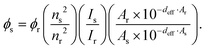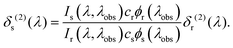Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A biocompatible macromolecular two-photon initiator based on hyaluronan†
Maximilian
Tromayer
ae,
Peter
Gruber
be,
Marica
Markovic
be,
Arnulf
Rosspeintner
c,
Eric
Vauthey
 c,
Heinz
Redl
de,
Aleksandr
Ovsianikov
be and
Robert
Liska
ae
c,
Heinz
Redl
de,
Aleksandr
Ovsianikov
be and
Robert
Liska
ae
aInstitute of Applied Synthetic Chemistry, TU Wien (Technische Universitaet Wien), Getreidemarkt 9/163/MC, 1060 Vienna, Austria
bInstitute of Materials Science and Technology, TU Wien (Technische Universitaet Wien), Getreidemarkt 9/308, 1060 Vienna, Austria
cPhysical Chemistry Department, Sciences II, University of Geneva, 30 Quai Ernest Ansermet, CH-1211 Geneva 4, Switzerland
dLudwig Boltzmann Institute - Experimental and Clinical Traumatology, Donaueschingenstraße 13, 1200 Vienna, Austria
eAustrian Cluster for Tissue Regeneration, Austria
First published on 29th November 2016
Abstract
The possibility of the direct encapsulation of living cells via two-photon induced photopolymerization enables the microfabrication of hydrogel scaffolds with high initial cell loadings and intimate matrix–cell contact. While highly efficient water-soluble two-photon initiators based on benzylidene ketone dyes have been developed, they exhibit considerable cyto- and phototoxicity. To address the problem of photoinitiator migration from the extracellular matrix into the cytosol, a two-photon initiator bound to a polymeric hyaluronan backbone (HAPI) was synthesized in this work. HAPI exhibited a distinct improvement of cytocompatibility compared to a reference two-photon initiator. Basic photophysical investigations were performed to characterize the absorption and fluorescence behavior of HAPI. Laser scanning microscopy was used to visualize and confirm the hindered transmembrane migration behavior of HAPI. The performance of HAPI was tested in two-photon polymerization at exceedingly high printing speeds of 100 mm s−1 producing gelatin-based complex 3D hydrogel scaffolds with a water content of 85%. The photodamage of the structuring process was low and viable MC3T3 cells embedded in the gel were monitored for several days after structuring.
Introduction
The process of two-photon induced polymerization (2PP) has attracted considerable interest because it enables 3D printing with a resolution in the sub-micrometer range. Parts containing ultra-small features like photonic crystals, cantilevers, optical waveguides and microelectronic components may thus be produced.1 Furthermore 2PP can be employed to fabricate 3D scaffolds as a structural support for cell growth in tissue engineering.2 This is of great interest because 2D matrices used in traditional cell culture systems do not accurately reproduce the cells’ natural environment and lead to significant differences in the structure, function or physiology compared to living tissue. Various studies3,4 have investigated and demonstrated that 3D-matrix adhesions enhanced cellular functional activities compared to 2D adhesions. Bokhari et al. have shown that HepG2 hepatocytes grown on 3D polystyrene scaffolds are less susceptible to certain toxicological challenges than those grown in 2D.3Usually such scaffolds are pre-fabricated with large enough pores and seeded with cells on the surface, which then migrate inside the scaffold, attach within the pores and proliferate there. Another popular approach is the encapsulation of cells within hydrogels, which can be cross-linked by different means, including photopolymerization. Cell encapsulation provides the advantages of high initial cell loading and more intimate cell–matrix contact, similar to that of the natural extracellular matrix (ECM).
Since biological tissue is relatively transparent at the laser wavelengths used for 2PP, arbitrary 3D structures can be created deep within aqueous media. Thus 2PP can in principle be employed to encapsulate living cells within 3D hydrogel structures by performing 2PP around them.5
Recently several specialized water-soluble two photon initiators (2PIs) have been developed and tested for their efficiency in the microfabrication of 3D hydrogel structures.6 While they possess a relatively high two-photon absorption (2PA) cross section compared to commercial water-soluble one-photon initiators like Irgacure 2959, and thus allow for structuring at high printing speeds and low laser intensities, there is still a need for novel initiators with improved cytocompatibility. Besides the mere cytotoxicity in darkness, the occurrence of additional phototoxicity upon irradiation is an important concern with PIs designed for biological applications.
The aforementioned phototoxicity can either be mediated by the active species that are generated from the PI itself, i.e. free radicals in the case of initiators for radical photopolymerization, or by other species that originate from secondary pathways. Among the non-radiative processes that lead to the relaxation of the involved excited triplet states of the PI, quenching by molecular oxygen under the formation of singlet oxygen (SO) and as a further consequence other reactive oxygen species (ROS) play an important role. Oxygen-activated species such as superoxide anions, hydroxyl radicals and singlet oxygen are by-products of oxygen-dependent reactions and have a wide potential for causing cell damage. Among these chemical entities, singlet oxygen is one of the most reactive, capable of damaging cells and tissues. Besides its relatively long lifetime in solution, from micro- to milliseconds, singlet oxygen behaves as a strong electrophile in solution and reacts avidly with molecules possessing regions of high electron density. The oxidative damage of cells mediated by singlet oxygen is common and DNA, proteins and lipids are all at risk.7 In fact, this is also made use of for medical purposes in photodynamic therapy.8 In the case of the encapsulation of living cells via 2PP using water soluble 2PIs, it is hypothesized that due to their size and low molecular weight, they are able to pass the cytoplasmic membrane and migrate inside the cells. Since the cells are highly transparent for the lasers used in 2PP, the 2PI molecules are excited within the cytoplasm and the generated radicals and ROS may damage vital structures inside the cells. Thus, limiting the diffusion of 2PIs through the cell membrane might be an efficient strategy to reduce the overall photodamage to the cells and tackle the problem of phototoxicity of the 2PP structuring process.
A promising approach designing molecules to realize this is increasing the size and molecular weight of the 2PI by modifying a polymeric backbone, covalently attaching several units of a suitable derivative based on a previously examined efficient low molecular weight 2PI.
Hyaluronan (HA) was chosen as a backbone for novel polymeric 2PIs in this present work. HA is a natural and vital part of the ECM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and is becoming increasingly important as a building block for the creation of new bio-materials with utility in tissue engineering and regenerative medicine.10–12 Furthermore there are suitable methods for chemical modification e.g. amidation,13,14 and it is a highly negatively charged polycarboxylate, which poses an additional hindrance to migration through the cytoplasmic membrane which also bears a net negative charge.15
9 and is becoming increasingly important as a building block for the creation of new bio-materials with utility in tissue engineering and regenerative medicine.10–12 Furthermore there are suitable methods for chemical modification e.g. amidation,13,14 and it is a highly negatively charged polycarboxylate, which poses an additional hindrance to migration through the cytoplasmic membrane which also bears a net negative charge.15
Two recent publications describe the synthesis and application of several efficient 2PIs based on cyclic dibenzylidene ketones – the first one16 focuses on 2PIs intended for polymerizable formulations based on organic solvents and resins, while the second one6 discusses derivatives bearing carboxyl-groups linked to their amino donor-functionalities leading to water-solubility and thus extending the range of application to hydrogel-based materials. Since the water solubility is already provided by the polycarboxylate HA-backbone and additional carboxyl groups in the 2PIs would only make the synthesis and purification more difficult, the 2PI component should bear simple alkylamino-groups that provide good organo-solubility until the last step of coupling to HA.
It has been reported in the literature that the quantum yield of singlet oxygen production from excited cyclic dibenzylidene ketones depends strongly on the size of the central ring, decreasing drastically for larger ring sizes in a series of cyclobutanone, cyclopentanone and cyclohexanone based derivatives.17
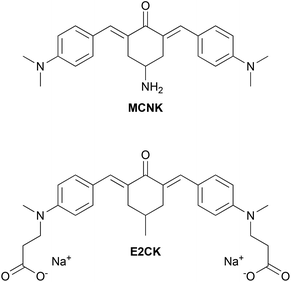
Because of the prospect of lower phototoxicity for a six-membered central ring, the high initiation efficiency previously reported for similar derivatives,6,16 several successful reports of amidations of HA in the literature using 1,1′-carbonyldiimidazole (CDI)14,18–20 as well as commercial availability of suitable starting materials, the 2PI in this work was based on an amino substituted cyclohexanone. Fig. 1 shows the basic structure of the amino-cyclohexanone 2PI MCNK prepared in this work, and the previously published6 cyclohexanone based reference 2PI E2CK.
After linking MCNK to HA, the complete polymer-bound 2PI was characterized by various spectroscopic methods, tested for cytotoxicity in darkness and finally applied in the 2PP encapsulation of living cells.
Experimental
Materials and methods
Syntheses of precursors and the polymer-based two-photon-initiator (HAPI)
Yield: 1.12 g (70% of theory).
Mp: 219–222 °C.
HR-MS m/z: [M + H]+ calculated for C29H38N3O3 476.2908; found 476.2932.
1H NMR (200 MHz, CDCl3): δ(ppm) = 7.88 (2H, s), 7.42 (4H, d, J = 8.9 Hz), 6.69 (4H, d, J = 8.9 Hz), 4.58–4.90 (1H, m), 4.09 (1H, bs), 2.72–3.25 (16H, m), 1.37 (9H, s).
13C NMR (50 MHz, CDCl3): δ(ppm) = 188.3, 155.1, 150.6, 139.9, 132.6, 128.1, 123.6, 111.6, 79.3, 45.2, 40.1, 34.4, 28.4.
Yield: 656 mg (95% of theory).
Mp: 158–160 °C.
HR-MS m/z: [M + H]+ calculated for C24H30N3O 376.2383; found 376.2394.
1H NMR (200 MHz, CDCl3): δ(ppm) = 7.82 (2H, s), 7.43 (4H, d, J = 9.0 Hz), 6.69 (4H, d, J = 9.0 Hz), 3.10–3.30 (3H, m), 3.00 (12H, s), 2.55–2.83 (2H, m), 1.39 (2H, s).
13C NMR (50 MHz, CDCl3): δ(ppm) = 188.8, 150.5, 138.5, 132.5, 129.6, 123.9, 111.6, 47.0, 40.1, 38.1.
Yield: 830 mg (79% of theory).
Mp: decomposition >250 °C.
HR-MS m/z: [M + H]+ calculated for C33H45N4O4 561.3435; found 561.3461.
1H NMR (200 MHz, CDCl3): δ(ppm) = 7.85 (2H, s), 7.37 (4H, d, J = 8.9 Hz), 6.64 (4H, d, J = 8.9 Hz), 6.49 (1H, d, J = 7.0 Hz), 4.86 (1H, bs), 4.25–4.49 (1H, m), 2.90–3.22 (18H, m), 2.13 (2H, t, J = 7.0 Hz), 1.70 (2H, quin, J = 6.8 Hz), 1.40 (9H, s).
13C NMR (50 MHz, CDCl3): δ(ppm) = 188.3, 172.1, 156.3, 150.6, 140.1, 132.7, 127.9, 123.5, 111.7, 79.1, 44.2, 40.1, 39.7, 33.9, 33.6, 28.4, 26.2.
Yield 94%.
Mp: 146–148 °C.
HR-MS m/z: [M + H]+ calculated for C28H37N4O2 461.2911; found 461.2919.
1H NMR (200 MHz, CDCl3): δ(ppm) = 7.85 (2H, s), 7.36 (4H, d, J = 8.8 Hz), 6.74 (1H, d, J = 7.8 Hz), 6.63 (4H, d, J = 8.8 Hz), 4.27–4.48 (1H, m, J = 4.7 and 4.7 and 7.8 Hz), 3.06 (4H, d, J = 4.7 Hz), 2.97 (12H, s), 2.57 (2H, t, J = 7.0 Hz), 2.14 (2H, t, J = 7.2 Hz), 1.62 (2H, quin, J = 7.0 Hz), 1.31 (2H, s).
13C NMR (50 MHz, CDCl3): δ(ppm) = 188.3, 172.5, 150.6, 140.1, 132.7, 127.9, 123.4, 111.6, 43.8, 41.4, 40.0, 34.2, 33.7, 29.0.
An aliquot of TBA-HA (125 mg, 200 μmol HA repetition units) was dissolved in dry DMSO (12 mL) under an argon atmosphere. After the addition of CDI (9.8 mg, 60 μmol) and methanesulfonic acid (1.46 mg, 15 μmol) stirring was continued for 18 h, then MGABA (55.6 mg, 120 μmol) was added and the clear orange solution was stirred for another 72 h. Brine (1.2 mL) was added dropwise to the reaction mixture and after 2 h of stirring at ambient temperature, acetone (35 mL) was added. The resulting orange precipitate was separated by centrifugation and washed with acetone (3 × 45 mL) by stirring vigorously and subsequent centrifugation. The precipitate was then dissolved in deionized water (25 mL), the resulting red solution dialyzed against deionized water and freeze-dried to obtain HAPI as a bright orange fibrous solid.
Yields: degradation step – 1.22 g (81% of theory), modification step – 83 mg (95% of theory).
M n (GPC of TBA-HA, 24 h degradation time): 50 kDa.
1H NMR (200 MHz, D2O) of HAPI: δ(ppm) = 7.57 (0.19H [varies with DS], bs), 7.01–7.45 (0.40H [varies with DS], m), 6.28–6.86 (0.39H [varies with DS], m), 4.19–4.64 (2H, m), 3.17–4.02 (10H, m), 2.52–3.03 (1.70 H [varies with DS], m), 1.92 (3H, bs).
Photophysics
Absorption spectra were recorded on a Cary 50 absorption spectrometer. Fluorescence spectra were recorded on a Jobin Yvon FluoroMax-4. The fluorescence spectra were corrected for the wavelength dependent sensitivity of the apparatus using a set of secondary emissive standards.21 The samples were measured in PBS-buffer (Roti-Cell, Roth). Emission quantum yields were determined using Rhodamine 6G in methanol as the reference (ϕr = 0.95) according toHere, nx, Ix and Ax are the refractive index, integrated emission intensity and absorbance at the excitation wavelength of x, with x being either the sample or reference. For the samples with quantum yields below 1%, the spectra were fitted with a lognorm function and the integral of the latter was used as Is.
Time resolved fluorescence experiments with an instrument response function of 200 ps were performed using a home-built single photon counting set-up, with excitation at 470 nm (LDH-P-C 470, PicoQuant), described in ref. 22.
Two-photon cross sections were determined via two-photon excitation spectra using a set-up similar to the one described in ref. 23 and detailed in ref. 24.
The two-photon cross section at a given wavelength, δs(2)(λ), was calculated as follows23
Here Ix(λ,λobs) is the (two-photon induced) fluorescence intensity at excitation wavelength λ and observation wavelength λobs for either sample or reference (x ∈ {s,r}). cx and ϕx(λobs) are the concentration and differential fluorescence quantum yield (at the observation wavelength) of the sample and reference. Rhodamine 6G in methanol was used as the reference for determining the absolute two-photon cross sections, while Coumarin 120 in ethanol, Coumarin 153 in methanol and LDS 698 in chloroform, were additionally used for assigning the bandshape.25
Cell culture
Mouse calvaria-derived preosteoblast cells (MC3T3-E1 Subclone 4) were obtained from ATCC-LGC Standards. MC3T3 were expanded in Alpha Minimum Essential Medium (αMEM) with ribonucleases, deoxyribonucleases, 2 mM L-glutamine, without ascorbic acid (Gibco), supplemented with 10% fetal bovine serum (Sigma) and 1% of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1 penicillin/streptomycin (Lonza). The cells were cultivated in an incubator in a humid atmosphere with 5% carbon dioxide at 37 °C. The medium was refreshed every second day.
000 U mL−1 penicillin/streptomycin (Lonza). The cells were cultivated in an incubator in a humid atmosphere with 5% carbon dioxide at 37 °C. The medium was refreshed every second day.
Evaluation of photoinitiator cytocompatibility
To evaluate the cytocompatibility of photoinitiators, PrestoBlue Cell Viability Reagent (Life Technologies) was used. For these tests 96-well plates were seeded with 5000 cells per well and incubated overnight for cells to attach. The cells were incubated with 100 μL of different dilutions of 2.0, 1.0 and 0.5 mM solutions of HAPI and E2CK for comparison. The procedure was performed under red light (620 nm LED) because of the light sensitivity and potential additional phototoxicity of the 2PIs. After 5 h of incubation with 2PIs, the culture medium was exchanged twice to remove 2PI residues and cell viability was evaluated. The resazurin-based reagent PrestoBlue was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with the medium and 100 μL were applied per well and incubated for 1 hour. Because of the reducing environment of viable cells, this reagent is transformed and turns red, becoming highly fluorescent. The fluorescence was measured with a plate reader (Synergy BioTek, excitation 560 nm, emission 590 nm). After correction for background fluorescence, the results of the cells exposed to different concentrations of the photoinitiator were compared to each other and to the controls (non-stimulated cells). It was assumed that the metabolic activity of the control not exposed to photoinitiators is 100%. A statistical evaluation of data was performed using software packages IBM SPSS Statistic 22 (SPSS Inc., Chicago, IL) and Excel 2013 (Microsoft Office). Results are presented as the average of repeated measurements ± standard deviation (SD). After verifying the normal distribution and homogeneity of variance, a one-way analysis of variance was used to compare means of the samples. If a significant difference was observed (p < 0.05), Bonferroni post hoc multiple comparison tests were performed to detect a significant difference between the samples.
10 with the medium and 100 μL were applied per well and incubated for 1 hour. Because of the reducing environment of viable cells, this reagent is transformed and turns red, becoming highly fluorescent. The fluorescence was measured with a plate reader (Synergy BioTek, excitation 560 nm, emission 590 nm). After correction for background fluorescence, the results of the cells exposed to different concentrations of the photoinitiator were compared to each other and to the controls (non-stimulated cells). It was assumed that the metabolic activity of the control not exposed to photoinitiators is 100%. A statistical evaluation of data was performed using software packages IBM SPSS Statistic 22 (SPSS Inc., Chicago, IL) and Excel 2013 (Microsoft Office). Results are presented as the average of repeated measurements ± standard deviation (SD). After verifying the normal distribution and homogeneity of variance, a one-way analysis of variance was used to compare means of the samples. If a significant difference was observed (p < 0.05), Bonferroni post hoc multiple comparison tests were performed to detect a significant difference between the samples.
