Rhodium(iii)-catalyzed and MeOH-involved regioselective mono-alkenylation of N-arylureas with acrylates†
Abstract
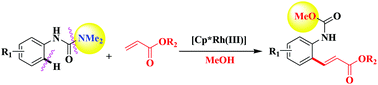
We herein disclose, for the first time, Rh(III)-catalyzed and MeOH-involved regioselective mono-alkenylation of arenes with acrylates using NHCONMe2 as the transformable directing group, giving direct access to diverse ortho-acrylated N-phenyl carbamates. The synthetic application of the obtained products to build privileged quinolin-2(1H)-ones and 3-(2-aminophenyl)acrylates has also been demonstrated in subsequent derivatization reactions.


 Please wait while we load your content...
Please wait while we load your content...