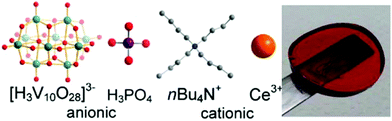
Instantaneous formation of polyoxometalate-based cerium vanadium oxide gels†
Andrey
Seliverstov
a,
Mojca
Rangus
b,
Martin
Hartmann
b,
Scott G.
Mitchell
c and
Carsten
Streb
*a
aInstitute of Inorganic Chemistry I, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: carsten.streb@uni-ulm.de; Web: http://www.strebgroup.net
bErlangen Catalysis Resource Center ECRC, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstr. 3, 91058 Erlangen, Germany
cInstituto de Ciencia de Materiales de Aragón (ICMA-CSIC), Universidad de Zaragoza, 50019-Zaragoza, Spain
First published on 25th November 2016
Abstract
The instantaneous formation of mechanically stable cerium vanadium oxide gels starting from soluble polyoxovanadates is reported together with initial application studies. Upon addition of phosphoric acid to solutions containing a vanadium oxide source (e.g. (nBu4N)4[V4O12]) and Ce3+, instantaneous formation (reaction time <1 s) of a vanadium oxide gel is observed. The gel shows unique mechanical and thermal stabilities (up to ∼180 °C). High permeability of the gel is observed, allowing its use for long-term acid delivery into aqueous media or for the adsorption of organic aromatic dye pollutants from solution. A range of spectroscopy and electron microscopy techniques provide insight into the gel formation and the gel composition: an intertwined 3D matrix of nanowires (d ∼ 10 nm) containing cerium, vanadium oxide and phosphate is identified as an inorganic matrix which enables the formation of the mechanically stable gel.
Introduction
Polyoxometalates (POMs) are molecular analogues of solid-state metal oxides.1 They have attracted vast interest in academia and industry due to their widespread application in (photo)-catalysis,2,3 energy storage,4,5 molecular electronics6 and nanomaterials science.7 POMs can be employed under homogeneous or heterogeneous conditions. While the use of POMs under homogeneous conditions provides detailed insight into reactivity and solution behaviour, many technological applications rely on heterogeneous systems, e.g. for facile separation and recycling.8 The need to embed POMs in heterogeneous systems has been recognized and various routes for POM heterogenization have been reported.9 One promising approach is the use of POMs as precursors for the formation of metal oxide gels. This approach allows the tuning of POM precursor properties on the molecular level; further fine-tuning of the materials’ properties is possible by incorporation of additional reactive components. POM-based gels with unique optical,10 electrochemical11,12 and thermo-/photoresponsive behaviour13 have been developed. Polyoxovanadates are particularly attractive precursors for gel formation as their conversion into nanostructured vanadium oxide gels is well-known.14 Vanadium oxide gels are technologically highly relevant materials15 and their use in solar energy conversion,16,17 (bio)sensors,18,19 porous materials20,21 and battery electrodes22,23 has been reported.Results and discussion
Gel synthesis
Here, we report a novel synthetic route which enables the instantaneous formation of a cerium vanadium-oxide based organogel. The gel is formed spontaneously when a basic organic solution containing a vanadate precursor (e.g. (nBu4N)3[H3V10O28]), nBu4NOH and Ce3+ is acidified using aqueous phosphoric acid (Fig. 1). Gelation occurs instantaneously (tgelation < 1 s) and a mechanically stable gel which can be cast into a desired shape (disk, cylinder, etc.) and manipulated using tweezers is formed. The high mechanical stability of the gel is shown, as the gel is self-supporting and can be cast or cut into the required shape (Fig. 1).The formation of the gel was discovered by serendipity when we investigated the synthesis of lanthanide-functionalized polyoxovanadate clusters in organic solution.24,25 It was observed that when a basic DMSO solution containing nBu4NOH, (nBu4N)3[H3V10O28] and Ce(NO3)3·6H2O is acidified with aqueous phosphoric acid, the solution viscosity instantaneously increases and a mechanically stable gel is obtained. Systematic analysis of the reaction parameters showed that both Ce3+ and phosphoric acid are required for gel formation: attempts to replace Ce3+ with a wide range of other metal cations, including lanthanides, alkali and alkali earth metals did not result in the formation of a gel. Gelation is therefore specific to Ce3+. While the exact role of Ce3+ is still unclear, we suggest that Ce3+ acts as an ionic cross-linker between the building blocks of the gel.26 Attempts to replace phosphoric acid with other mineral or organic acids (HClaq, HNO3, H2SO4, acetic acid, benzoic acid) did not result in gelation, indicating that phosphate anions are also critical for the gel formation. In contrast, the vanadium oxide source can be varied and gel formation is possible with a range of soluble vanadium oxide precursors such as (nBu4N)4[V4O12], (nBu4N)3[H3V10O28] or hydrolysed V2O5. As the synthesis is carried out under basic conditions (i.e. in the presence of nBu4NOH), the compounds are converted into metavanadate-type precursors,27–29 thus explaining their similar gel-forming reactivities upon acidification. For reasons of brevity, this report is focused on gels based on (nBu4N)3[H3V10O28].‡
It was further demonstrated that gel formation is achieved in a range of polar organic solvents e.g. dimethyl sulfoxide (DMSO), N,N′-dimethyl formamide (DMF) and water, giving access to vanadium oxide organogels and hydrogels. However, lower mechanical stability was observed for the hydrogel compared with the respective organogels, therefore this report is focused on DMSO-based gels.‡
Gel characterization
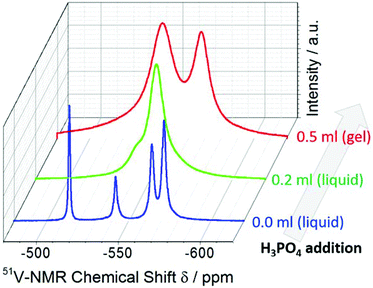
Upon H3PO4 addition (but still in the non-gelled form), one major signal arises at δ = −554 ppm (assigned to dinuclear [V2O7]4− species),30 while the resonance at δ = −542 ppm can be assigned to residual [VO4]3−.30 The data therefore suggest that the initial steps of acid-induced vanadium oxide poly-condensation are observed. Upon gelation, two distinct signals are observed by 51V-NMR spectroscopy at δ = −568 ppm and δ = −544 ppm, indicating that two vanadate coordination environments are present within the gel structure. The chemical shifts observed are in line with tetrahedrally coordinated vanadate centres in an all-oxo environment.30 Note that other possible vanadium coordination geometries would be observed at significantly different 51V-NMR chemical shifts: square pyramidal [VO5] units in solid-state vanadium oxide gels are observed at δ ca. 280 ppm,15 while octahedral [VO6] units are observed at δ ca. 400–500 ppm (e.g. in [V10O28]6−).3031P-NMR data show that in the gel, phosphate-based building blocks are observed, see the ESI.† The data therefore suggest that two distinct tetrahedral vanadate building blocks are present in the nanofibers together with phosphate groups, and future work will use high-resolution TEM, X-ray photoelectron spectroscopy and theoretical modelling to gain further insight into the detailed chemical structure.
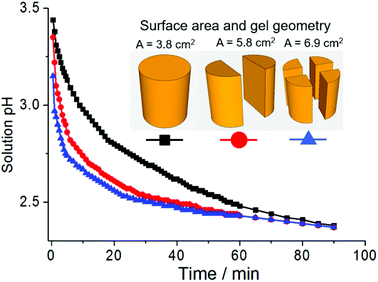
Gel properties and applications
Conclusions
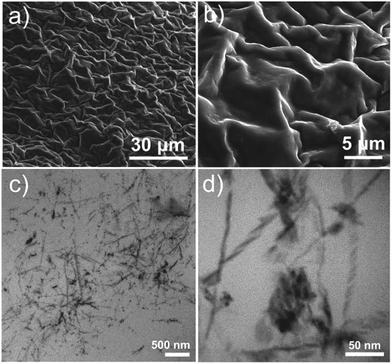
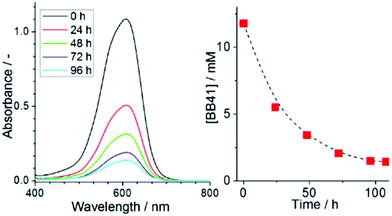
In summary, we report a facile route which allows the instantaneous formation of mechanically stable cerium vanadium oxide gels. Material characterization provides insight into the gel structure and it is shown that the gel is stabilized by intertwined vanadium oxide nanofibers (d ∼ 10 nm). Initial reactivity studies show that the gel features unprecedented thermal stability, does not de-gelate upon heating, can deliver protons into aqueous solution and shows promising pollutant adsorption properties in aqueous solution. Future studies will further explore the molecular-level structure of the gel and aim at further analysis of the adsorbent properties of the gel.Acknowledgements
Financial support by the Deutscher Akademischer Austauschdienst (DAAD) EU COST Action CM1203, Ulm University, Friedrich-Alexander-Universität Erlangen-Nürnberg and Fundación General CSIC (Programa ComFuturo) is gratefully acknowledged. The authors would like to acknowledge the use of The Advanced Microscopy Laboratory (LMA-INA).Notes and references
- L. Cronin, A. Müller and (guest eds.), Chem. Soc. Rev., 2012, 41, 7325–7648.
- C. L. Hill, J. Mol. Catal. A: Chem., 2007, 262, 2–6 CrossRef CAS.
- C. Streb, Dalton Trans., 2012, 41, 1651 RSC.
- A. Sartorel, M. Bonchio, S. Campagna and F. Scandola, Chem. Soc. Rev., 2013, 42, 2262–2280 RSC.
- Y. C. Ji, L. J. Huang, J. Hu, C. Streb and Y.-F. Song, Energy Environ. Sci., 2015, 8, 776–789 CAS.
- J. Lehmann, A. Gaita-Arino, E. Coronado and D. Loss, Nat. Nanotechnol., 2007, 2, 312–317 CrossRef CAS PubMed.
- D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CrossRef CAS PubMed.
- Y. Leng, J. Wang, D. Zhu, X. Ren, H. Ge and L. Shen, Angew. Chem., 2009, 121, 174–177 CrossRef.
- New and Future Developments in Catalysis: Hybrid materials, Composites and Organocatalysts, ed. S. Suib, Elsevier, Amsterdam, 2013 Search PubMed.
- S. Favette, B. Hasenknopf, J. Vaissermann, P. Gouzerh and C. Roux, Chem. Commun., 2003, 2664–2665 RSC.
- X. Tong and V. Thangadurai, J. Phys. Chem. C, 2015, 119, 7621–7630 CAS.
- R. Tsunashima, C. Richmond and L. Cronin, Chem. Sci., 2012, 3, 343 RSC.
- P. He, B. Xu, H. Liu, S. He, F. Saleem and X. Wang, Sci. Rep., 2013, 3, 1833 Search PubMed.
- J. Livage, Coord. Chem. Rev., 1998, 178–180(P), 999–1018 CrossRef CAS.
- J. Livage, Chem. Mater., 1991, 3, 578–593 CrossRef CAS.
- O. Dvorak and M. K. De Armond, Chem. Mater., 1992, 4, 1074–1077 CrossRef CAS.
- K. Zilberberg, S. Trost, J. Meyer, A. Kahn, A. Behrendt, D. Lützenkirchen-Hecht, R. Frahm and T. Riedl, Adv. Funct. Mater., 2011, 21, 4776–4783 CrossRef CAS.
- J. Liu, X. Wang, Q. Peng and Y. Li, Adv. Mater., 2005, 17, 764–767 CrossRef CAS.
- V. Glezer and O. Lev, J. Am. Chem. Soc., 1993, 115, 2533–2534 CrossRef CAS.
- G. T. Chandrappa, N. Steunou and J. Livage, Nature, 2002, 416, 702 CrossRef CAS PubMed.
- O. Durupthy, M. Jaber, N. Steunou, J. Maquet, G. T. Chandrappa and J. Livage, Chem. Mater., 2005, 17, 6395–6402 CrossRef CAS.
- N. A. Chernova, M. Roppolo, A. C. Dillon and M. S. Whittingham, J. Mater. Chem., 2009, 19, 2526–2552 RSC.
- G. Li, S. Pang, L. Jiang, Z. Guo and Z. Zhang, J. Phys. Chem. B, 2006, 110, 9383–9386 CrossRef CAS PubMed.
- A. Seliverstov and C. Streb, Chem. – Eur. J., 2014, 20, 9733–9738 CrossRef CAS PubMed.
- A. Seliverstov and C. Streb, Chem. Commun., 2014, 50, 1827–1829 RSC.
- Y. Zhang, N. Zhou, S. Akella, Y. Kuang, D. Kim, A. Schwartz, M. Bezpalko, B. M. Foxman, S. Fraden, I. R. Epstein and B. Xu, Angew. Chem., Int. Ed., 2013, 52, 11494–11498 CrossRef CAS PubMed.
- J. Tucher, L. C. Nye, I. Ivanovic-Burmazovic, A. Notarnicola and C. Streb, Chem. – Eur. J., 2012, 18, 10949–10953 CrossRef CAS PubMed.
- J. Forster, B. Rösner, R. H. Fink, L. C. Nye, I. Ivanovic-Burmazovic, K. Kastner, J. Tucher and C. Streb, Chem. Sci., 2013, 4, 418–424 RSC.
- K. Kastner, J. T. Margraf, T. Clark and C. Streb, Chem. – Eur. J., 2014, 20, 12269–12273 CrossRef CAS PubMed.
- D. Rehder, Bull. Magn. Reson., 1982, 4, 33–83 CAS.
- C. Streb, R. Tsunashima, D. A. MacLaren, T. McGlone, T. Akutagawa, T. Nakamura, A. Scandurra, B. Pignataro, N. Gadegaard and L. Cronin, Angew. Chem., Int. Ed., 2009, 48, 6490–6493 CrossRef CAS PubMed.
- M. Dave and C. Streb, Dalton Trans., 2015, 44, 18919–18922 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic and analytical details. See DOI: 10.1039/c6qi00457a |
| ‡ The gels employed for the application studies were prepared as follows: to a solution of (nBu4N)3[H3V10O28] (0.06 mmol) and CeCl3·7H2O (0.11 mmol) in DMSO (5 ml) was added a methanolic solution of TBAOH (1.5 M, 0.3 ml) at 80 °C. The solution was stirred for 5 min and H3PO4 (9 M, 0.45 ml) was added, resulting in the instantaneous formation of the gel which could be mechanically removed from the reaction vessel and cut into the desired shape using a scalpel. |
| This journal is © the Partner Organisations 2017 |