A novel radiation chemistry-based methodology for the synthesis of PEDOT/Ag nanocomposites
Zhenpeng
Cui
a,
Cecilia
Coletta
a,
Teseer
Bahry
a,
Jean-Louis
Marignier
a,
Jean-Michel
Guigner
b,
Matthieu
Gervais
c,
Sarah
Baiz
c,
Fabrice
Goubard
d and
Samy
Remita
*ae
aLaboratoire de Chimie Physique, LCP, UMR 8000, CNRS, Université Paris-Sud 11, Bât. 349, Campus d'Orsay, 15 avenue Jean Perrin, 91405 Orsay Cedex, France. E-mail: samy.remita@u-psud.fr
bInstitut de Minéralogie et de Physique des Milieux Condensés, IMPMC, UMR 7590, CNRS, Université Pierre et Marie Curie, Tour 23, Campus de Jussieu, 4 Place Jussieu, 75252 Paris Cedex 05, France
cLaboratoire Procédés et Ingénierie en Mécanique et Matériaux, PIMM, ENSAM, UMR 8006, CNRS, CNAM, 151 boulevard de l'hôpital 75013 Paris, France
dLaboratoire de Physicochimie des Polymères et Interfaces, LPPI, EA 2528, Université de Cergy-Pontoise, 5 mail Gay Lussac, Neuville sur Oise, 95031 Cergy-Pontoise Cedex, France
eDépartement CASER, Ecole SITI, Conservatoire National des Arts et Métiers, CNAM, 292 rue Saint-Martin, 75141 Paris Cedex 03, France
First published on 5th December 2016
Abstract
An original methodology based on radiation chemistry was used for the synthesis of hybrid organic–inorganic composites in aqueous solution. Starting from 3,4-ethylenedioxythiophene (EDOT) monomers and silver perchlorate salt, the preparation of PEDOT/Ag composites, made of poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymers and silver nanoparticles, was achieved through γ-radiolysis via different procedures. According to a two-step method, PEDOT polymers were first synthesized by reduction or oxidation of the EDOT monomers, and then silver nanoparticles were produced in the presence of PEDOT by the reduction of silver ions. According to a one-pot method, PEDOT polymers and silver nanoparticles were synthesized simultaneously thanks to the concomitant reduction of EDOT monomers and silver ions, as demonstrated via pulse radiolysis experiments. As highlighted using UV-Vis absorption spectrophotometry, ATR-FTIR spectroscopy and EDX spectroscopy, all the prepared PEDOT/Ag nanocomposites have the same chemical composition and are found to be doped, after lyophilization, with perchlorate anions. Nevertheless, due to the different synthesis procedures, the morphologies of the nanocomposites are found to be almost different as demonstrated using cryo-TEM and SEM microscopies. TGA revealed that, whatever their morphology, all the PEDOT/Ag composites are characterized by the same thermal stability, which is higher than that of pure PEDOT and which is enhanced upon increasing the amount of silver. Finally, four-point probe measurements demonstrated that the values of electrical conductivity of all the radiosynthesized PEDOT/Ag composites are in the same order of magnitude and remain close to those reported in the literature for PEDOT nanocomposites prepared according to other methodologies.
1. Introduction
Nanocomposites, composed of organic polymers and inorganic components, have attracted intensive research interest due to their remarkable properties.1,2 Conducting polymers (CPs), such as poly(3,4-ethylenedioxythiophene) (PEDOT), polypyrrole (PPy) and polyaniline (PANI), are organic polymers which exhibit inherent electrical conductivity due to the unique π-conjugated system in their structures,3–5 while noble metal (Au, Ag) nanoparticles consist of inorganic components which have already been widely studied and applied in a variety of fields.6,7 Nanocomposites formed by the combination of CPs with noble metal nanoparticles can not only inherit the excellent properties from their corresponding parent constituents but can also be characterized by novel properties with proper association.8–13 Such CP nanocomposites have potential applications in various areas including conducting materials, electrochromic devices, sensors, catalysts, etc.14–20Generally, the synthesis of CP nanocomposites can be achieved via traditional chemical and electrochemical routes.12,21,22 According to the synthetic methodologies, a two-step method or one-pot method is usually applied to synthesize CP nanocomposites.23,24 The latter usually have a core–shell structure, the composition of which fundamentally depends on the preparation procedure.25–27
γ-Ray radiolysis is very often used at ambient temperature and pressure in order to initiate, in the absence of any external chemical initiators, oxidation28,29 or reduction reactions,30,31 through the selective control of the initiator radicals, the nature of which depends on the medium (atmosphere, solvent, and potential solute). Radiolysis is known to be an interesting method for the synthesis of metal nanoparticles in aqueous solution,32–36 by using reducing species (such as hydrated electrons, e−aq) which enable metal ion reduction, and then metal atom aggregation. Also, radiolysis has recently been successfully used as a new alternative methodology for the preparation of nanostructured CPs in aqueous solution,37–42 by using oxidizing species (such as hydroxyl radicals, HO˙) which enable organic monomer oxidation, and then polymerization. Very recently, we also demonstrated that CP synthesis can also be achieved through a radiolytic reduction–polymerization route thanks to the use of e−aq as a reducing species.39
Starting from an aqueous solution containing both metal ions and organic monomers, our idea was to originally use radiation chemistry for the in situ production of hydrated electrons and hydroxyl radicals as reducing and oxidizing species, respectively, in order to synthesize CP/metal nanocomposites. More precisely, in the present work, we decided to focus on the PEDOT/Ag system. Indeed, PEDOT/Ag nanocomposites have already been successfully synthesized in the literature by means of either chemical or electrochemical methods and have also been shown to possess various potential applications.43–48 In addition, in our previous works, we succeeded in the radiation-induced synthesis, in aqueous solution, of shape controlled silver nanoparticles on the one hand,49,50 and of nanostructured conducting PEDOT polymers on the other hand.37,38 This evidently represents good omens for the successful synthesis of PEDOT/Ag composites by radiolysis.
In this work, starting from EDOT monomers and Ag+ silver ions dissolved in water, we describe simple γ-ray-based radiolytic methodologies which enable the synthesis of CP/metal nanocomposites. In fact, PEDOT/Ag nanocomposites were prepared by both two-step and one-pot methods. According to the two-step method, PEDOT polymers were first synthesized via either oxidation or reduction of the EDOT monomers and then, PEDOT/Ag nanocomposites were prepared by the further reduction of silver ions to metal nanoparticles. Differently, according to the one-pot method, polymerization and metal ion reduction were achieved in parallel, in one step. Pulsed radiolysis experiments were additionally performed in order to find the rate constants and also to understand the mechanism involved in such a one-pot procedure. Radiosynthesized PEDOT/Ag nanocomposites were characterized using different experimental techniques: UV-Vis absorption spectrophotometry, EDX spectroscopy and cryo-TEM microscopy in solution, and ATR-FTIR spectroscopy and SEM microscopy after deposition. Finally, the thermal stability and the electrical conductivity of radiosynthesized PEDOT/Ag nanocomposites were checked by TGA and a four-point probe technique, respectively, opening the way for many potential applications.
2. Experimental section
2.1. Materials
Ultrapure water (Millipore system, 18.2 MΩ cm) was used as the solvent. 3,4-Ethylenedioxythiophene, EDOT (≥98%, Sigma-Aldrich), was used as the organic monomer for PEDOT production. Silver perchlorate salt, AgClO4 (99%, Sigma-Aldrich), was used as the Ag+ source for silver nanoparticle synthesis. Isopropanol (≥98%, Sigma-Aldrich) and tert-butanol (99%, WR International S.A.S) were added to the solutions as hydroxyl radical scavengers. N2O and N2 (Air liquid Co.) were used to degas the aqueous solutions before γ-irradiation. Acetonitrile solvent (≥99.8%, Sigma Aldrich), containing NOBF4 (≥95%, Sigma Aldrich) as a dopant, was used in the electrical conductivity measurements.2.2. Radiolytic route
Within the nanosecond timescale, γ-irradiation of deoxygenated diluted aqueous solutions at neutral pH, under a N2 atmosphere, generates the following radiolytic species, which come from the direct effect of ionizing radiation on the more abundant species, namely water molecules:51–53H2O ![[long arrow, wavy then straight]](https://www.rsc.org/images/entities/char_e0f6.gif) HO˙, H˙, e−aq, H3O+, H2O2, H2 HO˙, H˙, e−aq, H3O+, H2O2, H2 | (1) |
In the presence of an alcohol, such as isopropanol, (CH3)2CHOH (at a relatively high concentration, 0.2 mol L−1), HO˙ and H˙ radicals are quantitatively scavenged, producing a reducing (CH3)2C˙OH secondary radical according to:55–57
| (CH3)2CHOH + HO˙ (or H˙) → (CH3)2C˙OH + H2O (or H2) | (2) |
| G(e−aq) = 2.8 × 10−7 mol J−1 | (3) |
| G((CH3)2C˙OH) = 3.4 × 10−7 mol J−1 | (4) |
When diluted aqueous solutions, containing Ag+ ions, are irradiated by γ-rays, both (CH3)2C˙OH and e−aq quantitatively act as reducing species towards silver ions, leading to silver atoms which aggregate into silver nanoparticles.58 As a consequence, under a N2 atmosphere, in the presence of isopropanol, the radiolytic yield, G-value, of silver ion reduction is:59
| Gred(Ag+) = 6.2 × 10−7 mol J−1 | (5) |
| Gred(EDOT) = 2.8 × 10−7 mol J−1 | (6) |
| e−aq + N2O + H2O → HO˙ + HO− + N2 | (7) |
| G(HO˙) = 5.6 × 10−7 mol J−1 | (8) |
| Gox(EDOT) = 5.6 × 10−7 mol J−1 | (9) |
| D (Gy) = C(mol L−1)/G(mol J−1) | (10) |
2.3. Solution preparation and γ-irradiation
Aqueous solutions containing EDOT (10 mM) and different concentrations of Ag+ ions (ranging from 0 to 20 mM) were prepared at room temperature, in the dark, to prevent any photochemical reaction. In all cases, the pH of the solution was found to be close to 7. EDOT concentration was always checked using UV-vis absorption spectroscopy.38 Also, the concentrations used in this work remained lower than the EDOT and AgClO4 solubilities in water.60,61 Note that such relatively low concentrations avoid direct ionization of the EDOT molecules and Ag+ ions due to the γ-rays.After degassing, all solutions were irradiated with a 60Co γ source (available at LCP laboratory, Paris-Sud University), at a dose rate of 5 kGy h−1, up to doses enabling the total consumption of EDOT molecules and Ag+ ions.
Starting from 10 mM in EDOT and 10 mM in silver ions, if we consider that, at high doses, EDOT polymerization into PEDOT, on the one hand, and Ag+ reduction to metal nanoparticles, on the other hand, are quantitative, then the weight ratio WPEDOT/WAg should amount, after radiolysis, to 1.3/1 whatever the preparation procedures.
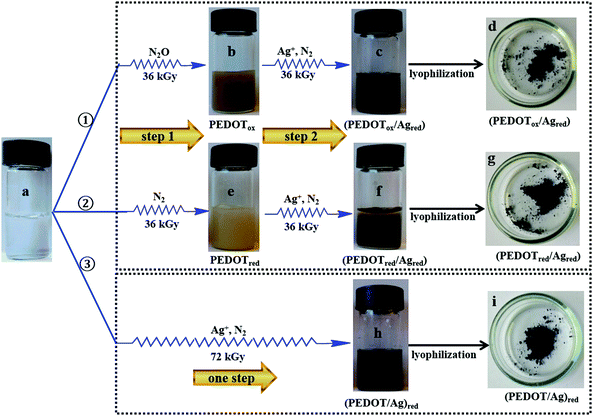
– Procedure ① (Fig. 1). In the first step, an aqueous solution of EDOT monomers (10 mM) was prepared and degassed with N2O for 20 min (sample a), and then irradiated by γ-rays at 36 kGy in order to quantitatively oxidize EDOT monomers and to synthesize PEDOT polymers (sample b). In the second step, the solution was added to AgClO4 (10 mM) and isopropanol (0.2 M), degassed with N2 for 20 min and then irradiated at 36 kGy in order to quantitatively reduce the silver ions (sample c).
– Procedure ② (Fig. 1). In the first step, an aqueous solution of EDOT monomers (10 mM) added to isopropanol (0.2 M) was first degassed with N2 for 20 min (sample a) and irradiated at 36 kGy in order to quantitatively reduce the EDOT monomers and synthesize PEDOT polymers (sample e). In the second step, the solution was added to AgClO4 (10 mM) and isopropanol (0.2 M), degassed again with N2 for 20 min and finally irradiated at 36 kGy in order to quantitatively reduce the silver ions (sample f).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and 0.2 M in isopropanol were prepared, degassed with N2 for 20 min and irradiated at 72 kGy (sample h). This dose is enough to ensure the total reduction of both EDOT molecules and Ag+ ions.
10) and 0.2 M in isopropanol were prepared, degassed with N2 for 20 min and irradiated at 72 kGy (sample h). This dose is enough to ensure the total reduction of both EDOT molecules and Ag+ ions.
According to this one-pot method, for the ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, we varied the irradiation dose from 0 to 72 kGy. Also, we changed the EDOT/Ag ratio, from 10
10, we varied the irradiation dose from 0 to 72 kGy. Also, we changed the EDOT/Ag ratio, from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, by varying the amount of AgClO4. Whatever the ratio of EDOT to Ag, the aqueous solutions were always added to isopropanol (0.2 M), degassed with N2 for 20 min and irradiated at 72 kGy, which enables in all cases the total reduction of both EDOT and Ag+.
20, by varying the amount of AgClO4. Whatever the ratio of EDOT to Ag, the aqueous solutions were always added to isopropanol (0.2 M), degassed with N2 for 20 min and irradiated at 72 kGy, which enables in all cases the total reduction of both EDOT and Ag+.
2.4. Kinetic study by pulse radiolysis
In order to understand the mechanism involved in the one-pot method and, more particularly, to determine the rate constant of reduction of EDOT molecules by hydrated electrons, pulse radiolysis experiments were carried out on a picosecond laser-triggered electron accelerator ELYSE at Paris-Sud University (Orsay) coupled with a time-resolved absorption spectroscopic detection system.62,63 The 15 ps pulses were characterized by a charge of 4–6 nC and an electron energy of 7.6 MeV, and were delivered at a repetition frequency of 5 Hz. The dose per pulse was derived from the absorbance of the hydrated electron at 660 nm with ε660nm,(e−aq) = 1.8 × 104 L mol−1 cm−1 and G3ns,(e−aq) = 3.45 × 10−7 mol J−1. For each pulse, the dose deposited in water was 40.5 Gy in the irradiated volume (i.e. [e−aq] ≈ 1.4 × 10−5 M).A N2-purged stock aqueous solution of 200 mL was used which circulated in the 10 × 10 mm fused silica irradiation cell at a flow rate of 100 mL min−1 by means of a peristaltic pump. The solution was then renewed between each pulse and, for each kinetic measurement, it was possible to apply up to 400 pulses.
Absorption measurements were performed using the white light beam of a homemade Xenon flash lamp.63 The light was focused through the sample collinearly with the electron beam and then directed onto the slit of a flat field spectrograph (Chromex 250IS), equipped with three 150 grooves per mm grating blazed at 300, 500 and 800 nm which dispersed the light on the entrance optics of a streak-camera (model C-7700 from Hamamatsu).41 The time-resolved absorbance of the sample was calculated using average images obtained on the streak-camera (with and without the electron pulses). The full spectra from 245 to 650 nm were obtained by two series of absorption measurements using two different optical filters (UG5 and GG325) to optimize the light intensity on a specific spectral domain (250–400 and 360–700 nm, respectively). Also, the spectral overlap between 360 and 400 nm enabled us to check the good stability and the reproducibility of all irradiation measurements.
In pulse radiolysis, an aqueous solution irradiated by a short pulse of high-energy electrons produces a significant concentration of radical species, according to reaction (1). In order to study the specific action of hydrated electrons, e−aq, towards EDOT molecules and in order to determine its rate constant, an aqueous solution containing 1 mM in EDOT and 0.2 M in tert-butanol, (CH3)3COH, was prepared, degassed with N2 and then studied via pulse radiolysis. As the isopropanol, tert-butanol is an efficient scavenger of HO˙ radicals (with a rate constant of 6.8 × 108 M−1 s−1),57 according to the following reaction:57,64
| (CH3)3COH + HO˙ → ˙CH2(CH3)2COH + H2O | (11) |
2.5. Characterization of PEDOT/Ag nanocomposites
During the cryo-TEM observations, in situ energy-dispersive X-ray (EDX) spectroscopy was used to check the chemical composition of our samples and to perform the elemental analysis of the radiosynthesized PEDOT/Ag nanocomposites in aqueous solution. An EDX JEOL Si(Li) detector mounted on the cryo-microscope was used with a resolution of 140 eV.
2.6. Analysis of the properties of the PEDOT/Ag nanocomposites
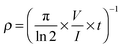 | (12) |
3. Results and discussion
3.1. Synthesis of PEDOT/Ag nanocomposites via the two-step method
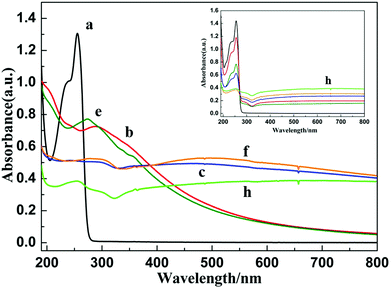
In this method, the first step corresponds to the synthesis, in aqueous solution, of PEDOT polymers by either oxidation (procedure ①) or reduction (procedure ②) of the EDOT monomers. An aqueous solution containing 10 mM in EDOT was prepared first. This aqueous solution was found to be transparent (Fig. 1, sample a). In this solution, EDOT monomers display two absorption maxima at 235 and 255 nm (Fig. 2, spectrum a) in agreement with the literature.37,38According to procedure ① (Fig. 1), in the first step, the transparent aqueous solution of EDOT monomers (10 mM) was degassed with N2O, and then irradiated at 36 kGy. Under these conditions, hydroxyl radicals generated by radiolysis should quantitatively oxidize the EDOT monomers. After irradiation, a yellow-brown suspension was observed (Fig. 1, sample b). Its absorption spectrum (Fig. 2, spectrum b) displays an absorption band at 290, a shoulder at 350 nm and a long absorption tail in the range of 400–800 nm, which were attributed, in the literature, to PEDOT oligomers and polymers.37–39 One can note, in this spectrum, the absence of absorption bands at 235 and 255 nm. This definitely proves that, after 36 kGy-irradiation, the EDOT monomers have completely disappeared, as expected. Indeed, they are quantitatively converted by oxidation to PEDOT polymers, called PEDOTox.
In the second step of procedure ① (Fig. 1), the irradiated solution was added to AgClO4 (10 mM) and isopropanol (0.2 M), degassed with N2, and then irradiated again at 36 kGy. Under these conditions, hydrated electrons generated by radiolysis should quantitatively reduce the Ag+ ions. After the second irradiation, a black suspension, which slowly precipitates, was clearly observed (Fig. 1, sample c). Its absorption spectrum (Fig. 2, spectrum c) displays the well-known plasmon absorption band of silver nanoparticles at around 450 nm.35 The asymmetric shape of the surface plasmon resonance (SPR) band could be due to the aggregation of silver nanoparticles or to their interaction (adsorption) with organic molecules.49,66–68 Note that the two shoulders at 290 and 350 nm, attributable to PEDOT, are still present in the absorption spectrum. This demonstrates that, according to procedure ①, both PEDOT (obtained by oxidation) and silver nanoparticles (obtained by reduction) are present in the aqueous medium. The corresponding composites will be called (PEDOTox/Agred).
In a different way, according to procedure ② (Fig. 1), the aqueous solution containing 10 mM in EDOT was degassed with N2, and then irradiated at 36 kGy. Under these conditions, hydrated electrons generated by radiolysis should quantitatively reduce the EDOT monomers. After irradiation, as in the case of procedure ①, a yellow suspension was observed (Fig. 1, sample e). Its UV-Visible absorption spectrum (Fig. 2, spectrum e) does not display bands at 235 and 255 nm, but highlights absorption bands in the UV region and a long absorption tail in the range of 400–800 nm, which were attributed in a previous work to PEDOT oligomers and polymers.39 Once again, we can conclude that, after 36 kGy-irradiation, but this time under a N2 atmosphere, EDOT monomers are quantitatively converted via reduction to PEDOT oligomers and polymers, called PEDOTred.
In the second step of procedure ② (Fig. 1), which is similar to the second step of procedure ①, the irradiated solution was added to AgClO4 and isopropanol, and then irradiated again at 36 kGy under a N2 atmosphere. At the end of the procedure, a black suspension, which sediments, was clearly observed (Fig. 1, sample f). Its absorption spectrum (Fig. 2, spectrum f), which, once again, displays the plasmon absorption band of silver nanoparticles at around 500 nm, is very similar to that obtained at the end of procedure ① (Fig. 2, spectrum e). This demonstrates that, according to procedure ②, both PEDOT (obtained by reduction) and silver nanoparticles (also obtained by reduction) are present in the aqueous medium. The corresponding composites will be called (PEDOTred/Agred).
Irradiated solutions, corresponding to the black suspensions of sample c and sample f, were dried via lyophilization in order to quantitatively eliminate water and isopropanol from the two dark powders obtained (Fig. 1, sample d and sample g, respectively). Naturally, due to the previous spectrophotometric results, these powders are expected to contain an organic component, PEDOT, in addition to an inorganic phase, Ag. In addition, perchlorate anions, ClO4−, should also remain in the sample after lyophilization. The PEDOT/Ag composite, contained in sample d, which is obtained at the end of procedure ① and which implies first the oxidation-polymerization of EDOT and second the reduction of silver ions, is called (PEDOTox/Agred). The second composite, contained in sample g, which is obtained at the end of procedure ② and which implies first the reduction–polymerization of EDOT and second the reduction of silver ions, is differently called (PEDOTred/Agred).
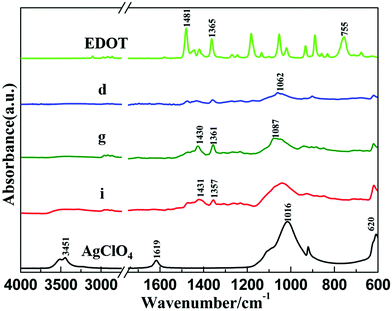
Both lyophilized samples (sample d and sample g) were characterized using ATR-FTIR spectroscopy in order to investigate the chemical nature of the solid phases and to confirm the presence of PEDOT polymers in the powders. The ATR-FTIR spectra of both powders, (PEDOTox/Agred) and (PEDOTred/Agred), are presented in Fig. 3 (spectrum d and spectrum g, respectively), in the wave number region 4000–600 cm−1, together with the spectra of pure non-irradiated EDOT and of pure AgClO4 salt.
In the ATR-FTIR spectrum of EDOT, the characteristic peak at 755 cm−1 corresponds to the C–H out-of-plane bending vibration in the thiophene ring.37 The stretching vibrations of C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the thiophene ring are also seen at 1365 and 1481 cm−1.69 ATR-FTIR spectra of (PEDOTox/Agred) (Fig. 3, spectrum d) and (PEDOTred/Agred) (Fig. 3, spectrum g) are in good agreement with the PEDOT spectra found in the literature.70 Indeed, all the characteristic bands of PEDOT are present, even if they appear slightly displaced. The bands at 1062 cm−1 (spectrum d) and 1087 cm−1 (spectrum g) are attributed to the ethylenedioxy group.37,39 The peaks at 1430 and 1361 cm−1 originating from C–C and C
C in the thiophene ring are also seen at 1365 and 1481 cm−1.69 ATR-FTIR spectra of (PEDOTox/Agred) (Fig. 3, spectrum d) and (PEDOTred/Agred) (Fig. 3, spectrum g) are in good agreement with the PEDOT spectra found in the literature.70 Indeed, all the characteristic bands of PEDOT are present, even if they appear slightly displaced. The bands at 1062 cm−1 (spectrum d) and 1087 cm−1 (spectrum g) are attributed to the ethylenedioxy group.37,39 The peaks at 1430 and 1361 cm−1 originating from C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations are also observed in both cases.70 Nevertheless, one can note the absence, in spectrum d and spectrum g, of the C–H vibration peak at 755 cm−1, which definitely proves that quantitative polymerization of EDOT, through an α,α′-coupling reaction, took place in both cases (procedures ① and ②), leading to organic/inorganic composites made of PEDOT.
C stretching vibrations are also observed in both cases.70 Nevertheless, one can note the absence, in spectrum d and spectrum g, of the C–H vibration peak at 755 cm−1, which definitely proves that quantitative polymerization of EDOT, through an α,α′-coupling reaction, took place in both cases (procedures ① and ②), leading to organic/inorganic composites made of PEDOT.
In the ATR-FTIR spectrum of AgClO4 salt, the stretching vibrations of coordinated and free ClO4− anions are observed at 1016 and 620 cm−1, while the peaks at 1619 and 3451 cm−1 are due to the bending and stretching vibrations of O–H groups, which is explained by the hygroscopic nature of the AgClO4 salt.71–73 In the ATR-FTIR spectra of (PEDOTox/Agred) (spectrum d) and (PEDOTred/Agred) (spectrum g), one can observe the presence of large intense bands around 1016 and 620 cm−1, which let us guess the presence of ClO4−, as doping anions, in the (PEDOTox/Agred) and (PEDOTred/Agred) dried composites. In fact, these anions come from silver perchlorate salt and are well-known to remain unreactive under the present radiolytic conditions.35
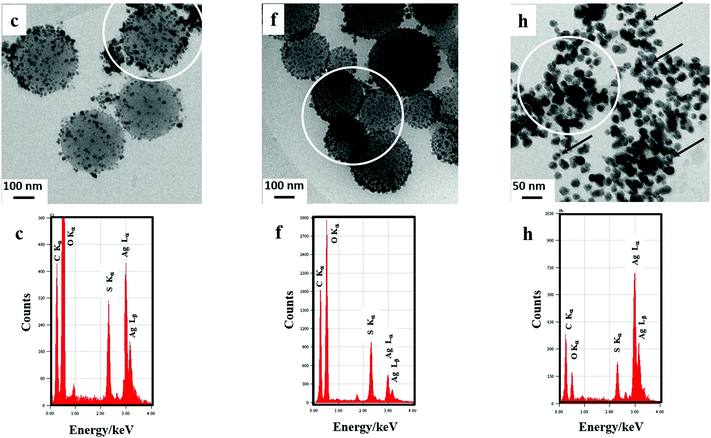
In order to investigate, in the liquid phase before any deposition, the structure and morphology of the (PEDOTox/Agred) and (PEDOTred/Agred) composites, the black suspensions obtained at the end of procedures ① and ② (sample c and sample f respectively) were observed using cryo-transmission electron microscopy just after irradiation and before any sedimentation (Fig. 4, image c and image f, respectively). The observations made on the (PEDOTox/Agred) and (PEDOTred/Agred) aqueous samples were totally similar, as can be seen when comparing images c and f. In both cases, representative images show the presence of low density globular structures forming polydisperse spherical nanoparticles with a diameter comprised between 100 and 500 nm. This result is in very good agreement with the observations we already made in the case of PEDOT synthesized, alone (in the absence of silver) by radiolysis, either by EDOT oxidation37,38 or by EDOT reduction.39 Thus, these low density globular objects are made of PEDOT polymers. Each globular structure should correspond to the self-assembly of independent amorphous PEDOT chains.
As can be seen in images c and f, smaller nanoparticles (from few nm to 50 nm), which appear much more contrasted (darker), are systematically found to be adsorbed onto the bigger globular PEDOT nanoparticles. The relatively high contrast of these small particles suggests their metallic nature.31 Since no other objects were observed during our cryo-TEM experiments, we suppose that these small spherical nanoparticles are made up of silver.
During cryo-TEM observations, in situ Energy-Dispersive X-ray (EDX) spectroscopy was used to check the chemical composition of our radiosynthesized materials in order to prove the presence of sulfur atoms (which are only present in PEDOT polymers, one sulfur atom per EDOT molecule) and of silver atoms (which would be the signature of silver nanoparticles). The aim was also to check the molar ratio between both atoms in the radiosynthesized PEDOT/Ag composites. EDX spectra (Fig. 4, spectrum c and spectrum f), which correspond, respectively, to the elemental analysis of the circled areas of image c and image f of Fig. 4, highlight the presence, in both samples, of the following chemical elements: O (Kα at 0.525 keV), C (Kα at 0.277 keV), S (Kα at 2.31 keV) and Ag (Lα at 2.98 keV and Lβ at 3.15 keV). The presence of oxygen and carbon atoms in both samples may be related to the presence of PEDOT polymers. Nevertheless, their preponderance in the EDX spectra is certainly due to the ice which surrounds all the observed nanomaterials and to the carbon-coated grid itself (which is used for cryo-TEM observations). In contrast to oxygen and carbon atoms, the detection of sulfur and silver elements definitely demonstrates, as expected, the concomitant presence, in the analyzed areas of images c and f of Fig. 4, of both PEDOT polymers and silver nanoparticles. A quantitative analysis enabled us to estimate the S and Ag atomic percentages in the circled areas of Fig. 4. We found 39% S and 61% Ag in spectrum c and 67% S and 33% Ag in spectrum f. Even if these percentages are not far from those expected (50% S and 50% Ag) starting from 10 mM in EDOT and 10 mM in AgClO4, they were found to be dependent on the analyzed area. In fact, sulfur and silver were systematically detected in all the nanoparticles observed using cryo-TEM. But their atomic percentage was found to vary from 20% to 80%. Note that sulfur and silver atoms were only observed in the nanomaterials and were never found in the surrounding aqueous medium.
As demonstrated using cryo-TEM microscopy and EDX spectroscopy, composites prepared using the two-step method are made of large spherical PEDOT nanoparticles at the surface of which smaller silver nanoparticles adsorb. The deposition and aggregation of these silver nanoparticles onto PEDOT organic compounds should explain their characteristic asymmetric plasmon absorption bands which were found using UV-Vis absorption spectroscopy (Fig. 2, spectra c and f).
Due to the two-step method used in procedures ① and ②, PEDOT polymers are first synthesized, in the absence of inorganic ions, and self-assemble via van der Waals interactions into globular organic nanoparticles. Silver nanoparticles are formed only in the second step. This explains their presence outside of the PEDOT nanoparticles. Nevertheless, in spite of all our cryo-TEM investigations, metal nanoparticles were never found isolated in the aqueous phase. They were always found adsorbed onto PEDOT spherical nanostructures. Maybe, in the second step, silver nanoparticles are radiolytically synthesized in the bulk of the aqueous solution and then diffuse and adsorb at the surface of PEDOT due to weak van der Waals interactions. But, more likely, prior to irradiation, silver ions first strongly interact with PEDOT particles and bind, as soft Lewis acids, with the sulfur atoms (soft Lewis bases) present all along the polymer chains. These coordinated silver ions are further reduced by hydrated electrons in the vicinity of the PEDOT nanoparticles.
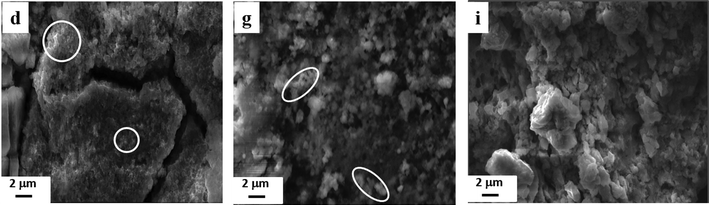
In order to check the morphology of the (PEDOTox/Agred) and (PEDOTred/Agred) composites, after the deposition procedure and the drying step, PEDOT-containing lyophilized powders (sample d and sample g) were deposited onto carbon tape adhered to aluminum mounts. The surface was then imaged using Scanning Electron Microscopy (Fig. 5, image d and image g, respectively).
The images d and g in Fig. 5 indicate the presence of very close-packed spheroid particles. Some of these particles are circled for clarity. These structures should come from the globular nanostructures already observed in aqueous solution using cryo-TEM (Fig. 4, image c and image f, respectively). All these observations are consistent with the reported characterization of PEDOT polymers synthesized via oxidation-polymerization or reduction polymerization routes.37,39 The composite nanoparticles observed using SEM after deposition are polydisperse in size with a diameter comprised between 100 and 500 nm. These SEM observations agree well with the size and the shape of the (PEDOTox/Agred) and (PEDOTred/Agred) particles previously observed in aqueous solution using cryo-TEM (Fig. 4). Therefore, deposition and drying do not seem to affect the nanostructuration of PEDOT/Ag nanocomposites.
3.2. Synthesis of PEDOT/Ag nanocomposites via the one-pot method
After the successful synthesis of PEDOT/Ag nanocomposites according to the two-step method (procedures ① and ②, Fig. 1), we tried to simplify the synthesis methodology by using a reduction-based one-pot method (procedure ③, Fig. 1). The aim was also to compare the morphology and the properties of the composites prepared by procedure ③ with those of nanocomposites differently prepared according to procedures ① and ②. For the success of the one-pot method, production of PEDOT and of silver nanoparticles should be concomitant. This is conceivable only if the reduction of silver ions and oxidation of EDOT monomers take place simultaneously.While, both (CH3)2C˙OH and e−aq act as reducing species towards silver ions, only hydrated electrons can reduce EDOT monomers,39 leading probably to EDOT anion radicals, EDOT˙−. As a consequence, the success of procedure ③ depends on the rate constant values of the following two reactions:
| Ag+ + e−aq → Ag0 | (13) |
| EDOT + e−aq → EDOT˙− | (14) |
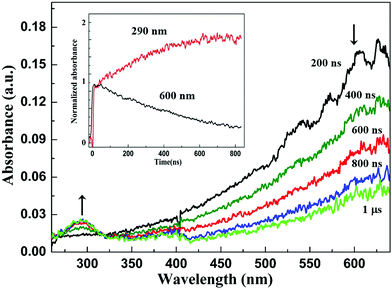
In order to study the specific action of hydrated electrons, e−aq, towards EDOT molecules and with the aim of determining the corresponding rate constant, an aqueous solution containing 1 mM in EDOT and 0.2 M in tert-butanol, (CH3)3COH, was prepared, degassed with N2 and then studied via pulse radiolysis. The transient absorption spectra of the irradiated solution were then recorded at different timescales from 200 ns to 1 μs after the electron pulse (Fig. 6). One can observe at 200 ns a large and intense absorption band at around 600 nm, which is the well-known signature of hydrated electrons generated in the medium due to water radiolysis. At longer times, this absorption band progressively decreases, demonstrating the decay of e−aq, while a weaker peak grows up at around 290 nm. Since no other bands are observed in the spectrum over the first 800 ns, one can conclude that the species absorbing at 290 nm comes from reaction (14) of the hydrated electrons with EDOT monomers and should correspond to the EDOT˙− anion radical.
The evolution with time, over 800 ns, of the absorbances at 290 and 600 nm is displayed in the inset of Fig. 6 (absorbances were normalized here for clarity). One can find that the time evolution of the absorbance of hydrated electrons at 600 nm fits well with a first-order decay. On the other hand, the growth of EDOT˙− at 290 nm, which is very well correlated with e−aq decay, fits well with a pseudo-first order reaction, in good agreement with reaction (14) since EDOT is present in excess in the irradiated medium (1 mM). The kinetic study of the decay at 600 nm and of the growth at 290 nm enabled us to find, twice, the same value for the effective rate constant of reaction (14): k14 = 3 × 109 L mol−1 s−1.
One can note that even if the value of k14 is relatively high, it remains ten times smaller than k13. This result means that starting from an aqueous solution containing equimolar amounts of EDOT monomers and Ag+ ions (10 mM in both for instance), the reduction of EDOT should be ten times slower than that of the silver ions. Therefore, according to procedure ③, PEDOT polymers and silver nanoparticles can be produced simultaneously. Nevertheless, the production of Ag nanoparticles should be faster than that of polymers.
According to procedure ③ (Fig. 1), aqueous solutions containing 10 mM in EDOT, 10 mM in AgClO4 (EDOT/Ag ratio = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and 0.2 M in isopropanol were prepared, degassed with N2 for 20 min and irradiated at 72 kGy. Let us remember that whatever the rate constants of reduction, this irradiation dose is enough to ensure the total reduction of both EDOT molecules and Ag+ ions.
10) and 0.2 M in isopropanol were prepared, degassed with N2 for 20 min and irradiated at 72 kGy. Let us remember that whatever the rate constants of reduction, this irradiation dose is enough to ensure the total reduction of both EDOT molecules and Ag+ ions.
After irradiation of the transparent aqueous solution containing both EDOT and silver ions, a brown-black suspension, which slowly precipitates, was observed (Fig. 1, sample h). Its absorption spectrum (Fig. 2, spectrum h) displays an asymmetric plasmon absorption band, between 400 and 800 nm, which is once again characteristic of silver nanoparticles.68 Nevertheless, the shape of the spectrum is quite different from that of spectra c and f. This could be due to the synthesis via procedure ③ of silver nanoparticles with a new morphology, which may interact differently with PEDOT polymers. One can also note in spectrum h, the absence of the absorption bands of EDOT at 235 and 255 nm. This proves that, after 72 kGy-irradiation, at the end of procedure ③, the EDOT monomers have completely disappeared, as expected.
The simultaneous production of PEDOT polymers and of silver nanoparticles according to this one-pot method was checked by following the evolution of the UV-visible absorption spectrum of the aqueous solution (inset of Fig. 2) as a function of the irradiation dose (0, 10, 20, 30, 50 and 70 kGy). One can note that even if the silver ion reduction is faster as demonstrated via pulse radiolysis, EDOT reduction is noticeable from the lower doses. Indeed, at 10 kGy, which is a dose lower than the dose which is necessary for the total reduction of 10 mM in Ag+ (16 kGy), the decay of EDOT at 235 and 255 nm is evident. Also, upon increasing the irradiation dose, the growth of the plasmon band of silver nanoparticles between 400 and 800 nm and the decay of the absorption bands of EDOT at 235 and 255 nm are concomitant. This definitely demonstrates that, according to procedure ③, PEDOT polymers and silver nanoparticles are formed simultaneously and are thus finally present in the aqueous medium of sample h. The corresponding composites will be called (PEDOT/Ag)red.
Irradiated solution, corresponding to the black suspension of sample h, was dried via lyophilization into a black powder (Fig. 1, sample i). Lyophilized sample i, which was expected to contain (PEDOT/Ag)red nanocomposites, was then characterized using ATR-FTIR spectroscopy. As observed in Fig. 3, its infrared spectrum (spectrum i) is found to be very similar to spectrum d and spectrum g. This definitely proves that, like sample d and sample g, sample i, obtained at the end of procedure ③, contains PEDOT polymers, silver nanoparticles as well as perchlorate anions.
According to this one-pot method, we changed the EDOT/Ag ratio, from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, by varying the concentration of AgClO4 from 5 mM to 20 mM. Whatever the ratio of EDOT to Ag, the aqueous solutions were all added to isopropanol (0.2 M), degassed with N2 for 20 min, and then irradiated at 72 kGy. This dose enables, in all cases, the total reduction of both EDOT and Ag+. In all cases, black suspensions were obtained after irradiation and black powders were obtained after lyophilization. The absorption spectra obtained after irradiation were all very close to spectrum h of Fig. 2 (obtained for the ratio 10
20, by varying the concentration of AgClO4 from 5 mM to 20 mM. Whatever the ratio of EDOT to Ag, the aqueous solutions were all added to isopropanol (0.2 M), degassed with N2 for 20 min, and then irradiated at 72 kGy. This dose enables, in all cases, the total reduction of both EDOT and Ag+. In all cases, black suspensions were obtained after irradiation and black powders were obtained after lyophilization. The absorption spectra obtained after irradiation were all very close to spectrum h of Fig. 2 (obtained for the ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Also, all the ATR-FTIR spectra were similar to spectrum i of Fig. 3 (results not shown). This indicates that, even if silver ion reduction is faster than that of EDOT monomers, (PEDOT/Ag)red composites can be formed according to the one-pot method whatever the EDOT/Ag ratio from 10
10). Also, all the ATR-FTIR spectra were similar to spectrum i of Fig. 3 (results not shown). This indicates that, even if silver ion reduction is faster than that of EDOT monomers, (PEDOT/Ag)red composites can be formed according to the one-pot method whatever the EDOT/Ag ratio from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.
20.
In order to investigate, in the liquid phase before any deposition, the structure and morphology of the (PEDOT/Ag)red composites, the black aqueous suspension obtained at the end of procedure ③ (sample h), was observed using cryo-transmission electron microscopy just after irradiation and before any sedimentation (Fig. 4, image h). (PEDOT/Ag)red nanocomposites appear as granular mixtures.45,75 Representative images of the (PEDOT/Ag)red aqueous sample are very different from those obtained in the case of (PEDOTox/Agred) and (PEDOTred/Agred) (Fig. 4, images c and f, respectively). Indeed, image h of Fig. 4 shows the presence of low density globular structures forming polydisperse spheroidal nanoparticles with a diameter between 10 and 50 nm. These organic particles, made of PEDOT polymers and obtained via the one-pot method, are ten times smaller than the PEDOT nanoparticles obtained via the two-step method. On the other hand, as can also be observed in image h of Fig. 4, smaller nanoparticles, which appear much more contrasted (darker), are found to be embedded into the core of the bigger globular PEDOT nanoparticles. Some of these dark nanoparticles are indicated in the figure by arrows. The relatively high contrast of these smaller particles suggests once again that they are made of silver.31,35 The presence of organic molds around these silver nanoparticles should explain their characteristic asymmetric plasmon absorption band which was found using UV-Vis absorption spectroscopy (Fig. 2, spectrum h).
The EDX spectrum (Fig. 4, spectrum h), which corresponds to the elemental analysis of the circled area of image h, highlights the presence, in sample h, of all the chemical elements already detected in spectrum c and spectrum f of Fig. 4, namely O, C, Ag and S. This definitely demonstrates, as expected, the presence, in the analyzed area of image h, of both PEDOT polymers and silver nanoparticles. In this area, the S and Ag atomic percentages were found to be 21% and 79%, respectively. Nevertheless, these percentages were found to be very dependent on the analyzed area.
In this one-pot method, we changed the EDOT/Ag ratio, from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, by varying the concentration of AgClO4 from 5 mM to 20 mM. Whatever the ratio of EDOT to Ag, the (PEDOT/Ag)red nanocomposites appear as granular mixtures with silver nanoparticles embedded into organic molds. This indicates that, whatever the EDOT/Ag ratio from 10
20, by varying the concentration of AgClO4 from 5 mM to 20 mM. Whatever the ratio of EDOT to Ag, the (PEDOT/Ag)red nanocomposites appear as granular mixtures with silver nanoparticles embedded into organic molds. This indicates that, whatever the EDOT/Ag ratio from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, the (PEDOT/Ag)red composites have the same morphology in aqueous solution. Nevertheless, when the amount of silver is higher, the size of the nanocomposites increases and a more important aggregation takes place (results not shown).67
20, the (PEDOT/Ag)red composites have the same morphology in aqueous solution. Nevertheless, when the amount of silver is higher, the size of the nanocomposites increases and a more important aggregation takes place (results not shown).67
In procedures ① and ②, PEDOT nanoparticles are first synthesized, and then in the second step, silver ions are reduced at the surface of these polymer nanoparticles. In contrast, in procedure ③, silver ion reduction takes place in parallel with EDOT reduction. Then, since silver reduction is faster than that of EDOT, metal nanoparticles can form prior to the existence of the PEDOT nanoparticles. In the one-pot method, according to procedure ③, all silver nanoparticles are found surrounded by an organic shell (Fig. 4, image h). This could mean that EDOT reduction and polymerization occur in the vicinity of silver nanoparticles. Such a growth mechanism could be explained by the strong interaction which exists between silver ions (or even atoms) and the sulfur atoms of the EDOT monomers. Or maybe, PEDOT polymers are formed far from the silver nanoparticles, in the bulk of the aqueous solution, and then diffuse and adsorb at their surface, due to weak van der Waals interactions, enabling the thermodynamic stabilization of silver particles and avoiding their aggregation and sedimentation. This effect of polymers on silver nanoparticles has already been reported in the literature.36
In order to characterize the morphology of the (PEDOT/Ag)red composites, after the drying and deposition procedures, the PEDOT-containing lyophilized powder (sample i) was observed using Scanning Electron Microscopy (Fig. 5, image i). In contrast to the observations made on images d and g of Fig. 5 concerning (PEDOTox/Agred) and (PEDOTred/Agred), respectively, no spherical nanoparticles were observed in image i, which may be due to the fact that their relatively small size avoids their observation during SEM microscopy. In fact, SEM characterization shows that the (PEDOT/Ag)red composites become a compact hybrid after deposition. Also, when varying the EDOT/Ag ratio, from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, as the proportion of silver increases, the (PEDOT/Ag)red composites become denser and form more compact blocks.
20, as the proportion of silver increases, the (PEDOT/Ag)red composites become denser and form more compact blocks.
3.3. Physico-chemical properties of the PEDOT/Ag nanocomposites
As demonstrated in Sections 3.1 and 3.2, PEDOT/Ag nanocomposites can be prepared via both the two-step method ((PEDOTox/Agred) and (PEDOTred/Agred) composites) and the one-pot method ((PEDOT/Ag)red composites) thanks to γ-radiolysis. The obtained PEDOT/Ag nanocomposites display very close UV-vis absorption spectra and similar ATR-FTIR spectra. All composites which are found to be made of PEDOT polymers and silver nanoparticles are shown to be doped with perchlorate anions.In contrast to their chemical composition which is the same, the morphology of PEDOT/Ag nanocomposites in aqueous solution and after deposition are almost different. In aqueous solution, while the (PEDOTox/Agred) and (PEDOTred/Agred) composites are made of large spherical PEDOT nanoparticles at the surface of which smaller silver nanoparticles adsorb, the (PEDOT/Ag)red composites are made of silver nanoparticles embedded in the core of small irregular spheroidal PEDOT nanoparticles. After deposition, while the (PEDOTox/Agred) and (PEDOTred/Agred) composites are made of very close-packed polydisperse spheroidal particles, the (PEDOT/Ag)red composites appear denser and form more compact blocks.
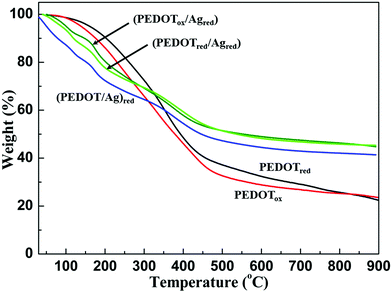
In order to check the influence of the morphology of PEDOT/Ag nanocomposites on their physico-chemical properties, thermal stability and composition analyses of lyophilized (PEDOTox/Agred), (PEDOTred/Agred) and (PEDOT/Ag)red composites were performed using thermogravimetric analysis (TGA). Also, in order to check the influence of the presence of the silver component on the stability of the radiosynthesized polymers, lyophilized PEDOT polymers prepared in the absence of silver ions according to procedures ① and ②, PEDOTox and PEDOTred, respectively (Fig. 1), were also analyzed using TGA from 50 to 900 °C.
The degradation curves of PEDOT polymers and PEDOT/Ag composites, which indicate the remaining weight of the powders as a function of temperature, are all displayed in Fig. 7. TGA curves of the PEDOT/Ag nanocomposites show quite similar tendencies in the whole temperature range. Before 150 °C, a small weight loss is caused by the evaporation of water and degradation of PEDOT oligomers. Then, a progressive weight loss occurs from 150 to 450 °C resulting from PEDOT fragmentation and carbon oxidation.76 After 450 °C, the degradation curves tend to be constant up to 900 °C. At this temperature, the remaining weights of the PEDOT/Ag nanocomposites are about 45%. The residues should contain silver nanoparticles, perchlorate and undecomposed PEDOT.46 The similarity in the degradation curves of the (PEDOTox/Agred), (PEDOTred/Agred) and (PEDOT/Ag)red composites means that the morphology of the composites has no clear influence on their thermal stability. This similarity in the curves is certainly due to the fact that the three materials have, as already demonstrated, the same chemical composition (even if the synthesis methodologies are different). Besides, when varying the EDOT/Ag ratio, from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 in the case of (PEDOT/Ag)red composites, as the proportion of silver perchlorate increases, one can note an increase in the remaining weight of the composites at 900 °C (results not shown). In particular, the remaining weight reaches 60% for the ratio 10
20 in the case of (PEDOT/Ag)red composites, as the proportion of silver perchlorate increases, one can note an increase in the remaining weight of the composites at 900 °C (results not shown). In particular, the remaining weight reaches 60% for the ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.
20.
TGA curves of pure PEDOT polymers, PEDOTox and PEDOTred, prepared in the absence of silver perchlorate, show quite similar tendencies in the whole temperature range (Fig. 7). Before 150 °C, in contrast to what is observed in the case of the composites, no weight loss is observed in the case of pure PEDOT. This could be explained by the existence of a smaller amount of PEDOT oligomers resulting from a higher level of polymerization. Above 150 °C and up to 450 °C, progressive weight loss occurs resulting from PEDOT fragmentation and carbon oxidation. Note that, in this range of temperatures, the degradation rate of pure PEDOT is faster than that of the PEDOT/Ag nanocomposites. This result indicates the superior thermal stability of PEDOT/Ag composites compared with pure PEDOT. Thus the presence of silver nanoparticles in the composites increases the thermal stability of PEDOT polymers.47 Above 450 °C, the degradation curves of pure PEDOT tend to be constant up to 900 °C, and only a small weight loss is observed. Besides, one can note that the final weight loss is more important in the case of pure PEDOT than in the case of the composites. This result is logical. Indeed, in the case of pure PEDOT, the residue should contain only undecomposed PEDOT, while in the case of the composites, it should also contain silver nanoparticles together with perchlorate. In conclusion, thanks to the presence of silver, PEDOT/Ag composites display a thermal stability which is globally higher than that of pure PEDOT over a wide range of temperatures.
Finally, in order to measure and to compare the electrical conductivities of the differently prepared PEDOT/Ag nanocomposites and in order to check the influence of the morphology and of the presence of silver, spin-coated films of (PEDOTox/Agred), (PEDOTred/Agred), (PEDOT/Ag)red, PEDOTox and PEDOTred were prepared and a four-point probe technique was used to measure the resistance of all the PEDOT and PEDOT/Ag films. Note that before the measurements, all the PEDOT and PEDOT/Ag solutions were doped with NOBF4 at a concentration of 10 mM in acetonitrile.
The electrical conductivities measured using the four-point probe technique are all reported in Table 1. The values found in the case of the PEDOT/Ag composites are in the same order of magnitude, even if that of (PEDOTred/Agred) appears slightly higher. These values are comparable to those already reported in the literature for PEDOT nanocomposites prepared according to other methods.24 Also, when varying the EDOT/Ag ratio, from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 10
5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 in the case of the (PEDOT/Ag)red composites, as the proportion of silver perchlorate increases, one can note a slight increase in the electrical conductivity from 3.2 × 10−3 to 3.9 × 10−3 S cm−1 (results not reported in Table 1).
20 in the case of the (PEDOT/Ag)red composites, as the proportion of silver perchlorate increases, one can note a slight increase in the electrical conductivity from 3.2 × 10−3 to 3.9 × 10−3 S cm−1 (results not reported in Table 1).
| Sample | Conductivity/S cm−1 |
|---|---|
| PEDOTox | 9.8 × 10−3 |
| PEDOTred | 3.4 × 10−3 |
| (PEDOTox/Agred) | 3.2 × 10−3 |
| (PEDOTred/Agred) | 5.6 × 10−3 |
| (PEDOT/Ag)red | 3.6 × 10−3 |
The measured electrical conductivities of PEDOTox and PEDOTred are also reported in Table 1. The value found in the case of PEDOTred (3.4 × 10−3 S cm−1) is somewhat lower than that of PEDOTox (9.8 × 10−3 S cm−1). Nevertheless, both values are close to those already reported in the literature for PEDOT polymers.77,78 Moreover, conductivities of pure PEDOT polymers and those of the PEDOT/Ag composites are found in the same order of magnitude. Definitely, this proves that neither the morphology of the composites, nor their chemical composition (amount of silver and perchlorate) have a clear influence on the electrical conductivity. The main factor which seems to determine the final conductivity is the amount of PEDOT conducting polymers, which is the same in all samples. Note that the oxidation or reduction methodologies used for the production of PEDOT polymers seem to have no influence.
4. Conclusion
Hybrid nanocomposites, made of organic conducting polymers and inorganic metal nanoparticles, have attracted intensive research interest due to their remarkable properties. These nanocomposites are generally synthesized via traditional chemical and electrochemical routes. Nevertheless, to our knowledge, radiolysis has never been used for the preparation of such conducting polymer-based nanocomposites. Herein, an original methodology based on radiation chemistry was used for the first time for the synthesis of hybrid organic–inorganic composites in aqueous solution. Starting from an aqueous solution containing both metal ions and organic monomers, our idea was to originally use gamma-radiolysis for the in situ production of hydrated electrons and hydroxyl radicals as reducing and oxidizing species, respectively, in order to synthesize hybrid CP/metal nanocomposites.Starting from EDOT monomers and silver perchlorate salt, the preparation of PEDOT/Ag composites, made of PEDOT conducting polymers and silver nanoparticles, was achieved by using different radiolytical procedures. According to the two-step method, PEDOT polymers were first synthesized by either oxidation or reduction of EDOT monomers and then, silver nanoparticles were produced in the presence of PEDOT by the reduction of silver ions. Differently, according to the one-pot method, polymerization and metal ion reduction were achieved in parallel, in one step, thanks to the concomitant reduction of EDOT monomers and of silver ions, as demonstrated by pulse radiolysis experiments.
As highlighted using UV-Vis absorption spectrophotometry, ATR-FTIR spectroscopy and EDX spectroscopy, all the prepared nanocomposites are made of PEDOT polymers and silver nanoparticles. Also, they are found to be doped with perchlorate anions. In contrast, the morphologies of the PEDOT/Ag nanocomposites are almost different and depend on the preparation procedure, as demonstrated, respectively, by cryo-TEM observations in aqueous solution and SEM microscopy after deposition. While composites prepared via the two-step method are made of large spherical PEDOT nanoparticles at the surface of which smaller silver nanoparticles adsorb, composites produced according to the one-pot method are made of silver nanoparticles embedded in the core of small irregular spheroidal PEDOT nanoparticles.
Four-point probe measurements demonstrated that the electrical conductivities of all radiosynthesized PEDOT/Ag composites, which are close to those reported in the literature for PEDOT nanocomposites prepared according to other methodologies, are in the same order of magnitude. Moreover, these conductivities are close to those measured in the case of pure PEDOT polymers synthesized by radiolysis in the absence of silver. This proves that neither the morphology, nor the presence of silver have a clear influence on the electrical conductivity of the composites. The main factor which seems to determine the final conductivity is the amount of PEDOT conducting polymers, which is the same in all our nanocomposites. More interestingly, TGA revealed that, whatever their morphology, all PEDOT/Ag composites are characterized by the same thermal stability, which remains higher, over a wide range of temperatures, than that of pure PEDOT synthesized by radiolysis. Also, this thermal stability can be enhanced by increasing the amount of silver into the composites.
We demonstrated, in previous papers,37,39,42 that PEDOT conducting polymers can be easily produced in aqueous solution by radiolysis. We succeeded in the present work in the preparation, thanks to radiation chemistry, of hybrid nanocomposites made of PEDOT conducting polymers and of silver nanoparticles. Even if the presence of silver nanoparticles does not seem to increase the electrical conductivity of the polymers, it certainly increases their thermal stability. Work is in progress in order to enhance the conductivity and the thermal stability of PEDOT polymers by improving their doping level, thanks to the increase in their conjugation length. Finally, the present results also encourage us to investigate synthesis via radiation chemistry of other hybrid CP/metal nanocomposites containing, for instance, polypyrrole as a conducting polymer and gold as an inorganic component.
Acknowledgements
We acknowledge the financial support from the China Scholarship Council (CSC).References
- J. Kao, K. Thorkelsson, P. Bai, B. J. Rancatore and T. Xu, Chem. Soc. Rev., 2013, 42, 2654–2678 RSC.
- C. Sanchez, B. Lebeau, F. Chaput and J. P. Boilot, Adv. Mater., 2003, 15, 1969–1994 CrossRef CAS.
- A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354–2371 RSC.
- C. Li, H. Bai and G. Shi, Chem. Soc. Rev., 2009, 38, 2397–2409 RSC.
- L. A. Kane-Maguire and G. G. Wallace, Chem. Soc. Rev., 2010, 39, 2545–2576 RSC.
- C. Zhu, D. Du, A. Eychmuller and Y. Lin, Chem. Rev., 2015, 115, 8896–8943 CrossRef CAS PubMed.
- X. X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- R. V. Salvatierra, M. M. Oliveira and A. J. G. Zarbin, Chem. Mater., 2010, 22, 5222–5234 CrossRef CAS.
- P. Xu, K. Chang, Y. I. Park, B. Zhang, L. L. Kang, Y. C. Du, R. S. Iyer and H. L. Wang, Polymer, 2013, 54, 485–489 CrossRef CAS.
- J. Liu, M. Li, Y. Q. Zhang, L. L. Yang and J. S. Yao, J. Appl. Polym. Sci., 2013, 129, 3787–3792 CrossRef CAS.
- K. E. Hnida, R. P. Socha and G. D. Sulka, J. Phys. Chem. C, 2013, 117, 19382–19392 CAS.
- R. Gangopadhyay and A. De, Chem. Mater., 2000, 12, 608–622 CrossRef CAS.
- X. F. Lu, W. J. Zhang, C. Wang, T. H. Wen and Y. Wei, Prog. Polym. Sci., 2011, 36, 671–712 CrossRef CAS.
- N. Vucaj, M. D. J. Quinn, C. Baechler, S. M. Notley, P. Cottis, P. Hojati-Talemi, M. V. Fabretto, G. G. Wallace, P. J. Murphy and D. R. Evans, Chem. Mater., 2014, 26, 4207–4213 CrossRef CAS.
- V. Stockhausen, P. Martin, J. Ghilane, Y. Leroux, H. Randriamahazaka, J. Grand, N. Felidj and J. C. Lacroix, J. Am. Chem. Soc., 2010, 132, 10224–10226 CrossRef CAS PubMed.
- E. Park, O. S. Kwon, S. J. Park, J. S. Lee, S. You and J. Jang, J. Mater. Chem., 2012, 22, 1521–1526 RSC.
- S. S. Kumar, C. S. Kumar, J. Mathiyarasu and K. L. Phani, Langmuir, 2007, 23, 3401–3408 CrossRef CAS PubMed.
- R. R. Yue, H. W. Wang, D. Bin, J. K. Xu, Y. K. Du, W. S. Lu and J. Guo, J. Mater. Chem. A, 2015, 3, 1077–1088 CAS.
- J. G. Wang, C. Zhang, Z. J. Du, H. Q. Li and W. Zou, RSC Adv., 2016, 6, 31782–31789 RSC.
- H. Ju and J. Kim, ACS Nano, 2016, 10, 5730–5739 CrossRef CAS PubMed.
- P. Xu, X. Han, B. Zhang, Y. Du and H. L. Wang, Chem. Soc. Rev., 2014, 43, 1349–1360 RSC.
- V. Armel, B. Winther-Jensen, R. Kerr, D. R. MacFarlaneb and B. Winther-Jensena, J. Mater. Chem., 2012, 22, 19767–19773 RSC.
- Z. L. Mo, D. D. Zuo, H. Chen, Y. X. Sun and P. Zhang, Eur. Polym. J., 2007, 43, 300–306 CrossRef CAS.
- S. K. Pillalamarri, F. D. Blum, A. T. Tokuhiro and M. F. Bertino, Chem. Mater., 2005, 17, 5941–5944 CrossRef CAS.
- S. Fujii, A. Aichi, K. Akamatsu, H. Nawafune and Y. Nakamura, J. Mater. Chem., 2007, 17, 3777–3779 RSC.
- X. M. Feng, H. P. Huang, Q. Q. Ye, J. J. Zhu and W. H. Hou, J. Phys. Chem. C, 2007, 111, 8463–8468 CAS.
- D. Muñoz-Rojas, J. Oró-Solé, O. Ayyad and P. Gómez-Romero, J. Mater. Chem., 2011, 21, 2078–2086 RSC.
- C. Hauville, H. Remita, P. Therond, A. Rouscilles, M. Couturier, D. Jore and M. Gardes-Albert, Radiat. Res., 1998, 150, 600–608 CrossRef CAS PubMed.
- N. Varmenot, S. Remita, Z. Abedinzadeh, P. Wisniowski, G. Strzelczak and K. Bobrowski, J. Phys. Chem. A, 2001, 105, 6867–6875 CrossRef CAS.
- S. Remita, P. Fontaine, E. Lacaze, Y. Borensztein, H. Sellame, R. Farha, C. Rochas and M. Goldmann, Nucl. Instrum. Methods Phys. Res., Sect. B, 2007, 263, 436–440 CrossRef CAS.
- J. Attia, S. Remita, S. Jonic, E. Lacaze, M.-C. Faure, E. Larquet and M. Goldmann, Langmuir, 2007, 23, 9523–9526 CrossRef CAS PubMed.
- Y. N. Rao, D. Banerjee, A. Datta, S. K. Das, R. Guin and A. Saha, Radiat. Phys. Chem., 2010, 79, 1240–1246 CrossRef CAS.
- L. He, L. F. Dumee, D. Liu, L. Velleman, F. H. She, C. Banos, J. B. Daviesc and L. X. Kong, RSC Adv., 2015, 5, 10707–10715 RSC.
- A. Balcerzyk, U. Schmidhammer, G. Horne, F. Wang, J. Ma, S. M. Pimblott, A. D. L. Lande and M. Mostafavi, J. Phys. Chem. B, 2015, 119, 10096–10101 CrossRef CAS PubMed.
- S. Remita, PhD thesis, Paris-Sud University, Orsay, 1995.
- S. Remita, J. M. Orts and J. M. Feliu, Chem. Phys. Lett., 1994, 218, 115–121 CrossRef CAS.
- Y. Lattach, C. Coletta, S. Ghosh and S. Remita, ChemPhysChem, 2014, 15, 208–218 CrossRef CAS PubMed.
- Y. Lattach, A. Deniset-Besseau, J. M. Guigner and S. Remita, Radiat. Phys. Chem., 2013, 82, 44–53 CrossRef CAS.
- Z. Cui, C. Coletta, R. Rebois, S. Baiz, M. Gervais, F. Goubard, P. H. Aubert, A. Dazzi and S. Remita, Radiat. Phys. Chem., 2016, 119, 157–166 CrossRef CAS.
- Z. P. Cui, C. Coletta, A. Dazzi, P. Lefrancois, M. Gervais, S. Neron and S. Remita, Langmuir, 2014, 30, 14086–14094 CrossRef CAS PubMed.
- C. Coletta, Z. P. Cui, P. Archirel, P. Pernot, J. L. Marignier and S. Remita, J. Phys. Chem. B, 2015, 119, 5282–5298 CrossRef CAS PubMed.
- C. Coletta, Z. P. Cui, A. Dazzi, J.-M. Guigner, S. Néron, J.-L. Marignier and S. Remita, Radiat. Phys. Chem., 2016, 126, 21–31 CrossRef CAS.
- J. Roncali, P. Blanchard and P. Frère, J. Mater. Chem., 2005, 15, 1589–1610 RSC.
- L. B. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef CAS.
- H. R. Jung and W. J. Lee, Solid State Ionics, 2011, 187, 50–57 CrossRef CAS.
- M. Mumtaz, E. Cloutet, C. Labrugère, G. Hadziioannou and H. Cramail, Polym. Chem., 2013, 4, 615–622 RSC.
- P. Xu, K. Chang, Y. L. Park, B. Zhang, L. L. Kang, Y. C. Du, R. S. Iyer and H. L. Wang, Polymer, 2013, 54, 485–489 CrossRef CAS.
- S. Radhakrishnan, C. Sumathi, A. Umar, S. Jae Kim, J. Wilson and V. Dharuman, Biosens. Bioelectron., 2013, 47, 133–140 CrossRef CAS PubMed.
- S. Remita, M. Mostafavi and M. O. Delcourt, New J. Chem., 1994, 18, 581–588 CAS.
- M. Mostafavi, S. Remita, M. O. Delcourt and J. Belloni, J. Chem. Phys., 1996, 93, 1828–1842 CAS.
- J. W. T. Spinks and R. J. Woods, Ber. Bunsenges. Phys. Cher., John Wiley & Sons, Inc., New York, United States, 1990, pp. 251–256 Search PubMed.
- C. Ferradini and J.-P. Jay-Gerin, Can. J. Chem., 1999, 77, 1542–1575 CrossRef CAS.
- C. Ferradini and J. P. Jay-Gerin, Res. Chem. Intermed., 2000, 26, 549–565 CrossRef CAS.
- E. J. Hart, Acc. Chem. Res., 1969, 2, 161–167 CrossRef CAS.
- L. Y. Song, M. Z. Wang, Y. H. Cong, W. J. Liu, X. W. Ge and Z. C. Zhang, Polymer, 2007, 48, 150–157 CrossRef CAS.
- J. Belloni, M. Mostafavi, H. Remita, J.-L. Marignier and M.-O. Delcourt, New J. Chem., 1998, 1239–1255 RSC.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- M. Mostafavi, G. R. Dey, L. Francüois and J. Belloni, J. Phys. Chem. A, 2002, 106, 10184–10194 CrossRef CAS.
- S. Remita, P. Fontaine, C. Rochas, F. Muller and M. Goldmann, EPJdirect, 2005, 34, 231–233 CAS.
- N. Sakmeche, S. Aeiyach, J.-J. Aaron, M. Jouini, J. C. Lacroix and P.-C. Lacaze, Langmuir, 1999, 15, 2566–2574 CrossRef CAS.
- W. M. Hanynes, Handbook of Chemistry and Physics, 2012, vol. 84, p. 112 Search PubMed.
- J. Belloni, H. Monard, F. Gobert, J. P. Larbre, A. Demarque, V. De Waele, I. Lampre, J. L. Marignier, M. Mostafavi, J. C. Bourdon, M. Bernard, H. Borie, T. Garvey, B. Jacquemard, B. Leblond, P. Lepercq, M. Omeich, M. Roch, J. Rodier and R. Roux, Nucl. Instrum. Methods Phys. Res., 2005, 539, 527–539 CrossRef CAS.
- J. L. Marignier, V. de Waele, H. Monard, F. Gobert, J. P. Larbre, A. Demarque, M. Mostafavi and J. Belloni, Radiat. Phys. Chem., 2006, 75, 1024–1033 CrossRef CAS.
- M. V. Piechowski, M.-A. Thelen, J. Hoigne and R. E. Biihler, Ber. Bunsenges. Phys. Chem., 1992, 96, 1448–1453 CrossRef.
- D. L. Teagarden and D. S. Baker, Eur. J. Pharm. Sci., 2002, 15, 115–133 CrossRef CAS PubMed.
- D. Paramelle, A. Sadovoy, S. Gorelik, P. Free, J. Hobley and D. G. Fernig, Analyst, 2014, 139, 4855–4861 RSC.
- A. Henglein and M. Giersig, J. Phys. Chem. B, 1999, 103, 9533–9539 CrossRef CAS.
- D. Rajesh and C. S. Sunandana, Results Phys., 2012, 2, 22–25 CrossRef.
- C. Kvarnström, H. Neugebauer, S. Blomquist, H. J. Ahonenc, J. Kankarec and A. Ivaska, Electrochim. Acta, 1999, 44, 2739–2750 CrossRef.
- S. Ghosh, H. Remita, L. Ramos, A. Dazzi, A. Deniset-Besseau, P. Beaunier, F. Goubard, P. H. Aubert, F. Brisset and S. Remita, New J. Chem., 2014, 38, 1106–1115 RSC.
- S. Q. He, J. G. Wu and Q. L. Zhang, Acta Chim. Sin., 1984, 42, 1183–1187 CAS.
- J. S. Yang and G. H. Hsiue, J. Membr. Sci., 1996, 120, 69–76 CrossRef CAS.
- Y. Chen, Y. H. Zhang and L. J. Zhao, Phys. Chem. Chem. Phys., 2004, 6, 537–542 RSC.
- P. C. Beaumont and E. L. Powers, Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med., 1983, 485–494 CrossRef CAS.
- S. Mahendia, A. K. Tomar and S. Kumar, J. Alloys Compd., 2010, 508, 406–411 CrossRef CAS.
- J. Wu, W. Cho, D. C. Martin, Z. Q. Feng, M. K. Leach, E. W. Franz, Y. I. Naim, Z. Z. Gu and J. M. Corey, Polymer, 2013, 54, 702–708 CrossRef PubMed.
- B. H. Jones, K.-Y. Cheng, R. J. Holmes and T. P. Lodge, Macromolecules, 2012, 45, 599–601 CrossRef CAS.
- R. Nagarajan, J. Kumar, F. F. Bruno, L. A. Samuelson and R. Nagarajan, Macromolecules, 2008, 41, 3049–3052 CrossRef.
| This journal is © the Partner Organisations 2017 |