Evaluation of 2PI transmembrane migration
MC3T3 cells were cultured in μ-dishes (35 mm diameter with glass bottom, high version, Ibidi GmbH, Martinsried, Germany) until the glass bottom was covered with a non-confluent monolayer of cells and then exposed to either E2CK or HAPI (as 0.1 mM solutions in PBS). After 5 min of incubation time, the autofluorescence of the 2PIs was visualized by laser scanning microscopy (Zeiss Axio Observer Z1 with an LSM 700 unit including an objective Zeiss EC Plan Neofluar 20×/0.5, ZEN11 software for evaluation) using the 488 nm laser for excitation.Two-photon-polymerization (2PP) printing and assay of encapsulated cells
The details of the 2PP microfabrication setup were reported previously.5 For the present work a femtosecond laser oscillator (MaiTai DeepSee by Spectra Physics) delivering 70 fs pulses at around 800 nm was used. The beam was focused into the sample with a water-immersion microscopy objective (32×/0.85).Methacrylamide-modified gelatin (Gel-MOD) with a degree of substitution of 72% used in this experiment was prepared in accordance with a previously reported protocol.26 Structures were written in a solution of 15% Gel-MOD in αMEM containing 1 mM HAPI, 10 mM MBCD and a cell density of 10 million cells per 1 mL. The cell containing hydrogel precursor solution was applied to μ-dishes (35 mm diameter with a glass bottom, high version, Ibidi GmbH, Martinsried, Germany) where the glass slide had been functionalized with methacrylate groups by cleaning and activating with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of conc. H2SO4 and H2O2 (30% in water), and then using 3-(trimethoxysilyl)propyl methacrylate (Sigma Aldrich) according to the literature.27 The yin–yang structures were 2PP-fabricated starting directly on the glass surface to ensure proper adhesion via covalent bonding between the methacrylated glass surface and the crosslinked hydrogel, operating the 2PP system at the following parameters: laser power after objective 60 mW, scanning speed 100 mm s−1, hatch 0.5 μm, layer spacing 0.5 μm. The excess hydrogel precursor solution was removed after 2PP structuring by replacing the supernatant solution above the structures three times with fresh αMEM, and incubating for 1 h at 37 °C in-between exchanges. The cells were stained by calcein-AM and propidium iodide (Life Technologies) as previously described,28 24 h and 5 days after 2PP structuring. Hydrogel constructs and encapsulated cells were visualized by laser scanning microscopy using the 488 nm and 555 nm lasers for excitation (Zeiss LSM 700 and ZEN11 software for evaluation).
1 mixture of conc. H2SO4 and H2O2 (30% in water), and then using 3-(trimethoxysilyl)propyl methacrylate (Sigma Aldrich) according to the literature.27 The yin–yang structures were 2PP-fabricated starting directly on the glass surface to ensure proper adhesion via covalent bonding between the methacrylated glass surface and the crosslinked hydrogel, operating the 2PP system at the following parameters: laser power after objective 60 mW, scanning speed 100 mm s−1, hatch 0.5 μm, layer spacing 0.5 μm. The excess hydrogel precursor solution was removed after 2PP structuring by replacing the supernatant solution above the structures three times with fresh αMEM, and incubating for 1 h at 37 °C in-between exchanges. The cells were stained by calcein-AM and propidium iodide (Life Technologies) as previously described,28 24 h and 5 days after 2PP structuring. Hydrogel constructs and encapsulated cells were visualized by laser scanning microscopy using the 488 nm and 555 nm lasers for excitation (Zeiss LSM 700 and ZEN11 software for evaluation).
Results and discussion
Synthesis and characterization
Classical aldol condensation reactions under alkaline catalysis are a powerful and cost-efficient tool to build 2PI chromophore systems.16 Since primary amines (necessary for coupling to HA) are reactive towards aldehydes and ketones, the prevention of unwanted side-reactions during storage or the aldol-condensation reaction requires the use of a protective group. Thus (4-oxocyclohexyl)carbamic acid tert-butyl ester (Boc-CNK) was used as a starting material, and condensed with 4-(dimethylamino)benzaldehyde. This forms a dibenzylidene bischalcone of the quadrupolar D–π-A–π-D structure with dimethylamino-groups as strong electronic donors (D) and the carbonyl group as the acceptor (A), promoting a high 2PA cross section.16 The reactants are heated in ethanol in the presence of potassium hydroxide, leading to the precipitation of the protected 2PI Boc-MCNK with a yield of 70%.The deprotection was performed under mild conditions29 by stirring a suspension of Boc-MCNK in DCM vigorously with aqueous phosphoric acid and afforded the deprotected 2PI component MCNK with a yield of 95%.
First coupling attempts between HA and MCNK achieved only low degrees of substitution (DS) of about 5% (determined from 1H-NMR measurements by comparing the integrals of the aromatic protons of MCNK with the N-acetyl protons of HA), presumably because of the steric hindrance of the amino group on the cyclohexanone ring. Thus MCNK was amidated via a CDI based method30 by first reacting 4-(tert-butoxycarbonylamino)butyric acid (Boc-GABA-OH) with CDI to generate an activated carboxyl derivative, then adding freebase MCNK to form an amide bond and obtain Boc-MGABA (79% yield). Subsequent deprotection by aqueous phosphoric acid29 afforded the 2PI component MGABA (94% yield), providing a sterically less hindered amino group for attachment to HA.
For the final coupling step14,18–20 to generate the polymeric hyaluronan-based photoinitiator (HAPI), commercial sodium hyaluronate with an average molar weight of 1.6 MDa was first converted to DMSO soluble tetrabutylammonium hyaluronate (TBA-HA) via acidification with an ion exchanger resin and subsequent neutralization with TBA-OH solution. Then a part of the carboxylate groups (the residual unsubstituted groups ensuring water solubility) was activated via reaction with CDI, and subsequently MGABA was added to form the polymeric 2PI HAPI. The reaction was quenched with excess sodium chloride solution and HAPI was precipitated and washed with acetone to remove unreacted MGABA. Removal of the low molecular weight HA as well as cytotoxic TBA-cations by dialysis and subsequent freeze-drying afforded ready to use HAPI. A schematic representation of the whole reaction sequence is depicted in Fig. 2.
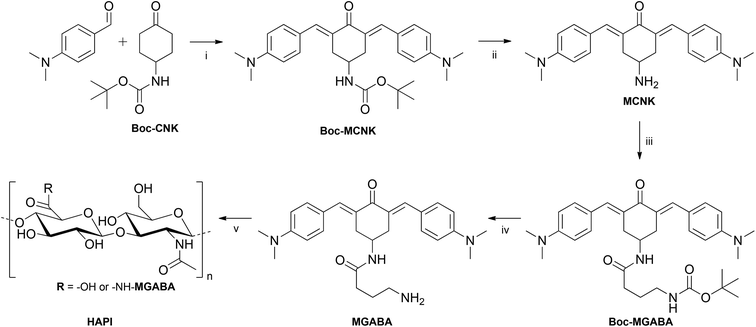 | ||
| Fig. 2 Synthetic pathway to HAPI. Conditions (i) KOH, EtOH; (ii) aq. H3PO4, DCM; (iii) CDI, Boc-GABA-OH, DCM, (iv) aq. H3PO4, DCM; (v) TBA-hyaluronan, CDI, MeSO3OH, DMSO. | ||
Preliminary coupling experiments between HA and MGABA achieved DS up to 10%, justifying the choice of the sterically more accessible amino group in comparison to MCNK. While HAPI prepared in this fashion was sufficiently water-soluble as a sodium salt, it could not be processed in 2PP structuring since a precipitation from the solution upon the addition of crosslinkable macromers like a modified gelatin (GelMod)5,26 occurred. As the first approach to improve the compatibility of HAPI with the macromers, the HAPI solubility was increased by using HA with a decreased molecular weight. While high molecular weight HA maintains homeostasis and potentially down-regulates inflammation, the generation of low molecular weight HA may act as an endogenous signal – likely mediated by cell surface receptors such as CD44 and TLR-4.31–33 Studies have demonstrated that low and very low molecular weight degradation products of HA may elicit various pro-inflammatory responses, such as a marked difference between 20 kDa and 50 kDa HA on the upregulation of TNF-α expression in keratinocytes,34 or macrophages that undergo phenotypic changes dependent on the molecular weight of HA that correspond to either the (1) pro-inflammatory response for very low molecular weight (digest and 5 kDa) HA or (2) pro-resolving response for high molecular weight (800 and 3000 kDa) HA.33 Thus while the HAPI solubility is expected to be the highest for smaller HA fragments, a compromise had to be made in degradation to avoid the generation of higher amounts of HA with molecular weights below 20–50 kDa.
Acidic degradation of commercial 1.6 MDa sodium salt in solution at pH 1 and 60 °C for 24 h, subsequent neutralization with TBA-OH and dialysis against deionized water to remove small HA fragments and excess TBA-Cl, afforded the TBA-salt of HA with a molecular weight of 50 kDa, which was converted to HAPI in the same fashion as the high molecular weight one.
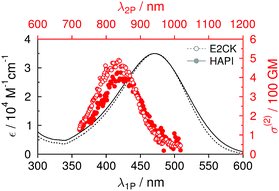
Photophysics
The one-photon absorption (1PA) spectrum of HAPI resembles both the spectral bandshape (maximum around 470 nm) and the maximum extinction coefficient (approx. 35 × 103 M−1 cm−1) of the reference 2PI (E2CK) almost to perfection (see Table 1 and Fig. 3). The lowest energy absorption band exhibits a small shoulder at higher energies (<400 nm), which, in analogy to previous findings,16,17 may be attributed to a one-photon forbidden (two-photon allowed) transition that has its origin in the excitonic interaction of the two branches of the D–π-A–π-D system.35 The fluorescence of E2CK and HAPI peaks around 650 nm, with fluorescence quantum yields in PBS being very low (around 0.2%). The associated fluorescence lifetimes are significantly below the time-resolution of our set-up (<100 ps). Whether this low emission efficiency and fast deactivation of the singlet state has its origin in an ultrafast internal conversion channel back to the ground state or an enhanced intersystem crossing channel to the triplet state is currently being investigated using ultrafast transient absorption spectroscopy.| λ abs [nm] | ε max [M−1 cm−1] | λ 2P [nm] | σ (2) [GM] | λ em [nm] | ϕ [10−3] | |
|---|---|---|---|---|---|---|
| E2CK | 471 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
830 | 480 | ∼650 | 2.5 |
| HAPI | 466 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
840 | 400 | ∼650 | 1.8 |
Two-photon (2P) induced fluorescence was used to record the corresponding two-photon excitation spectra and cross sections. Again, HAPI shows almost identical behavior to E2CK, with the 2PA maximum being around 830–840 nm and maximum 2PA cross-section values in the range of approx. 400–500 GM (see Table 1 and Fig. 3). The slightly lower maximum 2PA cross section of HAPI compared to E2CK could be the result of slightly different degrees of planarity of the 2PI chromophores (different substituents on the amino-donor groups and on the central cyclohexanone ring) as has been previously reported for similar derivatives.16 However, it is worth noting that this observed difference is also in the same order of magnitude as the potential error related to the very low fluorescence quantum yields as well as the error related to the NMR-determination of HAPI's DS, both of which affect the calculated 2P cross sections. The fact that the strong 2P transition shows up at lower wavelengths is in line with the above mentioned excitonic interaction of the two branches of the D–π-A–π-D system and has been extensively described36,37 and observed in the literature for structurally similar systems.17
2PI transmembrane migration
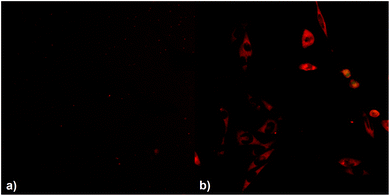
To investigate the effect of the covalent linking of the 2PI to HA on transmembrane migration from the ECM to the cytosol, MC3T3 cells were exposed to solutions of either HAPI or E2CK. The distribution of the 2PIs was then visualized by laser scanning microscopy (LSM) imaging, taking advantage of the autofluorescence of the 2PI chromophores. HAPI does not readily enter the cells and exhibits a weak fluorescence (Fig. 4(a), brightness digitally enhanced) in the surrounding medium, the cells themselves appearing comparatively dark against this background fluorescence. In the case of E2CK the picture is reversed (see Fig. 4(b)), with the cells readily taking up the 2PI and various structures inside the cells appearing brightly stained. This provides a strong indication that the transmembrane migration of the 2PI is effectively hindered by covalent linkage to HA.Cytotoxicity assay
During the 2PP sample preparation and printing process, the cells come into prolonged contact with the material containing dissolved 2PI, even if they are destined to be trapped in the cavities of a structure and are thus not directly exposed to laser radiation and laser-induced radicals. Thus besides phototoxicity, the cytocompatibility of the 2PIs without excitation by 2PA is also of interest. MC3T3 cells were exposed to the αMEM cell culture medium containing either HAPI or the water-soluble 2PI reference E2CK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 at various concentrations. After an exposure time of 5 hours, a representative time frame for the 2PP manufacturing even of relatively large structures, the metabolic activity of cells was evaluated with a PrestoBlue assay. While a comparison of HAPI with a control sample incubated under the same conditions but without 2PI showed no statistically significant difference, in the concentration range from 0.5 mM–2 mM the metabolic activity for E2CK was decreased versus the control by 40 ± 9% to 49 ± 9%, indicating a significant cytotoxicity of the reference compound but not HAPI (see Fig. 5).
6 at various concentrations. After an exposure time of 5 hours, a representative time frame for the 2PP manufacturing even of relatively large structures, the metabolic activity of cells was evaluated with a PrestoBlue assay. While a comparison of HAPI with a control sample incubated under the same conditions but without 2PI showed no statistically significant difference, in the concentration range from 0.5 mM–2 mM the metabolic activity for E2CK was decreased versus the control by 40 ± 9% to 49 ± 9%, indicating a significant cytotoxicity of the reference compound but not HAPI (see Fig. 5).
2PP encapsulation of cells
To examine the functionality of the HAPI in its dedicated application of living cell encapsulation, 3D polymeric hydrogel structures were printed via 2PP in a cell suspension containing dissolved HAPI and GelMod.While high molecular weight (1.6 MDa HA) HAPI was soluble in αMEM cell culture medium at the desired concentrations, upon mixing with GelMod solutions a phase separation occurred and HAPI formed a dense, deeply red gel-like precipitate, depriving the supernatant solution of the photoinitiator. An increase in the solubility was first attempted by using degraded lower molecular weight HA (50 kDa) as the starting material. This measure increased the compatibility significantly, but proved insufficient, so further stabilization of the solvation of the HAPI chains was attempted by adding methyl-β-cyclodextrin (MBCD), which is expected to form inclusion complexes with the apolar parts of the bound MGABA, thus weakening interactions like π–π stacking of the photoinitiator component. At a concentration of 10 mM MBCD was able to stabilize mixtures containing 15% GelMod and 1 mM low molecular weight HAPI so that 2PP structuring was successful. It should be noted that the literature38 suggests the cytotoxicity of MBCD at the required concentrations and our preliminary tests showed a greater reduction of the metabolic activity by 10 mM MBCD alone than by 2 mM E2CK. However in the case of actual 2PP encapsulation, cell survival was excellent (see Fig. 6) likely because MBCD predominantly binds to the large amount of GelMod present in the hydrogel precursor formulations, and thus does not interfere with the cells.
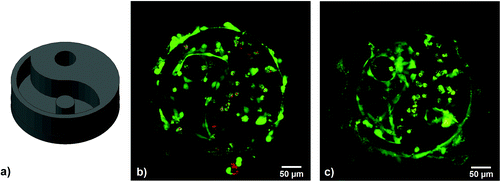
For 2PP encapsulation of live cells, a 3D yin–yang structure was written into a suspension of MC3T3 cells in αMEM cell culture medium containing 15% GelMod, 1 mM HAPI and 10 mM MBCD. The yin–yang structure (see Fig. 6) allows for an easy side-by-side comparison of cells that have been exposed to the laser and are encapsulated in the hydrogel and cells that have not been exposed but are trapped in the medium-filled cavity of the structure.5
Cell viability was assayed 24 h and 5 days after printing by live/dead-staining and LSM imaging, showing live cells in green and dead cells in red. The hydrogel itself also appears stained red due to the autofluorescence of residual 2PI. Throughout the examined period, cell survival was excellent, both in the polymerized parts and the cavities of the structure. After 5 days, the cells in the cavity had a stretched morphology and proliferated, while encapsulated cells were still alive but showed a round morphology, possibly due to physical confinement within the surrounding GelMod matrix (see Fig. 6).
Conclusions
The two-photon initiator precursor MGABA was developed, containing a donor–π-acceptor–π-donor structure motif for efficient two-photon absorption and a sterically accessible primary amino group to allow for high degrees of substitution in subsequent modification reactions on hyaluronan. A novel hyaluronan-based polymeric two-photon initiator (HAPI) was prepared and characterized.Laser scanning microscopy of cells incubated with either HAPI or the reference small molecule 2PI E2CK indicated that the macromolecular nature of HAPI indeed hinders 2PI transmembrane migration effectively.
The assay of cytotoxicity independent of two-photon excitation proved a superior biocompatibility of HAPI compared to the reference water-soluble two-photon initiator E2CK. 3D hydrogel structures containing living cells were successfully produced by the 2PP crosslinking of GelMod with HAPI in the presence of methyl-β-cyclodextrin as an additive to stabilize the hydrogel precursor solutions. The samples were followed up for at least 5 days using a live/dead-staining assay confirming the viability of the cells over this period. These results indicate the low phototoxicity and high efficiency of HAPI, as evidenced by a high scanning speed (100 mm s−1) during the 2PP process. While optimization of the solubility behavior and further investigation of the relationship between the structure of HAPI and two-photon initiation activity are desirable, the system shows excellent biocompatibility and is a promising basis for further developments of the encapsulation of live cells by two-photon induced polymerization.
Acknowledgements
The authors acknowledge the financial support by the European Research Council (Starting Grant-307701, A. O.), the Austrian Science Fund (FWF, Project P27555) and the Université de Genève. We would also like to acknowledge the support of Prof. Dr Sandra Van Vlierberghe and Prof. Dr Peter Dubruel (Polymer Chemistry and Biomaterials Group, Ghent University, Belgium) for providing methacrylated gelatin (GelMod), and Prof. Dr Oscar Hoffmann (Department of Pharmacology and Toxicology, University of Vienna, Austria) for providing the cells for biological experiments.References
- M. Rumi, S. Barlow, J. Wang, J. W. Perry and S. R. Marder, Two-photon absorbing materials and two-photon-induced chemistry, Adv. Polym. Sci., 2008, 213(Photoresponsive Polymers I), 1–95 CAS
.
- A. Ovsianikov, V. Mironov, J. Stampf and R. Liska, Engineering 3D cell-culture matrices: multiphoton processing technologies for biological and tissue engineering applications, Expert Rev. Med. Devices, 2012, 9(6), 613–633 CrossRef CAS PubMed
.
- M. Bokhari, R. J. Carnachan, N. R. Cameron and S. A. Przyborski, Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge, J. Anat., 2007, 211(4), 567–576 CAS
.
- E. Cukierman, R. Pankov, D. R. Stevens and K. M. Yamada, Taking cell-matrix adhesions to the third dimension, Science, 2001, 294(5547), 1708–1712 CrossRef CAS PubMed
.
- A. Ovsianikov, S. Mühleder, J. Torgersen, Z. Li, X.-H. Qin, S. Van Vlierberghe, P. Dubruel, W. Holnthoner, H. Redl, R. Liska and J. Stampfl, Laser Photofabrication of Cell-Containing Hydrogel Constructs, Langmuir, 2014, 30(13), 3787–3794 CrossRef CAS PubMed
.
- Z. Li, J. Torgersen, A. Ajami, S. Muehleder, X. Qin, W. Husinsky, W. Holnthoner, A. Ovsianikov, J. Stampfl and R. Liska, Initiation efficiency and cytotoxicity of novel water-soluble two-photon photoinitiators for direct 3D microfabrication of hydrogels, RSC Adv., 2013, 3(36), 15939–15946 RSC
.
- D. Decuyper-Debergh, J. Piette, C. Laurent and A. Van de Vorst, Cytotoxic and genotoxic effects of extracellular generated singlet oxygen in human lymphocytes in vitro, Mutat. Res. Lett., 1989, 225(1-2), 11–14 CrossRef CAS
.
- J. G. Parker, Optical monitoring of the generation of singlet oxygen during photodynamic treatment of tumors, Johns Hopkins APL Tech. Dig., 1990, 11(1–2), 185–190 CAS
.
- D. Vigetti, E. Karousou, M. Viola, S. Deleonibus, G. De Luca and A. Passi, Hyaluronan: Biosynthesis and signaling, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840(8), 2452–2459 CrossRef CAS PubMed
.
- J. A. Burdick and G. D. Prestwich, Hyaluronic Acid Hydrogels for Biomedical Applications, Adv. Mater., 2011, 23(12), H41–H56 CrossRef CAS PubMed
.
- M. N. Collins and C. Birkinshaw, Hyaluronic acid based scaffolds for tissue engineering—A review, Carbohydr. Polym., 2013, 92(2), 1262–1279 CrossRef CAS PubMed
.
- C. B. Highley, G. D. Prestwich and J. A. Burdick, Recent advances in hyaluronic acid hydrogels for biomedical applications, Curr. Opin. Biotechnol., 2016, 40, 35–40 CrossRef CAS PubMed
.
- C. E. Schante, G. Zuber, C. Herlin and T. F. Vandamme, Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications, Carbohydr. Polym., 2011, 85(3), 469–489 CrossRef CAS
.
-
D. Bellini and A. Topai, Preparation and use of hyaluronic acid amides or other derivatives, Fidia Advanced Biopolymers S.r.l., Italy, 2000, p. 36 Search PubMed
.
- J. N. Mehrishi and J. Bauer, Electrophoresis of cells and the biological relevance of surface charge, Electrophoresis, 2002, 23(13), 1984–1994 CrossRef CAS PubMed
.
- Z. Li, N. Pucher, K. Cicha, J. Torgersen, S. C. Ligon, A. Ajami, W. Husinsky, A. Rosspeintner, E. Vauthey, S. Naumov, T. Scherzer, J. Stampfl and R. Liska, A Straightforward Synthesis and Structure–Activity Relationship of Highly Efficient Initiators for Two-Photon Polymerization, Macromolecules, 2013, 46(2), 352–361 CrossRef CAS
.
- Q. Zou, Y. Zhao, N. S. Makarov, J. Campo, H. Yuan, D.-C. Fang, J. W. Perry and F. Wu, Effect of alicyclic ring size on the photophysical and photochemical properties of bis(arylidene)cycloalkanone compounds, Phys. Chem. Chem. Phys., 2012, 14(33), 11743–11752 RSC
.
- A. Borzacchiello, L. Mayol, A. Schiavinato and L. Ambrosio, Effect of hyaluronic acid amide derivative on equine synovial fluid viscoelasticity, J. Biomed. Mater. Res., Part A, 2010, 92A(3), 1162–1170 CAS
.
- M. D'Este, M. Alini and D. Eglin, Single step synthesis and characterization of thermoresponsive hyaluronan hydrogels, Carbohydr. Polym., 2012, 90(3), 1378–1385 CrossRef PubMed
.
- M. Pavan, D. Galesso, G. Menon, D. Renier and C. Guarise, Hyaluronan derivatives: Alkyl chain length boosts viscoelastic behavior to depolymerization, Carbohydr. Polym., 2013, 97(2), 321–326 CrossRef CAS PubMed
.
- J. A. Gardecki and M. Maroncelli, Set of Secondary Emission Standards for Calibration of the Spectral Responsivity in Emission Spectroscopy, Appl. Spectrosc., 1998, 52(9), 1179–1189 CrossRef CAS
.
- P.-A. Muller, C. Högemann, X. Allonas, P. Jacques and E. Vauthey, Deuterium isotope effect on the charge recombination dynamics of contact ion pairs formed by electron-transfer quenching in acetonitrile, Chem. Phys. Lett., 2000, 326(3–4), 321–327 CrossRef CAS
.
- N. S. Makarov, M. Drobizhev and A. Rebane, Two-photon absorption standards in the 550-1600 nm excitation wavelength range, Opt. Express, 2008, 16(6), 4029–4047 CrossRef CAS PubMed
.
- H. Ceymann, A. Rosspeintner, M. H. Schreck, C. Mutzel, A. Stoy, E. Vauthey and C. Lambert, Cooperative enhancement versus additivity of two-photon-absorption cross sections in linear and branched squaraine superchromophores, Phys. Chem. Chem. Phys., 2016, 18(24), 16404–16413 RSC
.
- S. de Reguardati, J. Pahapill, A. Mikhailov, Y. Stepanenko and A. Rebane, High-accuracy reference standards for two-photon absorption in the 680-1050 nm wavelength range, Opt. Express, 2016, 24(8), 9053–9066 CrossRef PubMed
.
- A. Ovsianikov, A. Deiwick, S. Van Vlierberghe, P. Dubruel, L. Möller, G. Dräger and B. Chichkov, Laser Fabrication of Three-Dimensional CAD Scaffolds from Photosensitive Gelatin for Applications in Tissue Engineering, Biomacromolecules, 2011, 12(4), 851–858 CrossRef CAS PubMed
.
- H. Garoff and W. Ansorge, Improvements of DNA sequencing gels, Anal. Biochem., 1981, 115(2), 450–457 CrossRef CAS PubMed
.
- M. Markovic, J. Van Hoorick, K. Hölzl, M. Tromayer, P. Gruber, S. Nürnberger, P. Dubruel, S. Van Vlierberghe, R. Liska and A. Ovsianikov, Hybrid Tissue Engineering Scaffolds by Combination of Three-Dimensional Printing and Cell Photoencapsulation, J. Nanotechnol. Eng. Med., 2015, 6(2), 021001–021001 CrossRef
.
- B. Li, M. Berliner, R. Buzon, C. K. F. Chiu, S. T. Colgan, T. Kaneko, N. Keene, W. Kissel, T. Le, K. R. Leeman, B. Marquez, R. Morris, L. Newell, S. Wunderwald, M. Witt, J. Weaver, Z. Zhang and Z. Zhang, Aqueous phosphoric acid as a mild reagent for deprotection of tert-butyl carbamates, esters, and ethers, J. Org. Chem., 2006, 71(24), 9045–9050 CrossRef CAS PubMed
.
- I. Smukste and D. B. Smithrud, Structure–Function Relationship of Amino Acid-[2]Rotaxanes, J. Org. Chem., 2003, 68(7), 2547–2558 CrossRef CAS PubMed
.
-
M. Farwick, P. Lersch and G. Strutz, Low Molecular Weight Hyaluronic Acid: Its Effects on Epidermal Gene Expression & Skin Ageing, Verlag für chemische Industrie, Augsburg, Germany, 2008, vol. 134 Search PubMed
.
- G. M. Campo, A. Avenoso, G. Nastasi, A. Micali, V. Prestipino, M. Vaccaro, A. D'Ascola, A. Calatroni and S. Campo, Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression, Biochim. Biophys. Acta, Mol. Basis Dis., 2011, 1812(9), 1170–1181 CrossRef CAS PubMed
.
- J. E. Rayahin, J. S. Buhrman, Y. Zhang, T. J. Koh and R. A. Gemeinhart, High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation, ACS Biomater. Sci. Eng., 2015, 1(7), 481–493 CrossRef CAS PubMed
.
- M. Farwick, G. Gauglitz, T. Pavicic, T. Köhler, M. Wegmann, K. Schwach-Abdellaoui, B. Malle, V. Tarabin, G. Schmitz and H. C. Korting, Fifty-kDa Hyaluronic Acid Upregulates Some Epidermal Genes without Changing TNF-α Expression in Reconstituted Epidermis, Skin Pharmacol. Physiol., 2011, 24(4), 210–217 CrossRef CAS PubMed
.
- C. Katan, S. Tretiak, M. H. V. Werts, A. J. Bain, R. J. Marsh, N. Leonczek, N. Nicolaou, E. Badaeva, O. Mongin and M. Blanchard-Desce, Two-Photon Transitions in Quadrupolar and Branched Chromophores: Experiment and Theory, J. Phys. Chem. B, 2007, 111(32), 9468–9483 CrossRef CAS PubMed
.
-
C. Katan, F. Terenziani, C. Le Droumaguet, O. Mongin, M. H. V. Werts, S. Tretiak and M. Blanchard-Desce, Branching of dipolar chromophores: effects on linear and nonlinear optical properties, 2005 Search PubMed
.
- F. Terenziani, A. Painelli, C. Katan, M. Charlot and M. Blanchard-Desce, Charge Instability in Quadrupolar Chromophores: Symmetry Breaking and Solvatochromism, J. Am. Chem. Soc., 2006, 128(49), 15742–15755 CrossRef CAS PubMed
.
- Y.-A. Choi, B. R. Chin, D. H. Rhee, H.-G. Choi, H.-W. Chang, J.-H. Kim and S.-H. Baek, Methyl-[beta]-cyclodextrin inhibits cell growth and cell cycle arrest via a prostaglandin E(2) independent pathway, Exp. Mol. Med., 2004, 36, 78–84 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6py01787h |
| This journal is © The Royal Society of Chemistry 2017 |