Open Access Article
Open Access ArticleRapid access to N-(indol-2-yl)amides and N-(indol-3-yl)amides as unexplored pharmacophores†
Tristan A.
Reekie‡
a,
Shane M.
Wilkinson‡
a,
Vivian
Law
a,
David E.
Hibbs
 b,
Jennifer A.
Ong
b and
Michael
Kassiou
b,
Jennifer A.
Ong
b and
Michael
Kassiou
 *a
*a
aSchool of Chemistry, University of Sydney, NSW 2006, Australia. E-mail: michael.kassiou@sydney.edu.au
bFaculty of Pharmacy, University of Sydney, NSW 2006, Australia
First published on 14th December 2016
Abstract
Preparation of N-(indol-2-yl)amides and N-(indol-3-yl)amides are scarce in the scientific literature due to unstable intermediates impeding current reported syntheses. We have employed cheap and readily available substrates in the Curtius rearrangement of indole-3-carboxazide to afford N-(indol-3-yl)amides. The reaction is observed for alkyl and aryl carboxylic acids and both N-substituted or 1H-indole derivatives are tolerated. This approach was extended to the preparation of N-(indol-2-yl)amides from the corresponding indole-2-carboxazides.
Introduction
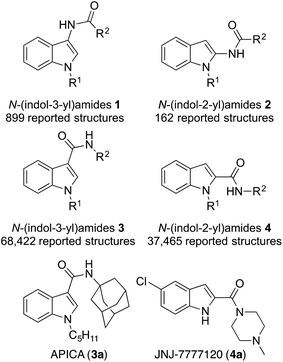
Despite what looks like a simple molecular construct, N-(indol-3-yl)amides 1 and N-(indol-2-yl)amides 2 are difficult motifs to prepare. Low yields are often reported for their preparation and only a limited number of structures are known to date. In the literature, only 162 molecules bearing the N-(indol-2-yl)amide 2 motif are known to exist and have been reported across 64 articles.1 Similarly, only 899 molecules bearing the N-(indol-3-yl)amide 1 motif have been reported across a total of 84 articles.1 In contrast, 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 and 68
465 and 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 reported structures are present in the literature for the reversed indole-3-carboxamide 3 and indole-4-carboxamide 4 motifs in 1225 and 3991 articles respectively. Additionally, compounds of type 3 and 4 have found many applications as pharmacophores. Examples include the multitude of cannabinoid receptor agonists, with APICA (3a) being the lead example,2 and JNJ-7777120 (4a) a selective antagonist at the histamine H4 receptor (Fig. 1).3 The rarity in literature and pharmacological applications of 1 and 2 is a consequence of lacking methodology to access these motifs. With the indole scaffold already being an important structure in drug discovery, improving the accessibility of structure classes 1 and 2 would open the door to new functionalization and could see these moieties incorporated into drug design strategies.
422 reported structures are present in the literature for the reversed indole-3-carboxamide 3 and indole-4-carboxamide 4 motifs in 1225 and 3991 articles respectively. Additionally, compounds of type 3 and 4 have found many applications as pharmacophores. Examples include the multitude of cannabinoid receptor agonists, with APICA (3a) being the lead example,2 and JNJ-7777120 (4a) a selective antagonist at the histamine H4 receptor (Fig. 1).3 The rarity in literature and pharmacological applications of 1 and 2 is a consequence of lacking methodology to access these motifs. With the indole scaffold already being an important structure in drug discovery, improving the accessibility of structure classes 1 and 2 would open the door to new functionalization and could see these moieties incorporated into drug design strategies.
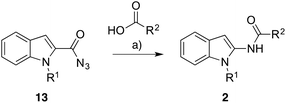
While only a few methods for the synthesis of N-(indolyl)amides 1 and 2 are known,4 the most commonly reported preparation is via an acylation 3- or 2-aminoindole respectively.5,6 However, we and others7 have found 2- and 3-aminoindoles and their precursors, 2-nitroindole and 3-nitroindole, to be unstable and poor yielding. The limited commercial availability of 2- or 3-aminoindole, and 2- or 3-nitroindole infer their apparent instability and futile synthesis. Common drawbacks to known alternative methods include a narrow substrate library or the generation of a specific subset of products (e.g. tertiary amides). As a feasible alternative to known methods, we have developed a mild and convenient synthetic route to N-(indolyl)amides 1 and 2 from easily available compounds and report our results herein.
Results and discussion
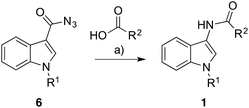
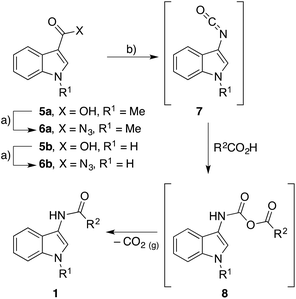
It is well documented that a Curtius rearrangement can produce an amide from a carboxylic acid,8 but to our knowledge, there are no reports of N-(indol-3-yl)amides 1 and N-(indol-2-yl)amides 2 being prepared in this fashion. N-Indol-3-yl ureas7a,9 and urethanes7a,10 have all been prepared via the Curtius rearrangement, as has 3-aminoindole, although its reported instability hindered its isolation and limited its subsequent use.7a We decided to investigate the use and scope of the Curtius rearrangement to conveniently and rapidly prepare N-(indol-3-yl)amides 1 and N-(indol-2-yl)amides 2 from easily accessible substrates.We began our exploration by converting the commercially available N-methyl indole-3-carboxylic acid (5a) into its carboxazide 6a by employing diphenylphosphorylazide (DPPA) with data matching that previously reported.11 With this in hand we investigated the conditions needed to facilitate the one-step amide formation with propionic acid (R2 = Et) (Scheme 1). The carboxazide 6a was heated at reflux in toluene to facilitate isocyanate 7a formation – which was complete after only 10 minutes (by TLC analysis). Propionic acid and triethylamine (0.05 equiv.) were added to the refluxing reaction and, after 2 h, the generation of two main products was observed, both presumably proceeding through intermediate 8a. The first product was identified as N-(1-methyl-indol-3-yl)propionamide 1a (37% yield) which confirmed the direct amide formation from indol-3-yl isocyanate 7a. The second constituent of the reaction was identified as N,N′-bis(1-methyl-indol-3-yl)urea 9. The formation of these bis-urea by-products has been reported for the analogous reaction between phenyl isocyanates and carboxylic acids.8a This component fortuitously precipitates from the reaction media upon cooling but can also be effortlessly resolved from the desired N-(indol-3-yl)amide with flash chromatography.
 | ||
| Scheme 1 Synthesis of N-(indol-3-yl)amides (1). Reaction conditions: (a) Et3N, CH2Cl2, rt, 15 min then DPPA, rt 1 h, 94% (R1 = Me), 97% (R1 = H); (b) refer to Table 1. | ||
With a moderate yield of the desired N-(indol-3-yl)amide 1a from this initial reaction, we explored what reaction conditions could be optimized. A slight improvement in yield (39%) and convenience was achieved by merging the reaction into a one-step reaction by refluxing the indol-3-yl carboxazide 6a with propionic acid and triethylamine. Altering the solvent from toluene to N,N-dimethylformamide saw an improvement in yield (49%) but isolation of the product became cumbersome.
An equal improvement in yield (49%) was observed when the equivalents of propionic acid was raised from two to five equivalents. Alternatively, the yields could be improved by conserving the carboxylic acid equivalents and performing the reactions at increased concentrations. When the reaction was performed at 10 mM (with respect to indol-3-yl carboxazide 6a) and 2 equivalents of propionic acid, a yield of 39% was observed. When the reaction was concentrated to 100 mM, a 56% yield was recorded and finally at 500 mM, a 64% yield was observed.
It has been reported that a proton transfer reagent accelerates the decomposition of mixed anhydrides such as 8.8b,c,12 To this end, the one-step reaction was repeated with an increased triethylamine concentration (from 0.05 to 0.2 equivalents) but no improvement in yield was observed. However, when triethylamine was replaced with 10 mol% of N,N-dimethylaminopyridine (DMAP), the desired N-(indol-3-yl)amide 1a was obtained in 86% yield.
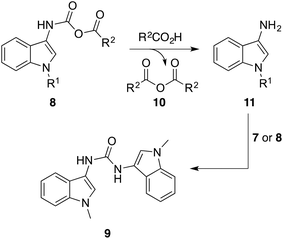
Despite the improved yields, the bis-urea 9 was still an unavoidable by-product in each of these reactions and accounted for the majority of the residual yield in each case. We first proposed it was due to water in the reaction but meticulous drying of all reagents and solvents or the incorporation of drying agents (4 Å molecular sieves) did not improve amide yields or decrease urea formation. We propose that the bis-urea 9 occurs due to the carboxylic acid substrate attacking the mixed anhydride intermediate 8 which results in a homogeneous anhydride of the carboxylic acid 10 and the 3-aminoindole 11 (Scheme 2). This 3-aminoindole 11 subsequently reacts with unreacted indol-3-yl isocyanate 7 or the mixed anhydride intermediate 8 to generate the undesired bis-urea 9. To validate this hypothesis, we reacted the indol-3-yl isocyanate 7a with ethyl magnesium bromide to circumvent the formation of the mixed anhydride intermediate 8 and thus avoid the bis-urea by-product 9. The desired N-(indol-3-yl)amide 1a was formed in 54% yield with no bis-urea product 9 observed. Due to no significant improvement on product yield, the initially explored one-pot, single-step thermal decomposition between the indol-3-yl isocyanate 7a and a carboxylic acid was chosen as the means of synthesizing N-(indol-3-yl)amides 1 given the simplicity and convenience of the method.
The substrate scope of this reaction was explored by substituting propionic acid for a variety of aliphatic and aryl carboxylic acids (Table 1). Steric hindrance appears to play a pivotal role in the amide formation. As steric bulk is increased around the carbon alpha to the carboxylic acid, we observe a reduction in yield when comparing one methyl group (1b 64%)13 to two methyl groups (1c 28%) to three methyl groups (1d 13%). This trend is also observed with phenyl groups (1g 75% vs.1h 53%) and the bulky adamantane group (1i 6% vs.1j 29%).
| Product | R1 | R2 | Yieldb, % |
|---|---|---|---|
| a Conditions: indol-3-yl carboxazide (0.5 M), carboxylic acid (2 equiv.), DMAP (0.1 equiv.), PhMe, reflux, 2 h. b Values in parentheses represent yields obtained when Et3N (0.05 equiv.) was used instead of DMAP (0.1 equiv.). c Trace product observed. | |||
| 1a | Me | Ethyl | 86 (64) |
| 1b | Me | Methyl | 69 (50) |
| 1c | Me | Iso-propyl | 58 (28) |
| 1d | Me | tert-Butyl | 3 (13) |
| 1e | Me | Cyclopentyl | 75 (27) |
| 1f | Me | Cyclohexyl | 43 (23) |
| 1g | Me | Benzyl | 88 (75) |
| 1h | Me | Diphenylmethyl | 62 (53) |
| 1i | Me | Adamantan-1-yl | 6 (6) |
| 1j | Me | Adamantan-1-ylmethyl | 41 (29) |
| 1k | Me | Phenyl | 28 (43) |
| 1l | Me | Pyridin-2-yl | 51 (13) |
| 1m | Me | Pyridin-3-yl | 41 (NRc) |
| 1n | Me | Pyridin-4-yl | NRc (NR) |
| 1o | Me | 4-Methoxyphenyl | 32 (8) |
| 1p | Me | 4-Methoxybenzyl | 90 (64) |
| 1q | Me | 4-Nitrophenyl | NRc(NR) |
| 1r | Me | 4-Nitrobenzyl | 67 (46) |
| 1s | Me | Ethenyl | 40 (23) |
| 1t | Me | Ethynyl | 45 (23) |
| 1u | H | Methyl | 7513 |
| 1v | H | Ethyl | 64 |
| 1w | H | Benzyl | 64 |
| 1x | H | Phenyl | 24 |
| 1y | H | Adamantan-1-yl | 31 |
| 1z | H | Adamantan-1-ylmethyl | 26 |
We were pleased that the rearrangement was also observed for sp2 and sp hybridized carbons with heteroaromatic rings tolerated. Yields were generally lower than the analogous sp3 hybridized substrates (for example benzyl 1g 75% vs. phenyl 1k 43% and benzyl 1p 64% vs. phenyl 1o 8%). Electronic effects also appear to control amide formation with electron-withdrawing substituents (1n, 1q) abolishing amide formation. In these cases, the bis-urea by-product 9 was the major product which further validates the urea-forming mechanism (Scheme 2). It is proposed that the electron-withdrawing substituents on the carboxylic acid activate the carboxy carbonyl of the mixed anhydride intermediate 8 for nucleophilic attack. Acrylic acid and propiolic acid both underwent amide formation (1r and 1s respectively) in similar yields.
To further demonstrate the potential of this procedure, the same reaction was performed on the 1H-indol-3-yl carboxazide 6b with a selection of carboxylic acids to give the corresponding amides (1u–1z). Under identical reaction conditions, reactions yields were generally lower but were still satisfactory given the scarcity of N-(1H-indol-3-yl) amides in the literature. The employment of the 1H-indole to this reaction allows for an N-substitution at a later stage of a synthesis and therefore allowing access to a greater range of N-(indolyl)amides.
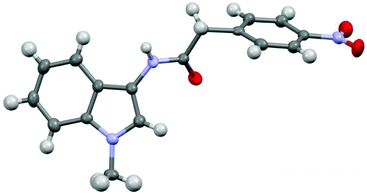
A crystal of the N-(1-methyl-indol-3-yl)-2-(4-nitrophenyl)acetamide (1r) was grown and single crystal X-ray analysis confirmed the reversed amide functionality (Fig. 2). The carbonyl bond in aryl and indole carboxamides typically align parallel to the aryl rings due to extended pi orbital interactions. However, in this case, the reversed amide is disconnected and, in its lowest energy conformation state, can adopt a less sterically-hindered twisted conformation.
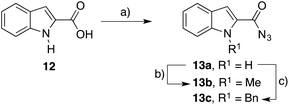
Given the successful preparation of N-(indol-3-yl)amides with the developed methodology, the focus was turned to preparation of N-(indol-2-yl) amides 2. Taking commercially available indole-2-carboxylic acid (12), it was converted to the 1H-carboxazide 13a in 93% yield using DPPA (Scheme 3). To prepare the N-methyl homologue 13b, we found it convenient to alkylate the 1H-carboxazide 13a with NaH and methyl iodide which resulted in the desired N-methyl indole-2-carboxazide (13b) in 64% yield.11
Subjecting the indole-2-carboxazides 13a and 13b to the identical conditions developed for the preparation of N-(indol-3-yl)amides 1 resulted in moderate to excellent yields of the N-(indol-2-yl)amides 2 (Table 2). The reaction yields followed similar trends in substrate reactivity to the indole-3-carboxazide reactions (Table 1) albeit lower yields were observed relative to the respective 3-position counterpart. Nevertheless, the rapid and convenient access to these N-(indol-2-yl)amides via this methodology is a vast improvement on current approaches. An interesting observation is that substitution effects of the acid do not seem to translate between 2- and 3-substitution. For example, the formation of 1p occurred effectively in 90% yield whereas the corresponding formation of 2d was lower with a yield of 50%.
| Product | R1 | R2 | Yieldb, % |
|---|---|---|---|
| a Conditions: indol-2-yl carboxazide (0.5 M), carboxylic acid (2 equiv.), DMAP (0.1 equiv.), PhMe, reflux, 2 h. b Values in parentheses represent yields obtained over two steps for the benzyl protection and rearrangement. | |||
| 2a | Me | Methyl | 52 |
| 2b | Me | Ethyl | 55 |
| 2c | Me | Benzyl | 68 |
| 2d | Me | 4-Methoxybenzyl | 50 |
| 2e | Me | 4-Nitrobenzyl | 71 |
| 2f | H | Methyl | 45 |
| 2g | H | Ethyl | 45 |
| 2h | H | Benzyl | 56 |
| 2i | H | 4-Methoxybenzyl | 91 |
| 2j | H | 4-Nitrobenzyl | 91 |
| 2k | Bn | Methyl | 72 (40) |
| 2l | Bn | Ethyl | 70 (39) |
| 2m | Bn | Benzyl | 66 (36) |
| 2n | Bn | 4-Methoxybenzyl | 62 (34) |
| 2o | Bn | 4-Nitrobenzyl | 59 (32) |
To further illustrate the utility of this reaction we sort to incorporate a protecting group onto the indole prior to rearrangement. Taking the 1H-indole-2-carboxazide 13a the benzyl group was successfully installed (55%), with tetrabutylammonium iodide (TBAI) proving to be essential to effect the transformation. The resulting N-benzyl indole-2-carboxazide 13c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 was subjected to the Curtius rearrangement with a selection of carboxylic acids (Table 2, entries k–o). However, over 2 steps, the yield of the desired 1-benzyl N-(indol-2-yl)amides (2k–2o) were lower in most cases than the corresponding reactions performed with the 1H-indole-2-carboxazide (2f–2j), indicating that, unless otherwise required, it was higher yielding, more economical and more efficient to simply perform the reaction with the 1H-indole. When the methyl (2a–e) or benzyl (2k–o) indole amides were characterized, NMR spectra showed the presence of rotamers. When solubility allowed, these could be suppressed through the use of deuterated methanol as the solvent (see the ESI† for more details).
11 was subjected to the Curtius rearrangement with a selection of carboxylic acids (Table 2, entries k–o). However, over 2 steps, the yield of the desired 1-benzyl N-(indol-2-yl)amides (2k–2o) were lower in most cases than the corresponding reactions performed with the 1H-indole-2-carboxazide (2f–2j), indicating that, unless otherwise required, it was higher yielding, more economical and more efficient to simply perform the reaction with the 1H-indole. When the methyl (2a–e) or benzyl (2k–o) indole amides were characterized, NMR spectra showed the presence of rotamers. When solubility allowed, these could be suppressed through the use of deuterated methanol as the solvent (see the ESI† for more details).
Conclusions
In summary, a new means of accessing N-(indol-3-yl)amides 1 and N-(indol-3-yl)amides 2 has been described. The precursory indolyl carboxazides 6 and 13 can be obtained on a high-yielding, multi-gram scale whilst the coupling carboxylic acids can be conveniently sourced from commercial supplies without prior activation or modification. Moderate yields are obtained across a wide substrate library that tolerate alkanoic, alkenoic, alkynoic and benzoic acids. This reaction is a mild and convenient route to N-(indol-3-yl)amides 1 and N-(indol-2-yl)amides 2 that avoids the isolation of unstable intermediates that impede previously reported methods. This work allows rapid access to a once limited indole motif and, with its wide substrate scope, expands the repertoire of indole-based drug design providing access to new chemical entities with potentially useful biological activity.Acknowledgements
This work was supported by National Health and Medical Research Council (APP1037979). We also thank Ms Caoimhe Clerkin for assistance with chemical synthesis.Notes and references
- American Chemical Society, Scifinder 2016, Accessed on 11th October 2016, Search filters, history and results are reported as supplementary information Search PubMed.
- (a) N. Uchiyama, M. Kawamura, R. Kikura-Hanajiri and Y. Goda, Forensic Toxicol., 2012, 30, 114 CrossRef CAS; (b) S. D. Banister, S. M. Wilkinson, M. Longworth, J. Stuart, N. Apetz, K. English, L. Brooker, C. Goebel, D. E. Hibbs, M. Glass, M. Connor, I. S. McGregor and M. Kassiou, ACS Chem. Neurosci., 2013, 4, 1081 CrossRef CAS PubMed.
- R. L. Thurmond, P. J. Desai, P. J. Dunford, W.-P. Fung-Leung, C. L. Hofstra, W. Jiang, S. Nguyen, J. P. Riley, S. Sun, K. N. Williams, J. P. Edwards and L. Karlsson, J. Pharmacol. Exp. Ther., 2004, 309, 404 CrossRef CAS PubMed.
- (a) Q. Shuai, G. Deng, Z. Chua, D. S. Bohle and C.-J. Li, Adv. Synth. Catal., 2010, 352, 632 CrossRef CAS; (b) F. M. Albini, R. Oberti and P. Caramella, J. Chem. Res., Synop., 1983, 4 CAS; (c) A. Pews-Davtyan and M. Beller, Org. Biomol. Chem., 2011, 9, 6331 RSC; (d) J. K. Augustine, R. Kumar, A. Bombrun and A. B. Mandal, Tetrahedron Lett., 2011, 52, 1074 CrossRef CAS; (e) E. Brachet, T. Ghosh, I. Ghosh and B. Konig, Chem. Sci., 2015, 6, 987 RSC.
- (a) M. Boes, Affectis Pharmaceuticals AG, Germany, WO2010118921A1, 2010, 80pp; (b) S. Roy, S. Roy and G. W. Gribble, Tetrahedron Lett., 2008, 49, 1531 CrossRef CAS.
- J. E. Mangette, X. Chen, R. Krishnamoorthy, A. Samuel Vellekoop, A. J. Csakai, F. Camara, W. D. Paquette, H.-J. Wang, H. Takahashi, R. Fleck and G. P. Roth, Tetrahedron Lett., 2011, 52, 1292 CrossRef CAS.
- (a) N. N. Suvorov, V. S. Velezheva, A. V. Yarosh, Y. V. Erofeev and T. N. Kozik, Chem. Heterocycl. Compd., 1975, 11, 959 CrossRef; (b) S. Roy and G. W. Gribble, Heterocycles, 2006, 70, 51 CrossRef CAS; (c) G. Berti, A. D. Settimo and E. Nannipieri, J. Chem. Soc. C, 1968, 2145 RSC.
- (a) I. S. Blagbrough, N. E. Mackenzie, C. Ortiz and A. I. Scott, Tetrahedron Lett., 1986, 27, 1251 CrossRef CAS; (b) K. Sasaki and D. Crich, Org. Lett., 2011, 13, 2256 CrossRef CAS PubMed; (c) A. C. Schuemacher and R. W. Hoffmann, Synthesis, 2001, 0243 CrossRef CAS.
- (a) J. F. Djung, R. J. Mears, C. A. G. N. Montalbetti, T. S. Coulter, A. Golebiowski, A. N. Carr, O. Barker, K. D. Greis, S. Zhou, E. Dolan and G. F. Davis, Bioorg. Med. Chem., 2011, 19, 2742 CrossRef CAS PubMed; (b) A.-R. A. H. Farghaly, J. Chin. Chem. Soc., 2004, 51, 147 CrossRef.
- (a) G. Xu, L. Zheng, Q. Dang and X. Bai, Synthesis, 2013, 743 CAS; (b) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Synthesis, 2008, 136 CrossRef CAS.
- R. K. Arigela, R. Kumar, T. Joshi, R. Mahar and B. Kundu, RSC Adv., 2014, 4, 57749 RSC.
- G. Höfle, W. Steglich and H. Vorbrüggen, Angew. Chem., Int. Ed. Engl., 1978, 17, 569 CrossRef.
- S. Roy and G. W. Gribble, Heterocycles, 2006, 70, 51 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1508492. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ob02622b |
| ‡ T.A.R and S.M.W contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |