Manipulation of cellular orientation and migration by internalized magnetic particles†
Jiaojiao
Liu‡
ab,
Xiaodong
Tian‡
c,
Meimei
Bao
ab,
Jingliang
Li
d,
Yujiang
Dou
e,
Bing
Yuan
*ab,
Kai
Yang
*ab and
Yuqiang
Ma
abf
aCenter for Soft Condensed Matter Physics and Interdisciplinary Research, Soochow University, Suzhou, 215006, P. R. China. E-mail: yuanbing@suda.edu.cn; yangkai@suda.edu.cn
bCollege of Physics, Optoelectronics and Energy, Soochow University, Suzhou, 215006, P. R. China
cDepartment of Thoracic Surgery, Chinese PLA General Hospital, Beijing, 100853, P. R. China
dInstitute for Frontier Materials, Deakin University, Geelong, Australia
eSchool of Electronic and Information Engineering, Soochow University, Suzhou, 215006, P. R. China
fNational Laboratory of Solid State Microstructures and Department of Physics, Nanjing University, Nanjing, 210093, P. R. China
First published on 9th December 2016
Abstract
The quick response of magnetic nanoparticles (MNPs) to an external field provides a unique way for cellular manipulation in a remote and non-contact mode. In this work, we demonstrate the modulation of cellular behaviors including orientation and migration based on internalized Fe3O4 nanoparticles in a particle-concentration dependent manner. After being treated with MNPs at low concentrations (e.g. 0.277 μg mL−1), the internalized particles separately distributed around the nuclei, and somewhat influenced the orientation of the cells along the direction of the external magnetic field. In contrast, when the concentration of MNPs was high enough (e.g. 2.770 μg mL−1), the particles formed clusters within the cells and moved towards the edges of the cell in the direction of the magnetic field, leading to an obvious morphological change and subsequently a directed migration of the cells. This result shows a facile way to manipulate cell behaviors with excellent biocompatibility and its potential application in the biomedical field such as in tissue engineering.
Morphological changes and migration of cells are regarded as central issues not only in many biological processes such as embryonic development, cell signaling and wound healing, but also in tissue engineering applications including cell capture and/or separation, multicellular construction of artificial tissue, etc.1–3 Thus, attempts to modulate these cellular behaviors are undoubtedly significant. Compared with the surface-aided cell growth control using patterned substrates,4–7 and fluidic or dielectrophoresis modulation of cells for transport, separation and characterization,8 straightforward applications of biofunctionalized magnetic nanoparticles (MNPs) that are capable of attaching to the cell membrane promise a unique way to modulate cellular behavior in a template-free, highly selective, remote and non-contact mode both in vitro and in vivo, due to the quick response of MNPs to an external magnetic field.9–14 However, it is realized based on the prerequisite of surface functionalization of MNPs for cell binding which involves complicated manipulation procedures including purification; moreover, it might cause some adverse effects such as cell twisting and detachment from the substrate during a long-term cellular modulation process.15–17 These limit its applications in certain fields. Herein, we report a simple but effective method for modulating cellular behavior including orientation and migration based on internalized MNPs.18,19 The influence of internalized MNPs on cell viability, functions (e.g. stem-cell differentiation,20 neural actuation,21,22 or endothelial progenitor cell (EPC) growth23–25), and magnetic resonance imaging (MRI) detection26 has been previously investigated. In this regard, modulation of cellular orientation or migration requires the use of a larger amount of particles without causing adverse effects on cell viability.27,28 This work demonstrates that Fe3O4 NPs are excellent candidates with negligible cytotoxicity even if a large number of particles are internalized into cells. It is significant to find that when exposed to an external magnetic field (e.g. 4000 G in this work), the internalized Fe3O4 particles have a strong ability to modulate the orientation, morphology and migration of human gastric carcinoma (MGC-803) cells, which occurs in a particle-concentration dependent manner.
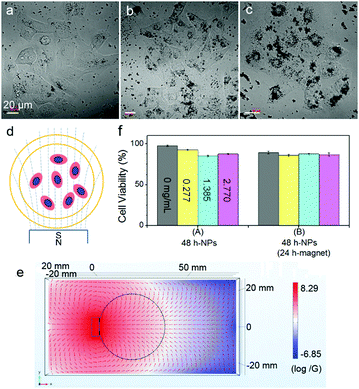
The cells showed surprising tolerance against Fe3O4 NPs under the conditions of particle concentration and magnetic intensity used in this work (Fig. 1). After co-incubation of cells with Fe3O4 NPs (170 ± 50 nm) at different concentrations of 0.277, 1.385 and 2.770 μg mL−1 at 37 °C for 24 h, the internalized particles, which can be obviously distinguished under both an AFM and an optical microscope (Fig. S1, see the ESI†), showed different ways of distributing inside the cells. At a high concentration of 2.770 μg mL−1, the particles accumulated into clusters of ∼7 μm on average; while at a low concentration of 0.277 μg mL−1, the particles homogeneously dispersed around the nuclei of the cells. The cells were exposed to a permanent magnet generating a magnetic field of 4000 G at the surface, and the magnetic field distribution in the system was simulated using Comsol software (Fig. 1d and e; for a 3D side view, refer to Fig. S2 in the ESI†).29 At all MNP concentrations, more than 85% of the cells survived even after being exposed to a magnet for 24 h (Fig. 1f). Importantly, most of the cells adhered and spread well on the substrate surface, which is distinctly different from the abnormal cell behaviors induced by membrane-bound MNPs after 4 h of magnetic attraction, as reported in previous work.16
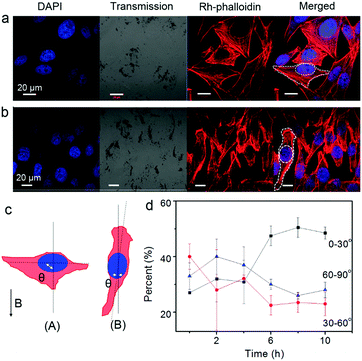
Furthermore, the orientation of the cells was pronouncedly influenced by an external magnetic field due to the internalized Fe3O4 NPs (Fig. 2). Cells at the central location of the Petri dish were selected for observation. The Fe3O4 particles (or particle aggregates) inside the cells showed a quick response to the external magnetic field. Upon applying a magnetic field, the particles, which once distributed around the nuclei of the cells roughly in an elliptical shape, aligned within seconds along the direction of the magnetic field. Interestingly, the morphology and orientation of the cells changed accordingly. One representative example showing the orientation angle θ of the cells (the acute angle) with respect to the magnetic field is shown in Fig. 2c. By analyzing the angle distribution of more than 30 cells from four independently repeated experiments both before and after magnetic treatment (Fig. 2d; ESI†), it was found that after 10 h of magnetic attraction, nearly half of the cells (∼48.5%) obviously oriented or reoriented themselves along the direction of the magnetic field (with an orientation angle θ ≤ 30°). In contrast, the percentage of the cells with other orientations (30° < θ ≤ 60° or 60° < θ ≤ 90°) decreased to around 25%. Note that at the initial moment, the cells orientated randomly with angles distributed almost uniformly among these three ranges. These results suggest that the internalized MNPs modulate the cells's orientation in the magnetic field.
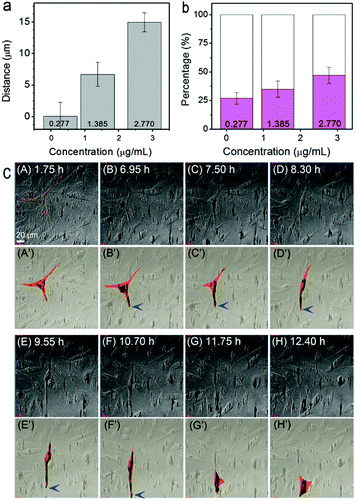
The migration behavior of cells with internalized MNPs was also strongly affected in the external magnetic field. For real-time monitoring of the influence of the magnet, cells pre-incubated with Fe3O4 NPs at different concentrations were transferred respectively into a cell cultivation incubator equipped on a confocal microscope for in situ magnetic treatments. A particle-concentration dependent rule in cell migration behavior was observed after a 12 h treatment. The mean moving distance of the cells in the magnetic direction (take the direction towards the magnet as positive) varied from ∼0.05 μm at 0.277 μg mL−1, to ∼15 μm at 2.770 μg mL−1 (averaged from more than 30 cells in four independently repeated experiments for each particle concentration, Fig. 3a). Actually, even upon magnet attraction, the motion of the cells was still complicated. (Besides moving towards the magnet, cells moving along the direction of the original filopodium, moving in complex orientations, or even motionless cells, were also observed.) Despite this, similar to the statistical result of the mean moving distance, the percentage of the amount of cells moving along the external magnetic field also depends on the particle concentration. For the cells pre-incubated with NPs at 2.770 μg mL−1, almost 50% of the cells were found moving towards the magnetic field direction (Fig. 3b, in magenta); while for the condition of 0.277 μg mL−1, the percentage decreased to approximately 27% (and mostly with a much smaller shifting distance). Such a particle-concentration dependent phenomenon in cell migration clearly indicates the influence of the loaded Fe3O4 NPs on the magnet-directed movement of the cells.
To further understand the influence of the loaded Fe3O4 particles on the morphology and behavior of the cells in a magnetic field, real-time tracking of cell movement was performed. Fig. 3c shows time-lapse tracking of cells (pre-incubated with 2.770 μg mL−1 Fe3O4 NPs) in a vertically downward magnetic field for over 12 h. Panels A–H are top-view snap-shot images (extracted from Video S1, ESI;† the magnet was applied at the time of 0 h). For clarity, the schematic morphology and migration tracking of one representative cell, being extracted from panels A–H, are shown in panels A′–H′. At t = 1.75 h, i.e. panel A′, the cell showed a characteristic asymmetric shape with three pseudopodia spreading over the substrate. Such a cell shape was maintained for almost 6 hours. At around t = 6.95 h, the internalized particles, which were initially separately distributed, were found to be accumulating and shifting downward within the cell, leading to the deformation of the cell shape. After the time of t = 7.5 h (panels C′ and D′), remarkable elongation of the cell occurred, with one filopodium prominently spreading forward. Then, at t = 9.55 or 10.7 h (panels E′ and F′), the cell migrated downward, probably driven by the front filopodium. At last, after t = 11.75 h (G′ and H′), the cell completed the movement and recovered to its original triangular shape and spread over the substrate again. Note that during the stages of cellular deformation and migration, the particles kept aggregating as clusters and remained at the leading top of the filopodium in the direction of the magnetic field (marked with blue arrows in Fig. 3c).
The real time observations demonstrated that aggregation and shifting of the MNPs were essential for the directed cell migration. For example, at a low particle concentration of 0.277 μg mL−1, the internalized particles, which had localized around the nucleus, tended to align but without much aggregation or intracellular movement. Accordingly, magnet-directed cellular migration was not obvious (Fig. S3, ESI†). In contrast, as demonstrated above, at a high MNP concentration of 2.770 μg mL−1, magnet directed particle migration occurred. Hence, it can be concluded that the directed movement of the internalized particle aggregates under a magnetic field governs the deformation and modulated migration of a cell. The modulating behavior of internalized Fe3O4 particles might involve a number of underlying mechanisms, such as cell membrane distortion due to a mechanical force exerted by the particle aggregates on the cell,29 the response in the cytoskeleton and some other related protein structures, and ultimately the realization of focal adhesion and formation of a directed pseudopodium.
Moreover, the influence of the magnet-directed cellular migration on the spatial distribution of the cells in the system was examined. Fig. S4 (ESI†) shows the result after 12 h of magnetic treatment of the cells pre-incubated with 2.770 μg mL−1 Fe3O4 particles. The cells were enriched at the bottom side of the Petri dish in the direction of the magnetic field. This result indicates the promising applications of such magnetically modulated cellular migration in the biomedical field including targeted delivery and tissue engineering.22,24 However, in a control experiment it was found that such MNP modulation on cellular orientation and migration was barely perceptible on round-shaped and less invasive cells such as HeLa (Fig. S5, ESI†).
In conclusion, modulation of cellular orientation and movements with internalized Fe3O4 particles in the direction of a magnetic field was investigated. The cells were firstly co-incubated with Fe3O4 NPs at different concentrations for particle internalization prior to magnetic field application. At low MNP concentrations (e.g. 0.277 μg mL−1), the internalized particles separated in the lysosomes surrounding the nucleus, and tended to arrange into orientated line segments in the magnetic field. Such orientated particle arrangements influenced the orientation of the cell along the magnetic direction to some extent, but hardly affected cellular migration. In contrast, at a high concentration (e.g. 2.770 μg mL−1), obvious aggregation with the internalized particles occurred. In the magnetic field, the aggregated particle clusters moved in the direction of the magnetic field and assembled at the leading edge of the cell, which mediated the migration of the cells. The modulated behavior of the internalized particles is supposed to be a key factor for the mediated orientation and migration of the cells in the magnetic field, which may promise wide applications for targeted delivery and tissue engineering in the future.
Acknowledgements
This work was financially supported by the National Science Foundation of China (No. 91427302, 21422404, 21374074, U1532108 and 11474155).Notes and references
- D. Dormann and C. J. Weijer, EMBO J., 2006, 25, 3480 CrossRef CAS PubMed.
- C. J. Weijer, J. Cell Sci., 2009, 122, 3215 CrossRef CAS PubMed.
- S. E. Le Devedec, K. A. Yan, H. de Bont, V. Ghotra, H. Truong, E. H. Danen, F. Verbeek and B. van de Water, Cell. Mol. Life Sci., 2010, 67, 3219 CrossRef CAS PubMed.
- A. Pathak and S. Kumar, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10334 CrossRef CAS PubMed.
- M. Nikkhah, F. Edalat, S. Manoucheri and A. Khadem-hosseini, Biomaterials, 2012, 33, 5230 CrossRef CAS PubMed.
- S. R. Krishna Vedula, M. C. Leong, T. L. Lai, P. Hersen, A. J. Kabla, C. T. Lim and B. Ladoux, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 12974 CrossRef PubMed.
- L. Lara Rodriguez and I. C. Schneider, Integr. Biol., 2013, 5, 1306 RSC.
- K. Khoshmanesh, S. Nahavandi, S. Baratchi, A. Mitchell and K. Kalantar-zadeh, Biosens. Bioelectron., 2011, 26, 1800 CrossRef CAS PubMed.
- T. D. Schladt, K. Schneider, H. Schild and W. Tremel, Dalton Trans., 2011, 40, 6315 RSC.
- E. Amstad, M. Textor and E. Reimhult, Nanoscale, 2011, 3, 2819 RSC.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Chem. Rev., 2012, 112, 5818 CrossRef CAS PubMed.
- H. Gu, P.-L. Ho, K. W. T. Tsang, L. Wang and B. Xu, J. Am. Chem. Soc., 2003, 125, 15702 CrossRef CAS PubMed.
- J. Dobson, Nat. Nanotechnol., 2008, 3, 139 CrossRef CAS PubMed.
- M. Colombo, S. Carregal-Romero, M. F. Casula, L. Gutierrez, M. P. Morales and I. B. Bohm, et al. , Chem. Soc. Rev., 2012, 41, 4306 RSC.
- V. H. Ho, K. H. Muller, A. Barcza, R. Chen and N. K. Slater, Biomaterials, 2010, 31, 3095 CrossRef CAS PubMed.
- M. J. Long, Y. Pan, H. C. Lin, L. Hedstrom and B. Xu, J. Am. Chem. Soc., 2011, 133, 10006 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995 CrossRef CAS PubMed.
- M. Bradshaw, T. D. Clemons, D. Ho, L. Gutiérrez, F. J. Lázaro, M. J. House, T. G. S. Pierre, M. W. Fear, F. M. Wood and K. S. Iyer, Nanoscale, 2015, 7, 4884 RSC.
- E. E. White, A. Pai, Y. Weng, A. K. Suresh, D. Van.Haute, T. Pailevanian, D. Alizadeh, A. Hajimiri, B. Badie and J. M. Berlin, Nanoscale, 2015, 7, 7780 RSC.
- D. Fayol, N. Luciani, L. Lartigue, F. Gazeau and C. Wilhelm, Adv. Healthcare Mater., 2013, 2, 313 CrossRef CAS PubMed.
- C. Riggio, M. P. Calatayud, C. Hoskins, J. Pinkernelle, B. Sanz and T. E. Torres, et al. , Int. J. Nanomed., 2012, 7, 3155 CAS.
- N. Alon, T. Havdala, H. Skaat, K. Baranes, M. Marcus and I. Levy, et al. , Lab Chip, 2015, 15, 2030 RSC.
- C. Wilhelma, L. Bal, P. Smirnov, I. Galy-Fauroux, O. Clement and F. Gazeau, et al. , Biomaterials, 2007, 28, 3797 CrossRef PubMed.
- B. Polyak, I. Fishbein, M. Chorny, I. Alferiev, D. Williams and B. Yellen, et al. , Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 698 CrossRef CAS PubMed.
- F. Gazeau and C. Wihelm, Future Med. Chem., 2010, 2, 397 CrossRef CAS PubMed.
- M.-Q. Wei, D.-D. Wen, X.-Y. Wang, Y. Huan, Y. Yang and J. Xu, et al. , Mol. Med. Rep., 2015, 11, 3814 CAS.
- H. A. Schreiber, J. Prechl, H. Jiang, A. Zozulya, Z. Fabry, F. Denes and M. Sandor, J. Immunol. Methods, 2010, 356, 47 CrossRef CAS PubMed.
- H. Markides, M. Rotherham and A. J. El Haj, J. Nanomater., 2012, 2012, 4873 Search PubMed.
- M. Marcus, M. Karni, K. Baranes, I. Levy, N. Alon and S. Margel, et al. , J. Nanobiotechnol., 2016, 14, 37 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and data analysis methods. Characterization and cellular uptake of Fe3O4 NPs. Real-time tracking (images and video) of cells with loaded particles in a magnetic field. Spatial redistribution of cells in a Petri dish with loaded MNPs in a magnet. See DOI: 10.1039/c6qm00219f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |