, and the PCE of PTB7-Th:ITIC was 6.80%.77 Hou and coworkers adopted a new conjugated polymer, PBDB-T with a strong aggregation effect in solution and a small ΔHOMO (HOMOd − HOMOa) to fabricate solar cell devices. Outstanding performance with a PCE of 11.21% and excellent thermal stability were obtained.78 Li and coworkers used ITIC as an acceptor in the J51-based nonfullerene PSCs; encouragingly, the non-fullerene PSCs based on J51:ITIC exhibited a high PCE of 9.26%.79 This group also designed and synthesized a new medium bandgap 2D-conjugated copolymer (J61) adopting linear alkylthio substituents on the thiophene conjugated side chain of the benzodithiophene (BDT) units. The devices based on J61:ITIC showed a PCE of up to 9.53%.80 Hou and coworkers rationally designed two new molecular acceptors by incorporating methyl-modified end groups on ITIC, namely IT-M and IT-DM, by increasing the energy levels slightly, without causing too much steric hindrance for intermolecular packing. Finally, a high PCE of 12.05% was obtained for PBDB-T:IT-M.9
Zhan and coworkers successfully developed ITIC-Th based on ITIC after altering the phenyl side chains to thienyl side-chains to downshift the molecular energy levels and facilitate compatibility with high-performance electron donors, especially moderate or WBG donors. The PSCs based on ITIC-Th and the low-bandgap (LBG) PTB7-Th and the WBG polymer donor PDBT-T1 exhibited PCEs of up to 8.7% and 9.6%, respectively.81 Furthermore, based on the IDT unit, Zhan and coworkers also reported the IC-C6IDT-IC acceptor with four n-hexyl side chains and a highly planar backbone, prepared in high yield using a one-step facile reaction. The PSCs based on PDBT-T1:IC-C6IDT-IC without additional treatments yield PCEs of up to 8.71%, demonstrating that the planar acceptor also exhibits good performance in PSCs.82
These electron-donating extended fused rings, IDT and IDTT, bearing extended π-frameworks and rigid coplanar structures are beneficial to enhance charge carrier mobility. In addition, this type of non-fullerene SMA possessing a narrow bandgap and appropriate energy level fits in well with the WBG polymers that usually exhibit a high molar absorption coefficient in the range 350–700 nm and high hole mobility. These properties endow the devices based on IDT:WBG polymers with high EQEs, which is favorable for the JSC and FF.
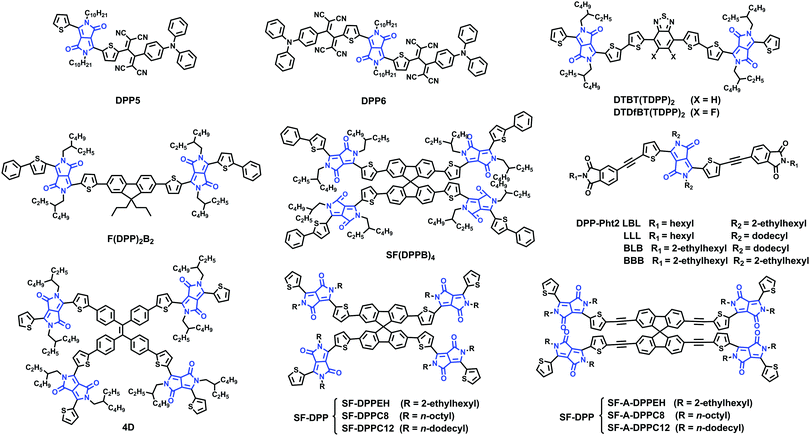
4. DPP based small molecule acceptors
Due to the strong electron withdrawing ability and high polarity of the DPP moiety, DPP-conjugated materials often exhibit broad and tunable optical absorption, high mobilities for holes and electrons, and an enhanced crystalline tendency, which are beneficial for high photocurrents and good fill factors in solar cells.83–87 Based on the above considerations, DPP has now become one of the most favored electron-deficient units used for constructing electron acceptors (Table 4).| Acceptor | Donor | Device structure | LUMO/HOMO (eV) | V OC (V) | J SC (mA cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Calculated from the onset oxidation and reduction potential. b Calculated from the method of photoelectron spectroscopy in air (PESA). | ||||||||
| DPP5 | P | ITO/PEDOT:PSS/BHJ/Al | −4.22/−5.54a | 0.92 | 8.23 | 0.52 | 3.90 | 88 |
| DPP6 | P | ITO/PEDOT:PSS/BHJ/Al | −4.36/−5.64a | 0.86 | 10.28 | 0.56 | 4.95 | 88 |
| DPP-Ph2 | P3HT | ITO/ZnO/BHJ/MoO3/Ag | −4.13/−5.88a | 0.89 | 5.91 | 0.50 | 3.28 | 89 |
| LBL | P3HT | ITO/ZnO/BHJ/MoO3/Ag | — | 0.89 | 5.80 | 0.50 | 3.23 | 90 |
| BBB | P3HT | ITO/ZnO/BHJ/MoO3/Ag | — | 0.15 | 2.72 | 0.25 | 0.12 | 90 |
| BLB | P3HT | ITO/ZnO/BHJ/MoO3/Ag | — | 0.50 | 4.40 | 0.40 | 1.10 | 90 |
| LLL | P3HT | ITO/ZnO/BHJ/MoO3/Ag | — | 0.14 | 0.51 | 0.25 | 0.02 | 90 |
| DTBT(TDPP)2 | PTB7 | ITO/ZnO/BHJ/MoO3/Ag | −4.18/−5.70 | 0.83 | 7.77 | 0.47 | 3.03 | 91 |
| DTDfBT(TDPP)2 | PTB7 | ITO/ZnO/BHJ/MoO3/Ag | −4.33/−5.85 | 0.81 | 12.1 | 0.51 | 5.00 | 91 |
| F(DPP)2B2 | P3HT | ITO/PEDOT:PSS/BHJ/PFN/Al | −3.39/−5.21a | 1.18 | 5.35 | 0.50 | 3.17 | 92 |
| 4D(TPE-DPP4) | P3HT | ITO/PEDOT:PSS/BHJ/Ca/A | −3.81a/−5.53b | 1.18 | 5.17 | 0.64 | 3.86 | 94 |
| SF(DPPB)4 | P3HT | ITO/PEDOT:PSS/BHJ/PFN/Al | −3.51/−5.26a | 1.14 | 8.29 | 0.55 | 5.16 | 95 |
Sharma and coworkers reported two small molecules, unsymmetrical DPP5 and symmetrical DPP6, substituted by triphenylamine based TCBD. DPP5 and DPP6 exhibited LBG with excellent absorption ability in the regions 500–800 and 500–900 nm, respectively. The devices based on optimized active layers of DPP5 and DPP6 and conjugated copolymer P showed PCEs of 3.90% and 4.95%, respectively (Fig. 6).88
Cabanetos and coworkers reported two electron acceptors built by attaching phthalimide groups on DPP through the use of acetylenic linkages. This permitted the extension of the effective conjugation, lowering the energy level of the frontier orbitals and reducing the steric interactions between thiophene units and phenyl rings. Once blended with P3HT, promising PCEs of 3.28% for DPP-Pht2 based devices were achieved.89 On the basis of this work, Cabanetos and coworkers also investigated the effect of side chains on the electronic and photovoltaic properties of DPP-Pht2 with different alkyl groups (linear and branched solubilizing groups on the phthalimide end or the DPP core, namely LBL, LLL, BLB and BBB). It turns out that BBB and LBL acceptors with branched side chains exhibit enhanced molecular light absorption and electronic mobility by favoring the formation of J-aggregates and more favorable phase separation with P3HT. Thus, the corresponding solar cells based on BBB and LBL had better PCEs (of 3.2%, 1.1%) than solar cells based on LLL and BLB (PCEs of 0.1%, 0.02%).90
Jo and coworkers synthesized two small-molecule electron acceptors using 4,7-dithien-2-yl-2,1,3-benzothiadiazole (DTBT) or fluoro-substituted DTBT (DTDfBT) as a core and DPP as a flanking unit to investigate the effect of fluorine substitution on the photovoltaic performance of NF-PSCs. Both electron acceptors exhibited a low bandgap of 1.5 eV with broad absorptivity in the range of 500–800 nm, high electron affinity, favorable energy levels, and high crystallinity due to the coplanar backbone structures and extended conjugation. As a result, the PSCs based on PTB7:DTDfBT(TDPP)2 exhibited a higher PCE of 5.00% than the DTBT(TDPP)2 based solar cells. The PTB7:DTDfBT(TDPP)2 based PSCs benefit from enhanced crystallinity and the resulting higher electron mobility of the fluorinated electron acceptor and thereby suppressed bimolecular recombination.91
Chen and coworkers designed and synthesized a bipolar DPP derivative F(DPP)2B2, in which a fluorene ring functioned as the core, two DPP units as the arms, and two benzene rings as the end-groups. This molecule showed an intense absorption band in the range of 550–700 nm. The solar cells based on P3HT:F(DPP)2B2 exhibited a PCE of 3.17% with a VOC of 1.18 V and those based on F(DPP)2B2:PC71BM showed a PCE of 3.26%.92 They then also developed a ternary PSC system P3HT:F(DPP)2B2:PC61BM using the bipolar F(DPP)2B2 molecule as the energy cascade material and obtained a best PCE of 3.92%, corresponding to ∼23% improvement. This was attributed to the enhanced light absorption in the 550–700 nm range, exciton dissociation and charge carrier transport by the introduction of the third bipolar component.93
Through the conjunction of TPE and DPP functionalities, a novel four-directional NFA (denoted as 4D) was designed, synthesized, and characterized. A PCE of 3.86% was obtained in solution-processable BHJ devices based on P3HT:4D with a very high VOC of 1.18 V.94
Chen and coworkers synthesized SF(DPPB)4 with SF as the core and four benzene end-capped DPP as arms. This molecule with cross-shaped molecular geometry was beneficial for suppressing strong intermolecular aggregation in a P3HT:SF(DPPB)4 blend. It exhibited energy levels that match well with those of P3HT, and thus the devices gave a PCE of 5.16% with an extremely high VOC of 1.14 V. Furthermore, the devices exhibited excellent thermal stability at 150 °C for 3 h.95 On the basis of the SF(DPPB)4 molecule, Chen and coworkers also investigated the effects of substituted alkyl side chains on molecular packing, crystallinity, and BHJ film morphology. They found the following: (1) the unique star-shaped molecular structure resulting from the spiro linkage imparted those molecules with high solubility and suppressed aggregation. (2) Small perturbations of the chemical structure, for example, the choice of different side chains and the C–C single bond or C–C triple bond connection, led to quite different physical properties, since the lack of structure order in SF-A-DPP thin films substantially reduces their electron transport characteristics. (3) In BHJ blends, SF-DPPEH crystallizes well after thermal annealing, while SF-DPPC8 and SF-DPPC12 show low crystallinity. (4) The branched 2-ethylhexyl (EH) group provided SF-DPPEH, which interacts less with P3HT, leading to an increase in the VOC. Thus, together with moderate crystallization of the acceptors, nanoscale phase separation (20–30 nm), and thus less-pronounced geminate recombination led to an increase in the device performance of P3HT:SF-DPPEH blends up to 3.63%.96
In conclusion, the DPP unit has a strong electron affinity and a planar, well-conjugated lactam skeleton, giving rise to strong π–π interactions. Molecules based on the DPP building block usually display a strong aggregation tendency in films. Hence, many strategies for constructing the twisted molecule have been proposed. The devices based on these electron acceptors have a high VOC and low voltage loss.
5. Conclusion and outlook
After decades of development, non-fullerene electron acceptors for PSCs have attracted considerable attention. Significant progress has been made, and the PCE of non-fullerene based polymer solar cells now exceeds 12%. Those developments demonstrated that some new non-fullerene acceptors have outperformed fullerenes in devices and will promote further development of the PSC field.However, NFAs still present challenges. First, the relatively low electron mobility and strong recombination losses seriously limit the development of NF-OSCs. Therefore, it is meaningful to design acceptors with an appropriate aggregation nature to form pure domains with a preferable charge transport pathway and decrease the bimolecular recombination possibility. Second, several examples demonstrate that some existing electron donors that are not suitable for the fullerene derivatives fit in well with the NFA materials. Hence, much effort should be devoted to the exploration of new donors that could form more appropriate alignment with the NFAs and endow the solar cells with low voltage losses as well as fast and efficient charge separation. Third, it must be noted that the lack of systematic perception about the exciton diffusion/separation process about the NFA based solar cells limits the further improvement of solar cells. Thus, much effort is urgently needed to explore further possible new donors and understand the charge separation process.
Benefiting from the diversification and implementability of chemical modification on non-fullerene acceptor materials along with the ongoing efforts to control the BHJ morphology, device engineering, and the availability of new donor materials, NFAs will become competitive with their fullerene counterparts and the dominant acceptors.
Acknowledgements
For financial support of this research, we thank the 973 Program (Grant 2014CB643500 and 2013CB933503), the National Natural Science Foundation of China (51673202, 21428304, and 21225209), NSFC-DFG Joint Project TRR61, and the Chinese Academy of Sciences (XDB12010400).Notes and references
- http://www.laserfocusworld.com/ .
- A. Richter, M. Hermle and S. W. Glunz, Photovoltaics, 2013, 4, 1184 CrossRef.
- C. Tang, Appl. Phys. Lett., 1986, 48, 183 CrossRef CAS.
- N. S. Saricifici, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474 Search PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russel and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- J. Huang, J. H. Carpenter, C. Li, J. Yu, H. Ade and A. K. Y. Jen, Adv. Mater., 2016, 28, 967 CrossRef CAS PubMed.
- S. Li, L. Ye, W. Zhao, S. Zhao, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423 CrossRef CAS PubMed.
- C. Zhan and J. Yao, Chem. Mater., 2016, 28, 1948 CrossRef CAS.
- C. Zhan, X. Zhang and J. Yao, RSC Adv., 2015, 5, 9300 Search PubMed.
- C. B. Nielsen, S. Holliday, H. Y. Chen, S. J. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803 CrossRef CAS PubMed.
- Y. Lin and X. Zhan, Adv. Energy Mater., 2015, 5, 1501063 CrossRef.
- W. Jiang, Y. Li and Z. Wang, Acc. Chem. Res., 2014, 47, 3135 CrossRef CAS PubMed.
- Z. Chen, A. Lohr, C. Saha-Moller and F. Wurthner, Chem. Soc. Rev., 2009, 38, 564 RSC.
- C. Li and H. Wonneberger, Adv. Mater., 2012, 24, 613 CrossRef CAS PubMed.
- V. Kamm, G. Battagliarin, I. A. Howard, W. Pisula, A. Mavrinskiy, C. Li, K. Müllen and F. Laquai, Adv. Energy Mater., 2011, 1, 297 CrossRef CAS.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2016, 49, 175 CrossRef CAS PubMed.
- P. E. Hartnett, A. Timalsina, H. S. Matte, N. Zhou, X. Guo, W. Zhao, A. Facchetti, R. P. Chang, M. C. Hersam, M. R. Wasielewski and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 16345 CrossRef CAS PubMed.
- X. Zhang, C. Zhan and J. Yao, Chem. Mater., 2015, 27, 166 CrossRef CAS.
- C. Zhan, X. Zhang and J. Yao, RSC Adv., 2015, 5, 93002 RSC.
- Y. Lin, Y. Wang, J. Wang, J. Hou, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2014, 26, 5137 CrossRef CAS PubMed.
- S. Liu, C. Wu, C. Li, S. Liu, K. Wei, H. Chen and A. K. Y. Jen, Adv. Sci., 2015, 2, 1500014 CrossRef PubMed.
- Y. Lin, Y. Wang, J. Wang, J. Hou, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2014, 26, 5137 CrossRef CAS PubMed.
- X. Zhang, Z. Lu, L. Ye, C. Zhan, J. Hou, S. Zhang, B. Jiang, Y. Zhao, J. Huang, S. Zhang, Y. Liu, Q. Shi, Y. Liu and J. Yao, Adv. Mater., 2013, 25, 5791 CrossRef CAS PubMed.
- W. Jiang, L. Ye, X. Li, C. Xiao, F. Tan, W. Zhao, J. Hou and Z. Wang, Chem. Commun., 2014, 50, 1024 RSC.
- L. Ye, W. Jiang, W. Zhao, S. Zhang, Y. Cui, Z. Wang and J. Hou, Org. Electron., 2015, 17, 295 CrossRef CAS.
- C.-H. Wu, C.-C. Chueh, Y.-Y. Xi, H.-L. Zhong, G.-P. Gao, Z.-H. Wang, L. D. Pozzo, T.-C. Wen and A. K. Y. Jen, Adv. Funct. Mater., 2015, 25, 5326 CrossRef CAS.
- D. Sun, D. Meng, Y. Cai, B. Fan, Y. Li, W. Jiang, L. Huo, Y. Sun and Z. Wang, J. Am. Chem. Soc., 2015, 137, 11156 CrossRef CAS PubMed.
- D. Meng, D. Sun, C. Zhong, T. Liu, B. Fan, L. Huo, Y. Li, W. Jiang, H. Choi, T. Kim, J. Y. Kim, Y. Sun, Z. Wang and A. J. Heeger, J. Am. Chem. Soc., 2016, 138, 375 CrossRef CAS PubMed.
- H. Zhong, C. Wu, C. Li, J. Carpenter, C. C. Chueh, J. Y. Chen, H. Ade and A. K. Jen, Adv. Mater., 2016, 28, 951 CrossRef CAS PubMed.
- P. E. Hartnett, H. S. S. Matte, N. D. Eastham, N. E. Jackson, Y. Wu, L. X. Chen, M. A. Ratner, R. P. H. Chang, M. C. Hersam, M. R. Wasielewski and T. J. Marks, Chem. Sci., 2016, 7, 3543 RSC.
- Y. Zhong, M. T. Trinh, R. Chen, W. Wang, P. P. Khlyabich, B. Kumar, Q. Xu, C. Y. Nam, M. Y. Sfeir, C. Black, M. L. Steigerwald, Y. L. Loo, S. Xiao, F. Ng, X. Y. Zhu and C. Nuckolls, J. Am. Chem. Soc., 2014, 136, 15215 CrossRef CAS PubMed.
- Y. Zhong, M. T. Trinh, R. Chen, G. E. Purdum, P. P. Khlyabich, M. Sezen, S. Oh, H. Zhu, B. Fowler, B. Zhang, W. Wang, C.-Y. Nam, M. Y. Sfeir, C. T. Black, M. L. Steigerwald, Y.-L. Loo, F. Ng, X. Y. Zhu and C. Nuckolls, Nat. Commun., 2015, 6, 8242 CrossRef CAS PubMed.
- Q. Yan, Y. Zhou, Y. Zheng, J. Pei and D. Zhao, Chem. Sci., 2013, 4, 4389 RSC.
- J. Zhao, Y. Li, H. Lin, Y. Liu, K. Jiang, C. Mu, T. Ma, J. Hu, D. Yu and H. Yan, Energy Environ. Sci., 2015, 8, 520 CAS.
- J. Liu, S. Chen, D. Qian, B. Gautam, G. Yang, J. Zhao, J. Bergqvist, F. Zhang, W. Ma, H. Ade, O. Inganas, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 16089 CrossRef CAS.
- Y. Lin, Y. Wang, J. Wang, J. Hou, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2014, 26, 5137 CrossRef CAS PubMed.
- S. Li, W. Liu, C. Li, F. Liu, Y. Zhang, M. Shi, H. Chen and T. P. Russell, J. Mater. Chem. A, 2016, 4, 10659 CAS.
- J. Lee, R. Singh, D. H. Sin, H. G. Kim, K. C. Song and K. Cho, Adv. Mater., 2016, 28, 69 CrossRef CAS PubMed.
- Y. Liu, C. Mu, K. Jiang, J. Zhao, Y. Li, L. Zhang, Z. Li, J. Lai, H. Hu, T. Ma, R. Hu, D. Yu, X. Huang, B. Tang and H. Yan, Adv. Mater., 2015, 27, 1015 CrossRef CAS PubMed.
- Y. Liu, J. Lai, S. Chen, Y. Li, K. Jiang, J. Zhao, Z. Li, H. Hu, T. Ma, H. Lin, J. Liu, J. Zhang, F. Huang, D. Yu and H. Yan, J. Mater. Chem. A, 2015, 3, 13632 CAS.
- H. Lin, S. Chen, H. Hu, L. Zhang, T. Ma, J. Lai, Z. Li, A. Qin, X. Huang, B. Tang and H. Yan, Adv. Mater., 2016, 28, 8546 CrossRef CAS PubMed.
- D. Zhao, Q. Wu, Z. Cai, T. Zheng, W. Chen, J. Lu and L. Yu, Chem. Mater., 2016, 28, 1139 CrossRef CAS.
- Q. Wu, D. Zhao, A. Schneider, W. Chen and L. Yu, J. Am. Chem. Soc., 2016, 138, 7248 CrossRef CAS PubMed.
- S. Rajaram, R. Shivanna, S. Kandappa and K. Narayan, J. Phys. Chem. Lett., 2012, 3, 2405 CrossRef CAS PubMed.
- L. Ye, K. Sun, W. Jiang, S. Zhang, W. Zhao, H. Yao, Z. Wang and J. Hou, ACS Appl. Mater. Interfaces, 2015, 7, 9274 CAS.
- N. Liang, K. Sun, Z. Zheng, H. Yao, G. Gao, X. Meng, Z. Wang, W. Ma and J. Hou, Adv. Energy Mater., 2016, 6, 1600060 CrossRef.
- G. Gao, X. Zhang, D. Meng, A. Zhang, Y. Liu, W. Jiang, Y. Sun and Z. Wang, RSC Adv., 2016, 6, 14027 RSC.
- W. Chen, X. Yang, G. Long, X. Wan, Y. Chen and Q. Zhang, J. Mater. Chem. C, 2015, 3, 4698 RSC.
- S. V. Bhosale, S. V. Bhosale and S. K. Bhargava, Org. Biomol. Chem., 2012, 10, 6455 CAS.
- S. L. Suraru and F. Wurthner, Angew. Chem., 2014, 53, 7428 CrossRef CAS PubMed.
- F. Wurthner and M. Stolte, Chem. Commun., 2011, 47, 5109 RSC.
- R. Kim, P. S. K. Amegadze, I. Kang, H.-J. Yun, Y.-Y. Noh, S.-K. Kwon and Y.-H. Kim, Adv. Funct. Mater., 2013, 23, 5719 CrossRef CAS.
- K. D. Deshmukh, T. Qin, J. K. Gallaher, A. C. Y. Liu, E. Gann, K. O'Donnell, L. Thomsen, J. M. Hodgkiss, S. E. Watkins and C. R. McNeill, Energy Environ. Sci., 2015, 8, 332 CAS.
- T. Earmme, Y. J. Hwang, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2014, 26, 6080 CrossRef CAS PubMed.
- Y. J. Hwang, T. Earmme, B. A. Courtright, F. N. Eberle and S. A. Jenekhe, J. Am. Chem. Soc., 2015, 137, 4424 CrossRef CAS PubMed.
- J. W. Jung, J. W. Jo, C. C. Chueh, F. Liu, W. H. Jo, T. P. Russell and A. K. Jen, Adv. Mater., 2015, 27, 3310 CrossRef CAS PubMed.
- C. Lee, H. Kang, W. Lee, T. Kim, K. H. Kim, H. Y. Woo, C. Wang and B. J. Kim, Adv. Mater., 2015, 27, 2466 CrossRef CAS PubMed.
- Y. Liu, L. Zhang, H. Lee, H.-W. Wang, A. Santala, F. Liu, Y. Diao, A. L. Briseno and T. P. Russell, Adv. Energy Mater., 2015, 5, 1500195 CrossRef.
- O. K. Kwon, J.-H. Park, S. K. Park and S. Y. Park, Adv. Energy Mater., 2015, 5, 1400929 CrossRef.
- O. K. Kwon, M. A. Uddin, J. H. Park, S. K. Park, T. L. Nguyen, H. Y. Woo and S. Y. Park, Adv. Mater., 2016, 28, 910 CrossRef CAS PubMed.
- H. Li, T. Earmme, S. Subramaniyan and S. A. Jenekhe, Adv. Energy Mater., 2015, 5, 1402041 CrossRef.
- H. Li, T. Earmme, G. Ren, A. Saeki, S. Yoshikawa, N. M. Murari, S. Subramaniyan, M. J. Crane, S. Seki and S. A. Jenekhe, J. Am. Chem. Soc., 2014, 136, 14589 CrossRef CAS PubMed.
- H. Li, Y. J. Hwang, B. A. Courtright, F. N. Eberle, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2015, 27, 3266 CrossRef CAS PubMed.
- Y. J. Hwang, H. Li, B. A. Courtright, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2016, 28, 124 CrossRef CAS PubMed.
- J. Zhang, X. Zhang, H. Xiao, G. Li, Y. Liu, C. Li, H. Huang, X. Chen and Z. Bo, ACS Appl. Mater. Interfaces, 2016, 8, 5475 CAS.
- J. Zhang, X. Zhang, G. Li, H. Xiao, W. Li, S. Xie, C. Li and Z. Bo, Chem. Commun., 2016, 52, 469 RSC.
- S. Chatterjee, Y. Ie, M. Karakawa and Y. Aso, Adv. Funct. Mater., 2016, 26, 1161 CrossRef CAS.
- H. Bai, Y. Wang, P. Cheng, J. Wang, Y. Wu, J. Hou and X. Zhan, J. Mater. Chem. A, 2015, 3, 1910 CAS.
- Y. Wu, H. Bai, Z. Wang, P. Cheng, S. Zhu, Y. Wang, W. Ma and X. Zhan, Energy Environ. Sci., 2015, 8, 3215 CAS.
- Y. Li, X. Liu, F. Wu, Y. Zhou, Z. Jiang, B. Song, Y. Xia, Z. Zhang, F. Gao, O. Inganas, Y. Li and L. Liao, J. Mater. Chem. A, 2016, 4, 5890 CAS.
- Y. Z. Lin, Z. G. Zhang, H. Bai, J. Wang, Y. Yao, Y. Li, D. Zhu and X. Zhan, Energy Environ. Sci., 2015, 8, 610 CAS.
- H. Lin, S. Chen, Z. Li, J. Lai, G. Yang, T. McAfee, K. Jiang, Y. Li, Y. Liu, H. Hu, J. Zhao, W. Ma, H. Ade and H. Yan, Adv. Mater., 2015, 27, 7299 CrossRef CAS PubMed.
- H. Yao, Y. Chen, Y. Qin, R. Yu, Y. Cui, B. Yang, S. Li, K. Zhang and J. Hou, Adv. Mater., 2016, 28, 8283 CrossRef CAS PubMed.
- Y. Li, L. Zhong, F. Wu, Y. Yuan, H. Bin, Z. Jiang, Z. Zhang, Z. Zhang, Y. Li and L. Liao, Energy Environ. Sci., 2016, 9, 3429 CAS.
- Y. Lin, J. Wang, Z. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganas, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734 CrossRef CAS PubMed.
- L. Gao, Z. G. Zhang, H. Bin, L. Xue, Y. Yang, C. Wang, F. Liu, T. P. Russell and Y. Li, Adv. Mater., 2016, 28, 8288 CrossRef CAS PubMed.
- H. Bin, Z.-G. Zhang, L. Gao, S. Chen, L. Zhong, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 4657 CrossRef CAS PubMed.
- Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C. J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973 CrossRef CAS PubMed.
- K. H. Hendriks, G. H. L. Heintges, V. S. Gevaerts, M. M. Wienk and R. A. Janssen, Angew. Chem., Int. Ed., 2013, 52, 8341 CrossRef CAS PubMed.
- R. S. Ashraf, I. Meager, M. Nikolka, M. Kirkus, M. Planells, B. C. Schroeder, S. Holliday, M. Hurhangee, C. B. Nielsen, H. Sirringhaus and I. McCulloch, J. Am. Chem. Soc., 2015, 137, 1314 CrossRef CAS PubMed.
- H. Choi, S.-J. Ko, T. Kim, P.-O. Morin, B. Walker, B. H. Lee, M. Leclerc, J. Y. Kim and A. J. Heeger, Adv. Mater., 2015, 27, 3318 CrossRef CAS PubMed.
- W. Li, K. H. Hendriks, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2013, 135, 5529 CrossRef CAS PubMed.
- W. Li, K. Hendriks, M. M. Wienk and R. A. J. Janssen, Acc. Chem. Res., 2016, 49, 78 CrossRef CAS PubMed.
- Y. Patil, R. Misra, M. L. Keshtov and G. D. Sharma, J. Phys. Chem. C, 2016, 120, 6324 CAS.
- P. Josse, C. Dalinot, Y. Jiang, S. Dabos-Seignon, J. Roncali, P. Blanchard and C. Cabanetos, J. Mater. Chem. A, 2016, 4, 250 CAS.
- P. Josse, A. Labrunie, C. Dalinot, S. M. McAfee, S. Dabos-Seignon, J. Roncali, G. C. Welch, P. Blanchard and C. Cabanetos, Org. Electron., 2016, 37, 479 CrossRef CAS.
- J. W. Jung and W. H. Jo, Chem. Mater., 2015, 27, 6038 CrossRef CAS.
- H. Shi, W. Fu, M. Shi, J. Ling and H. Chen, J. Mater. Chem. A, 2015, 3, 1902 CAS.
- W. Liu, H. Shi, L. Zuo, L. Wang and H. Chen, Org. Electron., 2015, 25, 219 CrossRef CAS.
- A. Rananaware, A. Gupta, J. Li, A. Bilic, L. Jones, S. Bhargava and S. V. Bhosale, Chem. Commun., 2016, 52, 8522 RSC.
- S. Li, W. Liu, M. Shi, J. Mai, T. Lau, J. Wan, X. Lu, C. Li and H. Chen, Energy Environ. Sci., 2016, 9, 604 CAS.
- X. Wu, W. Fu, Z. Xu, M. Shi, F. Liu, H. Chen, J. Wan and T. P. Russell, Adv. Funct. Mater., 2015, 25, 5954 CrossRef CAS.
| This journal is © the Partner Organisations 2017 |