Visible-light controlled catalytic Cu2O–Au micromotors†
Dekai
Zhou‡
ab,
Yuguang C.
Li‡
 a,
Pengtao
Xu
a,
Nicholas S.
McCool
a,
Longqiu
Li
*b,
Wei
Wang
c and
Thomas E.
Mallouk
a,
Pengtao
Xu
a,
Nicholas S.
McCool
a,
Longqiu
Li
*b,
Wei
Wang
c and
Thomas E.
Mallouk
 *a
*a
aDepartments of Chemistry, Biochemistry and Molecular Biology, and Physics, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: tem5@psu.edu
bSchool of Mechatronics Engineering, Harbin Institute of Technology, Harbin 150001, China. E-mail: longqiuli@hit.edu.cn
cSchool of Materials Science and Engineering, Harbin Institute of Technology, Shenzhen Graduate School, Shenzhen, Guangdong 58055, China
First published on 28th November 2016
Abstract
Visible light driven Cu2O–Au micromotors exhibit rapid on/off switching and speed control. Electrochemical measurements confirm that the light-induced movement of the Cu2O–Au micromotors involves a self-electrophoresis mechanism modulated by the photoconductivity of Cu2O. This study extends the utilization of the electromagnetic spectrum for micro/nanomotors into the visible range.
Autonomous micro/nanomotors are objects that can convert energy carried on board or supplied from their environment into mechanical motion. This idea was first demonstrated by Paxton et al., who showed that platinum–gold nanorods could swim at speeds of several microns per second by decomposing hydrogen peroxide in aqueous solutions.1 Since then, many different kinds of synthetic micro/nanomotors have been developed.2–5 Different forms of input energy such as chemical,1,6,7 magnetic,8–12 electrical,13,14 acoustic15–23 and thermal24,25 have been adapted to drive these motors. Gao and coworkers developed the first tubular micro-rockets that produced bubbles through an inner-surface-catalytic reaction to achieve self-propulsion in hydrogen peroxide solutions.26 Wang and coworkers later demonstrated that asymmetrically shaped microrods could be induced to translate, rotate, align and assemble in response to ultrasonic standing waves in the MHz frequency range.23 These fundamental studies have enabled a growing number of interesting motor functions and applications, such as drug or cargo delivery11,16,17,27 and water remediation.28–31 Wang and coworkers showed that metallic nanorods could be powered by resonant ultrasound to exhibit axial propulsion inside living Hela cells.22 This capability was then adapted to the detection of trace biomolecules in cells in vitro.32 Gao and coworkers have also used zinc-based micromotors in an in vivo mouse model to examine the distribution, retention, delivery of cargo, and the acute toxicity profile.33
Among the different propulsion mechanisms, light is one of the most interesting energy sources and has important prospects for applications that involve photo-activated and controlled micro/nanomotors. For example, Mou and Enachi demonstrated light-controlled bubble-propelled micromotors.34,35 Esplandiu and Hong et al., have developed two kinds of light-activated micropump systems with semiconductor materials.36,37 Dong and coworkers demonstrated the efficient propulsion of UV light-driven TiO2–Au Janus micromotors in aqueous solution.38 Xuan and coworkers described fuel-free Janus mesoporous silica nanoparticle motors that were activated by near-infrared (NIR) laser light.39 Palacci et al. realized self-organization of photoactivated colloidal particles.40 Recently, Chen, Li and Dai proposed different methods for controlling the direction of motion of photo-activated micromotors.41–43 For photo-driven micro/nanomotors, there are usually two major advantages: first, the speed of the micro/nanomotors can be controlled by the light intensity; secondly, light-driven micro/nano-motors can achieve rapid on/off control. Most of the photo-driven micro/nanomotors reported to date are activated either by UV or NIR light, which limits their utility, especially in environmental, sensing, and biomedical diagnostic applications. Here we describe a visible light-driven microscale Janus Cu2O–Au micromotor that displays autonomous motion in low-concentration hydrogen peroxide solutions. By controlling the light intensity, the velocity of the Cu2O–Au micromotors can be adjusted on demand, and the motion can be rapidly started or stopped by controlling the light source.
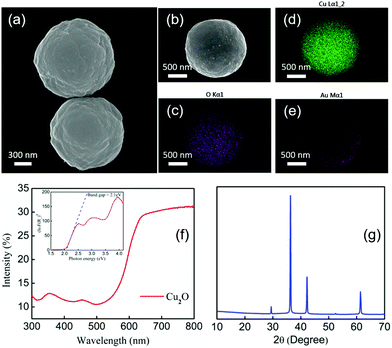
Cu2O microparticles were synthesized by previously reported procedures (ESI†).44Fig. 1(a) shows the morphology of the Cu2O particles, which are roughly spherical in shape with a diameter of about 1.2 μm. A 30 nm thick Au layer was evaporated onto the Cu2O microparticles making half-covered Janus spheres, as shown in the SEM image in Fig. 1(b). Fig. 1(c), (d) and (e) show EDS mapping of the elemental composition of the Cu2O–Au microparticles, which contain copper, gold and oxygen as expected from the synthesis. Copper and oxygen are evenly distributed whereas Au is concentrated on one side only.
Diffuse reflectance UV-visible spectra of the Cu2O particles on glass substrates were measured to confirm their visible light absorption properties. Fig. 1(f) shows that the onset of absorption is at about 580 nm. A band gap of 2.1 eV was calculated from the Kubelka–Munk function by means of a Tauc plot,45 which is shown in the inset of Fig. 2(f). The band gap of the Cu2O particles is consistent with their previously reported semiconducting properties.46,47Fig. 2(g) shows X-ray diffraction patterns of the as-synthesized Cu2O microparticles drop-cast onto a zero-background plate. Cu2O was the major product with only a trace amount (<5%) of CuO detected by Rietveld refinement analysis.
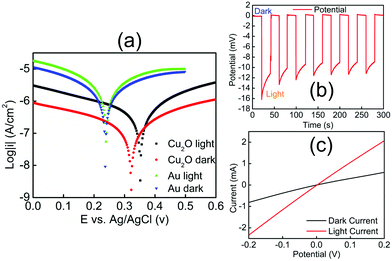
Electrochemical experiments (Fig. 2) provided evidence that Cu2O–Au micromotors are propelled by light-induced self-electrophoresis in H2O2 solutions (ESI†).48 The generation and consumption of protons on the opposite sides of the Cu2O–Au micromotors induces a proton concentration gradient, and the resulting electric field propels them with the Au side leading (see ESI, Video S-1†). When the Cu2O microparticles are irradiated by visible light, the density of mobile electrons and holes increases, resulting in photoconduction and enhancement of the rate of the bipolar electrochemical decomposition of H2O2 at Cu2O and Au. To confirm this mechanism of motility, Cu2O microparticles without the gold coating were tested under the same conditions and only Brownian motion was observed under visible light (Video S-2†). A general consensus is that high salt concentrations decrease the velocity of self-electrophoretic micromotors either by increasing the conductivity of the double layer, or by inhibiting the reaction on the metal surface. Such effects are considered unique to electrophoretically driven particles, and are therefore popularly used to differentiate self-electrophoretis from diffusiophoresis.49,50 In our experiments Cu2O–Au micromotors exhibited only Brownian motion when illuminated in H2O2 solutions in the presence of 5 mM NaNO3 (Video S-3†), which is again consistent with electrophoretic propulsion.
To better understand the mechanism of motility and effect of visible light, we measured the mixed potentials of Au and Cu2O microelectrodes in 2.5 v% H2O2 solutions with and without illumination (ESI†). Fig. 2(a) shows Tafel plots for these electrodes. The mixed potential of the Au electrode is +0.24 V vs. Ag/AgCl in both the light and the dark, consistent with earlier measurements.48 From the Tafel plot, the potential difference between Au and Cu2O is about 80 mV in the dark. When illuminated, the Cu2O electrode shifts in the positive direction by about 30 mV, indicating a small photovoltage generated at the Cu2O–solution interface. Because the potential of the Cu2O electrode is positive relative to Au, electrons flow from Au to Cu2O, and thus Au acts as the anode in the bipolar electrochemical decomposition of H2O2. This is consistent with the observation that Au is the leading end of the Cu2O–Au micromotors. Fig. 2(b) shows the j–V curve of a Cu2O–Au solid junction, where Cu2O was electrodeposited onto a fluorine-doped tin oxide (FTO)-coated glass slide and Au was sputter-deposited onto the Cu2O surface. The resistance of the Cu2O–Au electrode decreased by a factor of about 3.5 under illumination, consistent with the photoconductivity of the insulating Cu2O layer between the conductive FTO and Au layers. Fig. 2(c) shows a light-chopping experiment with the same Cu2O–Au electrode, showing a photovoltage on the order of 10–15 mV. This is consistent with the shift in the mixed potential of Cu2O observed in Fig. 2(a). Based on these data we can conclude that the dramatic increase in motility of Cu2O–Au Janus particles arises from two effects, the photoconductivity of Cu2O and the increase in the mixed potential difference under illumination.
To explore the parameters that control the mobility of the Cu2O–Au micromotors, we examined the effects of H2O2 concentration and light intensity on motor velocity. Fig. 3(a) shows the velocity at different concentrations of H2O2. The speed of the Cu2O–Au micromotors was close to 4 μm s−1 in 0.1 v% H2O2 and increased to 6 μm s−1 at 3 v% H2O2 concentration. As the concentration increased from 0.1 v% to 3 v%, we do not observe a proportional increase in the motor speed, suggesting that the conductivity of the Janus particle is rate-limiting. By changing neutral density (ND) filters, we varied the light intensity between 13.6 mW mm−2 and 5.8 mW mm−2. From Fig. 3(b), we observe a linear relationship between motor speed and light intensity. This is consistent with the conclusion that the photoconductivity of the motors is rate-limiting. Fig. 4(a)–(f) illustrates the rapid on/off motion of the Cu2O–Au micromotors. By tracking Video S-4,† the mean square displacement (MSD) of a Cu2O–Au micromotor was calculated with and without illumination (Fig. S-2†). The ballistic speed of the Au–Cu2O micromotors at short time scales was 6.9 μm s−1 under illumination, while the Brownian rotational time was found to be 2.3 seconds. A rather linear trajectory with a persistence length of ∼16 μm is therefore expected. Without illumination, the MSD is linear indicating Brownian motion.51 The highly repeatable and simple on/off motion control of the Cu2O–Au micromotors under visible light may be useful in analytical applications where switching control is needed.
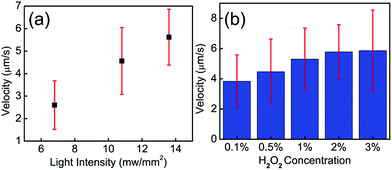 | ||
| Fig. 3 (a) Speed of the Cu2O–Au particles under different H2O2 concentrations. (b) Speed of the Cu2O–Au particles under different light intensities, tested in 2.5 v% H2O2. | ||
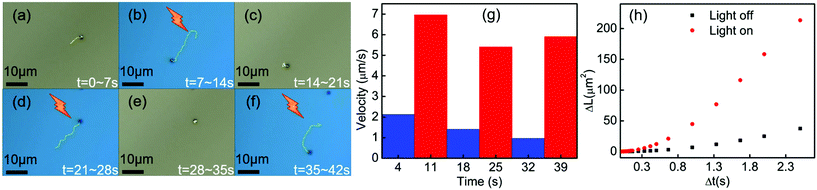 | ||
| Fig. 4 (a)–(f) Time-lapse tracking line images of the Au–Cu2O micromotors in 2.5 v% H2O2 with visible light and in the dark, taken every 7 s at a light intensity of 13.6 mW mm−2. (g) Measured speed of the micromotors from the time lapse images (see Video S-4†). (h) Mean squared displacement of Au–Cu2O micromotors with and without illumination (see Video S-4†), trajectories were showed on (a) and (b). | ||
In conclusion, we have synthesized visible light-activated micromotors that can achieve rapid on/off control and variable speed by modulating the light intensity. The light-activated self-electrophoresis mechanism of the micromotors was confirmed by electrochemical methods. The utilization of visible light could enable the use of micro/nanomotors in analytical and environmental applications, as well as in fundamental studies of the interactions of powered particles.
Acknowledgements
This work was supported by the National Science Foundation under MRSEC grant number DMR- 1420620. W. Wang is grateful for the financial support from National Natural Science Foundation of China (Grant No. 11402069) and government of Shenzhen (Grant No. KQCX20140521144102503). D. Zhou, and L. Li, are grateful for the financial support from Nation Natural Science Foundation of China (51521003 and 51175129) and Program of Introducing Talents of Discipline to Universities (B07018) and Self-Planned Task of State Key Laboratory of Robotics and System (HIT) (SKLRS201607C).Notes and references
- W. F. Paxton, K. C. Kistler, C. C. Olmeda, A. Sen, St. S. K. Angelo, Y. Cao, T. E. Mallouk, P. E. Lammert and V. H. Crespi, J. Am. Chem. Soc., 2004, 126(41), 13424–13431 CrossRef CAS PubMed.
- W. Wang, W. Duan, S. Ahmed, T. E. Mallouk and A. Sen, Nano Today, 2013, 8(5), 531–554 CrossRef CAS.
- W. Wang, W. Duan, S. Ahmed, A. Sen and T. E. Mallouk, Acc. Chem. Res., 2015, 48(7), 1938–1946 CrossRef CAS PubMed.
- K. K. Dey, F. Wong, A. Altemose and A. Sen, Curr. Opin. Colloid Interface Sci., 2016, 21, 4–13 CrossRef CAS.
- W. Gao and J. Wang, ACS Nano, 2014, 8(4), 3170–3180 CrossRef CAS PubMed.
- M. Pumera, Nanoscale, 2010, 2(9), 1643–1649 RSC.
- S. Fournier-Bidoz, A. C. Arsenault, I. Manners and G. A. Ozin, Chem. Commun., 2005,(4), 441–443 RSC.
- R. Dreyfus, J. Baudry, M. L. Roper, M. Fermigier, H. A. Stone and J. Bibette, Nature, 2005, 437(7060), 862–865 CrossRef CAS PubMed.
- A. Ghosh and P. Fischer, Nano Lett., 2009, 9(6), 2243–2245 CrossRef CAS PubMed.
- P. Fischer and A. Ghosh, Nanoscale, 2011, 3(2), 557–563 RSC.
- S. Tottori, L. Zhang, F. Qiu, K. K. Krawczyk, A. Franco-Obregón and B. J. Nelson, Adv. Mater., 2012, 24(6), 811–816 CrossRef CAS PubMed.
- T. Li, J. Li, H. Zhang, X. Chang, W. Song, Y. Hu, G. Shao, E. Sandraz, G. Zhang, L. Li and J. Wang, Small, 2016, 12(44), 6098–6105 CrossRef CAS PubMed.
- P. Calvo-Marzal, S. Sattayasamitsathit, S. Balasubramanian, J. R. Windmiller, C. Dao and J. Wang, Chem. Commun., 2010, 46(10), 1623–1624 RSC.
- S. T. Chang, V. N. Paunov, D. N. Petsev and O. D. Velev, Nat. Mater., 2007, 6(3), 235–240 CrossRef CAS PubMed.
- F. Nadal and E. Lauga, Phys. Fluids, 2014, 26(8), 082001 CrossRef.
- D. Kagan, M. J. Benchimol, J. C. Claussen, E. Chuluun-Erdene, S. Esener and J. Wang, Angew. Chem., Int. Ed., 2012, 51(30), 7519–7522 CrossRef CAS PubMed.
- S. Ahmed, W. Wang, L. O. Mair, R. D. Fraleigh, S. Li, L. A. Castro, M. Hoyos, T. J. Huang and T. E. Mallouk, Langmuir, 2013, 29(52), 16113–16118 CrossRef CAS PubMed.
- V. Garcia-Gradilla, J. Orozco, S. Sattayasamitsathit, F. Soto, F. Kuralay, A. Pourazary, A. Katzenberg, W. Gao, Y. Shen and J. Wang, ACS Nano, 2013, 7(10), 9232–9240 CrossRef CAS PubMed.
- S. Ahmed, D. T. Gentekos, C. A. Fink and T. E. Mallouk, ACS Nano, 2014, 8(11), 11053–11060 CrossRef CAS PubMed.
- A. L. Balk, L. O. Mair, P. P. Mathai, P. N. Patrone, W. Wang, S. Ahmed, T. E. Mallouk, J. A. Liddle and S. M. Stavis, ACS Nano, 2014, 8(8), 8300–8309 CrossRef CAS PubMed.
- V. Garcia-Gradilla, S. Sattayasamitsathit, F. Soto, F. Kuralay, C. Yardımcı, D. Wiitala, M. Galarnyk and J. Wang, Small, 2014, 10(20), 4154–4159 CAS.
- W. Wang, S. Li, L. Mair, S. Ahmed, T. J. Huang and T. E. Mallouk, Angew. Chem., 2014, 126(12), 3265–3268 CrossRef.
- W. Wang, L. A. Castro, M. Hoyos and T. E. Mallouk, ACS Nano, 2012, 6(7), 6122–6132 CrossRef CAS PubMed.
- H. R. Jiang, N. Yoshinaga and M. Sano, Phys. Rev. Lett., 2010, 105(26), 268302 CrossRef PubMed.
- L. Baraban, R. Streubel, D. Makarov, L. Han, D. Karnaushenko, O. G. Schmidt and G. Cuniberti, ACS Nano, 2013, 7(2), 1360–1367 CrossRef CAS PubMed.
- W. Gao, S. Sattayasamitsathit, J. Orozco and J. Wang, J. Am. Chem. Soc., 2011, 133(31), 11862–11864 CrossRef CAS PubMed.
- X. Ma, K. Hahn and S. Sanchez, J. Am. Chem. Soc., 2015, 137(15), 4976–4979 CrossRef CAS PubMed.
- L. Soler and S. Sanchez, Nanoscale, 2014, 6(13), 7175–7182 RSC.
- J. Orozco, V. García-Gradilla, M. D'Agostino, W. Gao, A. Cortés and J. Wang, ACS Nano, 2013, 7(1), 818–824 CrossRef CAS PubMed.
- W. Gao, X. Feng, A. Pei, Y. Gu, J. Li and J. Wang, Nanoscale, 2013, 5(11), 4696–4700 RSC.
- M. Guix, J. Orozco, M. García, W. Gao, S. Sattayasamitsathit, A. Merkoçi, A. Escarpa and J. Wang, ACS Nano, 2012, 6(5), 4445–4451 CrossRef CAS PubMed.
- B. Esteban-Fernández de Ávila, A. Martín, F. Soto, M. A. Lopez-Ramirez, S. Campuzano, G. M. Vásquez-Machado, W. Gao, L. Zhang and J. Wang, ACS Nano, 2015, 9(7), 6756–6764 CrossRef PubMed.
- W. Gao, R. Dong, S. Thamphiwatana, J. Li, W. Gao, L. Zhang and J. Wang, ACS Nano, 2015, 9(1), 117–123 CrossRef CAS PubMed.
- F. Mou, Y. Li, C. Chen, W. Li, Y. Yin, H. Ma and J. Guan, Small, 2015, 11(21), 2564–2570 CrossRef CAS PubMed.
- M. Enachi, M. Guix, V. Postolache, V. Ciobanu, V. M. Fomin, O. G. Schmidt and I. Tiginyanu, Small, 2016, 12(39), 5497–5505 CrossRef CAS PubMed.
- M. J. Esplandiu, A. Farniya and A. Bachtold, ACS Nano, 2015, 9(11), 11234–11240 CrossRef CAS PubMed.
- Y. Hong, M. Diaz, U. Cordova-Figueroa and A. Sen, Adv. Func. Mater., 2010, 20(10), 1568–1576 CrossRef CAS.
- R. Dong, Q. Zhang, W. Gao, A. Pei and B. Ren, ACS Nano, 2016, 10(1), 839–844 CrossRef CAS PubMed.
- M. Xuan, Z. Wu, J. Shao, L. Dai, T. Si and Q. He, J. Am. Chem. Soc., 2016, 138(20), 6492–6497 CrossRef CAS PubMed.
- J. Palacci, S. Sacanna, A. P. Steinberg, D. J. Pine and P. M. Chaikin, Science, 2013, 339(6122), 936–940 CrossRef CAS PubMed.
- W. Li, X. Wu, H. Qin, Z. Zhao and H. Liu, Adv. Funct. Mater., 2016, 26(18), 3164–3174 CrossRef CAS.
- C. Chen, F. Mou, L. Xu, S. Wang, J. Guan, Z. Feng, Q. Wang, L. Kong, W. Li, J. Wang and Q. Zhang, Adv. Mater., 2016 DOI:10.1002/adma.201603374.
- B. Dai, J. Wang, Z. Xiong, X. Zhan, W. Dai, C.-C. Li, S.-P. Feng and J. Tang, Nat. Nanotechnol., 2016 DOI:10.1038/nnano.2016.187.
- Z. Zhang, H. Che, J. Gao, Y. Wang, X. She, J. Sun, P. Gunawan, Z. Zhong and F. Su, Catal. Sci. Technol., 2012, 2(6), 1207–1212 CAS.
- J. H. Nobbs, Rev. Prog. Color. Relat. Top., 1985, 15(1), 66–75 CrossRef.
- M. Hara, T. Kondo, M. Komoda, S. Ikeda, J. N. Kondo, K. Domen, M. Hara, K. Shinohara and A. Tanaka, Chem. Commun., 1998,(3), 357–358 RSC.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10(6), 456–461 CrossRef CAS PubMed.
- Y. Wang, R. M. Hernandez, D. J. Bartlett, J. M. Bingham, T. R. Kline, A. Sen and T. E. Mallouk, Langmuir, 2006, 22(25), 10451–10456 CrossRef CAS PubMed.
- S. Ebbens, D. A. Gregory, G. Dunderdale, J. R. Howse, Y. Ibrahim, T. B. Liverpool and R. Golestanian, Europhys. Lett., 2014, 106(5), 58003 CrossRef.
- A. Brown and W. Poon, Soft Matter, 2014, 10(22), 4016–4027 RSC.
- W. Duan, M. Ibele, R. Liu and A. Sen, Eur. Phys. J. E: Soft Matter Biol. Phys., 2012, 35(8), 77 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr08088j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |