Flexible all-solid-state supercapacitors based on polyaniline orderly nanotubes array†
Huihua
Li‡
,
Juan
Song‡
,
Linlin
Wang
,
Xiaomiao
Feng
*,
Ruiqing
Liu
,
Wenjin
Zeng
,
Zhendong
Huang
,
Yanwen
Ma
* and
Lianhui
Wang
Key Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials, National Jiangsu Syngerstic Innovation Center for Advanced Materials (SICAM); Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamxmfeng@njupt.edu.cn; iamywma@njupt.edu.cn
First published on 17th November 2016
Abstract
Flexible all-solid-state supercapacitors are crucial to meet the growing needs for portable electronic devices such as foldable phones and wearable electronics. As promising candidates for pseudocapacitor electrode materials, polyaniline (PANI) orderly nanotube arrays are prepared via a simple template electrodeposition method. The structures of the final product were characterized using various characterization techniques, including scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), and X-ray photoelectron spectroscopy (XPS). The obtained PANI nanotube film could be directly used as a flexible all-solid-state supercapacitor electrode. Electrochemical results show that the areal capacitance of a PANI nanotube-based supercapacitor with the deposition cycle number of 100 can achieve a maximum areal capacitance of 237.5 mF cm−2 at a scan rate of 10 mV s−1 and maximum energy density of 24.31 mW h cm−2 at a power density of 2.74 mW cm−2. In addition, the prepared supercapacitor exhibits excellent flexibility under different bending conditions. It retains 95.2% of its initial capacitance value after 2000 cycles at a current density of 1.0 mA cm−1, which displays its superior cycling stability. Moreover, the prepared flexible all-solid-state supercapacitor can power a light-emitting-diode (LED), which meets the practical applications of micropower supplies.
1. Introduction
With ever-increasing environmental pollution and the depletion of fossil fuels, the growth of sustainable and clean energy sources is increasingly becoming more important, which greatly influences the world economy and environment.1 Electrochemical capacitors, also called supercapacitors (SCs), have attracted significant attention in the field of energy storage devices because SCs can provide greater power density, longer cycling stability, and higher energy density than that of the conventional capacitors.2,3 Moreover, SCs have many other advantages such as small size, light-weight, easy to handle, short charging time, low cost, and environmental benignancy.4 As is known, conventional SCs use incompressible package materials and liquid electrolytes. Liquid-electrolyte SCs suffer possible leakage of baneful electrolytes and unpopular dislocation of the electrode position, which results from the liquid electrolyte entering the electrodes or flowing out during the compressing–releasing process. Unlike conventional SCs, all-solid-state SCs that are based on the solid electrolytes prepared without the complicated post-processing, avoiding to the possible leakage of electrolytes and the usage of additional package materials are considered as the most promising for energy storage.5–8 Recently, the rapid advancement of portable and wearable electronic equipment as well as the increasing demand for roll-up displays and self-powered sensor networks have promoted the development of flexible energy conversion and storage devices.4,9,10 Flexible all-solid-state SCs possessing excellent mechanical properties and simplified configuration are becoming a research emphasis of the energy storage devices field, which have dramatic potential as new energy storage devices for flexible, safe, portable electronics with an environmentally friendly nature.11–13As promising electrode materials for pseudocapacitors, conducting polymers, which hold the great advantages of light weight and excellent mechanical flexibility, have been attracting significant attention for application in electronic devices. The most commonly used conducting polymers include polypyrrole (PPy),14,15 poly(3,4-ethylenedioxythiophene) (PEDOT),16,17 and polyaniline (PANI).18,19 PANI is considered to be one of the most promising electrode materials because of its low cost, easy synthesis, and high theoretical specific pseudocapacitance.20–23 To date, numerous studies on the fabrication of flexible all-solid-state SCs using PANI have been reported. For example, a type of all-solid-state supercapacitor using wavy shaped PANI/graphene electrodes were reported.24 In that work, PANI was deposited onto the surface of internal graphene sheets via pulsed electrodeposition, and the as-fabricated wavy shaped supercapacitor exhibited a maximum specific capacitance of 261 F g−1. However, the supercapacitor only retained about 89% of its initial capacitance over 1000 charge–discharge cycles. A flexible supercapacitor based on a composite electrode combined with cloth-supported single-walled carbon nanotubes and PANI nanowire arrays was obtained by the dip coating and chemical polymerization method.25 Although the composite electrode had excellent flexibility, the preparation process was complicated. PET/Au/PANI composite electrodes were prepared through in situ electrodeposition, which could be used for flexible all-solid-state SCs.26 The areal capacitance of the all-solid-state SCs only achieved a value of 51.7 mF cm−2. High-performance flexible SCs using single-walled carbon nanotube/PANI films have been fabricated by a floating chemical vapor deposition method.27 The specific capacitance of the hybrid film could achieve 236 F g−1. However, the preparation process of the SCs was not environmentally friendly because of the use of organic solvents. A metal–organic framework interwoven by the electrochemically-deposited PANI was designed for application as a flexible solid-state supercapacitor.2 This symmetric flexible solid-state supercapacitor exhibited an excellent areal capacitance of 2146 mF cm−2 at 10 mV s−1. However, it retained 80% of its initial capacitance after 2000 cycles, which showed that it has poor long-term stability.
As is known, morphologies of the active materials have a huge influence on the electrochemical performance, which relies on different charge-transport rates. The nanotube-like structure of conductive polymers is one of the ideal structures that can enhance the device performance. For example, Ppy/PANI nanotube composite electrodes were fabricated via in situ polymerization.28 Electrochemical experiments showed that the largest specific capacitance achieved was 416 F g−1. However, this supercapacitive performance can be dramatically enhanced if highly ordered nanotube array are substituted for the abovementioned nanotubes as electrode materials.17,29–32 Electrochemical energy storage based on Ppy/PANI double-walled nanotube arrays was reported to be obtained via an electrodeposition method.33 The large specific capacitance of the SCs could achieve 693 F g−1. These results show that the intensive nanotube array structure with a high surface area can shorten the diffusion distance for ion transport in the redox processes, thus leading to a high charge/discharge capacity rate. Although PANI-based SCs are well documented, flexible all-solid-state SCs with PANI orderly nanotube array films have been rarely reported.
Herein, we have developed a facile template electrodeposition method to fabricate PANI orderly nanotube array films that could be directly used for symmetrical, flexible, and all-solid-state SCs electrode. In this method, PANI nanotubes are deposited on an Au/PC membrane through cyclic voltammetry. This ‘green’ method without toxic solvents, reductive agents, and oxidants, is simple, fast, and environmentally friendly. The obtained PANI nanotube array film can act as a flexible all-solid-state supercapacitor electrode using a mixture of polyvinyl alcohol (PVA) and sulfuric acid (H2SO4) to serve as a solid state electrolyte. The prepared SCs can achieve a maximum areal capacitance of 237.5 mF cm−2 and an energy density of 24.31 mW h cm−2. The capacitance remains almost the same when the bending angle is close to 180°, which shows that the prepared SCs have impressive flexibility. The SCs have a good electrochemical performance due to the unique PANI array structure in which ions could easily access the active material. With its dramatic performance, the device could act as a promising candidate for the portable/wearable electronic devices and flexible energy storage systems.
2. Experimental
2.1 Materials
Cyclopore polycarbonate (PC) membrane was purchased from Millipore. Sodium sulfate (Na2SO4) was obtained from the Chinese Medicine Group Chemical Reagent Co. Ltd. Sulfuric acid (H2SO4) and aniline were purchased from Shanghai Ling-feng chemical reagents Co. All reagents and solvents were of analytical grade and used as received without further treatment.2.2 Preparation of PANI orderly nanotubes array
A thin PC membrane coated with gold (85 nm) using an electron beam evaporation system served as the current collector. PANI nanotubes were prepared through cyclic voltammetry at a scan rate of 50 mV s−1 with potential windows ranging from −0.4 to 1.0 V by different deposition cycle numbers (60, 80, 100, 120, or 140 cycles) in an electrolyte solution containing 0.1 M H2SO4, 0.5 M Na2SO4, and 0.1 M aniline. During the electrodeposition process, Au/PC membrane was used as the working electrode, and an Ag/AgCl electrode and platinum wire were used as the reference and counter electrodes, respectively.2.3 Fabrication of the flexible all-solid-state supercapacitors
First, the PVA/H2SO4 gel electrolyte was prepared by the following process. Briefly, 1 g of 98% concentrated H2SO4 and 1 g of PVA powder were added to 10 mL of deionized water, and then heated to 85 °C with vigorous stirring until the solution became unobscured and transparent (about 2 h). After cooling down to room temperature, the gel was then dropwise added onto two flexible PANI nanotubes electrodes, and the two electrodes were assembled into a supercapacitor. The PVA/H2SO4 gel membrane worked as both a solid electrolyte and a separator.2.4 Material characterization
The morphologies of the PANI orderly nanotubes array were examined by scanning electron microscopy (SEM, S-4800). The FT-IR spectra of the products in KBr pellets were obtained using a Bruker VECTOR22 Fourier transform spectrometer. X-ray photoelectron spectroscopic (XPS) measurements were conducted using an ESCA-LAB MK II X-ray photoelectron spectrometer. To test the electrochemical properties of the samples, a typical two-electrode test cell was used for the electrochemical measurements on a CHI660C electrochemical working station (Chenhua, Shanghai, China). The as-prepared film was cut into 0.4 × 1.5 cm2 squares. The electrochemical behaviors of the supercapacitor systems were estimated by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and galvanostatic charge–discharge (GCD). All of the measurements were manipulated at room temperature within the potential windows ranging from −0.6 to 0.6 V.3. Results and discussion
The PANI nanotube array was successfully synthesized in an Au coated PC film through a facile one-step electrodeposition method. Fig. 1 shows the preparation process for the PANI nanotube electrodes integrated on a PC film and the fabrication of the flexible all-solid-state SCs. A commercially available PC membrane with a symmetrical cylinder pore structure acted as the template. An 85 nm gold layer, which could improve the electrical conductivity of the PC film, was sputtered on one side of the porous membrane. The obtained Au-coated PC film was used as the working electrode. When the Au-coated PC film was subjected to potential scanning in the range from −0.4 to +1.0 V in an electrolyte solution containing 0.1 M H2SO4, 0.5 M Na2SO4, and 0.1 M aniline, aniline could be oxidized and polymerized in the pores of the film to form the PANI orderly nanotube array. After cutting the PANI nanotube film into two electrodes with the same shape and size, all-solid-state two-ply supercapacitors were assembled by mounting two parallel PANI electrodes onto a flexible PET substrate using a PVA/H2SO4 gel electrolyte. Following these process, a red light-emitting diode was lit. The PANI nanotube device would be most beneficial for practical application in micropower supplies.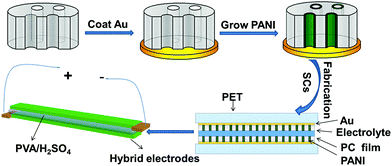 | ||
| Fig. 1 Schematic for the fabrication process of the PANI nanotubes array and the all-solid-state SCs. | ||
Fig. 2 shows the SEM images of the PANI nanotubes with different magnifications by different deposition cycle numbers. From this figure, we can see that the obtained PANI nanotubes show a good array structure. Moreover, the thickness of the PANI nanotubes increases with the increase in the deposition cycle numbers from 60 to 120 cycles, and the PANI nanotubes simultaneously present the array configuration. When the deposition cycle number increased from 120 to 140, the thickness of the PANI orderly nanotubes barley changed. For orderly nanotube arrays, it has been reported that the ion diffusion along the orientation parallel to the nanotubes array can be extraordinarily fast.33 The speed of ion diffusion can nearly achieve the same speed as that in the bulk electrolyte. Moreover, the orderly nanotubes array can reduce the resistance of the electrolyte penetration/diffusion.
The chemical compositions of the deposited PANI nanotubes were determined via Fourier transform infrared (FT-IR) spectroscopy. Fig. 3 shows the FT-IR spectra of the PANI nanotubes with different deposition cycle numbers in the range of 60–140 cycles. As shown in Fig. 3, the bands at 1555 and 1493 cm−1 could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the quinoid and benzenoid rings, respectively. The bands of the aromatic C–N, C
C stretching vibrations of the quinoid and benzenoid rings, respectively. The bands of the aromatic C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C–H stretching vibrations at 1299, 1142, and 794 cm−1, respectively, could be clearly observed.34 These results are in accordance with those for the characteristic vibrations of PANI, which indicates that PANI was successfully prepared on the Au-PC film.
N, and C–H stretching vibrations at 1299, 1142, and 794 cm−1, respectively, could be clearly observed.34 These results are in accordance with those for the characteristic vibrations of PANI, which indicates that PANI was successfully prepared on the Au-PC film.
X-ray photoelectron spectroscopy (XPS) was employed to further investigate the composition of the product. The N 1s XPS spectra of the PANI nanotubes electrode are given in Fig. 4A. The high-resolution XPS of the N 1s region of hybrids could be fitted by three different electronic states including the quinoid amine with the binding energy (BE) at 397.3 eV (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), the benzenoid amine with the BE centered at 398.1 eV (–NH–), and the nitrogen cationic radical with the BE at 399.0 eV (N˙+).19 Additionally, peaks for Au 4f centered at 82.1 and 87.2 eV (Fig. 4B) could also be observed, which agree well with the other reports on Au.35,36 These results indicate the formation of the PANI and Au PC film.
N–), the benzenoid amine with the BE centered at 398.1 eV (–NH–), and the nitrogen cationic radical with the BE at 399.0 eV (N˙+).19 Additionally, peaks for Au 4f centered at 82.1 and 87.2 eV (Fig. 4B) could also be observed, which agree well with the other reports on Au.35,36 These results indicate the formation of the PANI and Au PC film.
To evaluate the conductivity of the PANI nanotube electrodes with different deposition cycle numbers, electrochemical impedance spectroscopy (EIS) was conducted from 100 kHz to 0.01 Hz. All the plots display a semicircle in the high-frequency region and an inclined line in the low-frequency region, which correspond to the charge-transfer impedance on the electrode/electrolyte interface and the ion diffusion process within the electrodes, respectively.37 At the high frequency region, the equivalent series resistances (ESR) of the PANI nanotubes deposited for 60, 80, 100, 120, and 140 cycles were about 6.49, 5.93, 4.31, 12.3, and 13.2 Ω cm−2, respectively. The PANI nanotube electrode as-obtained by the 100 deposited cycles (named PANI/Au/PC-100) possesses a minimum ESR of 4.31 Ω cm−2, which may be due to the appropriate thickness of the wall (Fig. 5).
The electrochemical performances of the PANI nanotube electrodes with different deposition cycle numbers were further characterized by cyclic voltammetry (CV) and GCD using PVA/H2SO4 as the gel electrolyte. Fig. 6A shows the CV curves of the PANI nanotube electrodes with different deposition cycle numbers (0–140 cycles) at a constant scan rate of 100 mV s−1 in the potential windows ranging from −0.6 to 0.6 V. Two pairs of obvious redox peaks are observed owing to the redox transition of the PANI polymer chains, which indicates the typical behavior of pseudocapacitive SCs.26 The first pair is attributed to the redox transition of PANI between the leucoemeraldine form and emeraldine form, whereas the second pair is ascribed to the emeraldine–pernigraniline transformation.38 The areal capacitances (Cs) were calculated from the CV curves using the following eqn (1):39
 | (1) |
| Cs = (It)/(sV) | (2) |
Fig. 7A shows the CV results of PANI/Au/PC-100 at scan rates ranging from 10 to 100 mV s−1. The CV curves display obvious couples of redox peaks, and a highly symmetrical shape appears upon an increase in the scan rate, which exhibits the typical pseudocapacitive shape.26 The values of areal capacitances versus different scan rates were calculated according to eqn (1), as shown in Fig. 7B. It could be seen that as the scan rate decreased, the areal capacitance of the fabricated supercapacitor increased and could achieve a maximum areal capacitance of 237.5 mF cm−2 at a scan rate of 10 mV s−1. In addition, a good supercapacitor is expected to provide a high energy density or high specific capacitance at high charge–discharge rates (current densities). The GCD curves at different current densities with symmetry and a good linear profile manifest the good capacitive performance of the all-solid-state device, as shown in Fig. 7C. Fig. 7D shows the areal capacitances at different current densities of the device calculated according to eqn (2). The device exhibits a maximum areal capacitance of 130.4 mF cm−2 at a current density 0.1 mA cm−2. This areal capacitance is much higher than that of some reported works.26,41,42
The excellent mechanical flexibility of the PC film leads to high mechanical stability and a capacitance performance, which displays a little change with large bending angles close to 180°. The CV performances of the device under different bending conditions at a scan rate of 100 mV s−1 are shown in Fig. 8A. The CV curves show a similar shape at different curvatures due to the excellent flexibility of the electrode material, suggesting that bending has almost no effect on its capacitive performance. Fig. 8B presents the areal capacitances under different bending conditions of the device. The areal capacitances under the bending angles close to 180° remained at 91% of the initial capacitive value, revealing that the supercapacitor has high mechanical stability.
Cycle stability is another important parameter for supercapacitors. The cycle stability of the supercapacitor based on PANI/Au/PC-100 was determined by GCD tested at a current density of 1.0 mA cm−1 for 2000 cycles. The result shows that the device still retained about 95.2% of its initial capacitance after 2000 cycles, indicating excellent long-term cycling stability. This cycling stability is much higher than that reported in some other works.41,43 As is known, PANI based SCs usually suffer from poor cycling stability due to swelling and shrinking of PANI during the charge–discharge process. The good cycling stability achieved here is attributed to the unique PANI orderly nanotube array structure, which is beneficial for facilitating the interfacial electron transport. This structure could be maintained well after 2000 cycles, which could be seen from the corresponding SEM images (Fig. S1†).
EIS of PANI/Au/PC-100 after 2000 cycles (Fig. S2†) shows that the ESR is about 8.81 Ω cm−2, and there are no obvious changes compared with the initial value. Another reason for this might be that the integrated structure causes the active materials to be sealed in the PC membrane and elastic solid-state gel electrolyte, which results in a good protective effect to reduce the damage of swelling and shrinking during the charging–discharging of PANI. To prove the practical applications of the device in flexible energy storage, one device was used to light up a red light-emitting-diode (LED) (the inset in Fig. 9A). After being charged to 2.5 V for 70 s, the supercapacitor can successfully operate a red LED light (the lowest working voltage is 1.5 V). The discharged volumetric energy density W (mW h cm−3) and power density P (W cm−3) of the flexible all-solid-state PANI/Au/PC-100 electrode are the significant parameters required for the practical applications, which could be calculated from the GCD curves according to the following equations:41,44
| W = 0.5C(ΔE)2/3600V | (3) |
| P = W/ΔtV | (4) |
4. Conclusions
In conclusion, the flexible all-solid-state SCs based on PANI orderly nanotube array electrodes integrated on a flexible cyclopore Au-coated PC membrane have been successfully fabricated. The fabricated SCs have the maximum areal capacitance of 237.5 mF cm−2 at a scan rate of 10 mV s−1 and maximum energy density of 24.31 mW h cm−3 at a power density of 2.74 mW cm−2. Moreover, the fabricated all-solid-state SCs have excellent mechanical flexibility, long-time stability, and can light-up a red light-emitting diode. These exciting findings demonstrate that SCs fabricated with the method used in this research hold a great promise for use as high-performance flexible energy storage devices in the future.Acknowledgements
This work is jointly supported by the Ministry of Education of China (no. IRT1148), NSFC (20905038, 61504066), Synergistic Innovation Center for Organic Electronics and Information Displays, Jiangsu Province “Six Talent Peak” (2015-JY-015), Jiangsu Provincial NSF (BK20141424, BK20150838), the Program of NUPT (NY214088), and the Open Research Fund of State Key Laboratory of Bioelectronics (I2015010), the Southeast University.References
- Y. Wang, J. Zeng, J. Li, X. Cui, A. M. Al-Enizi, L. Zhang and G. Zheng, J. Mater. Chem. A, 2015, 3, 16382 RSC.
- L. Wang, X. Feng, L. Ren, Q. Piao, J. Zhong, Y. Wang, H. Li, Y. Chen and B. Wang, J. Am. Chem. Soc., 2015, 137, 4920 CrossRef CAS PubMed.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326 CrossRef CAS PubMed.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867 Search PubMed.
- B. Liu, B. Liu, X. Wang, X. Wu, W. Zhao, Z. Xu, D. Chen and G. Shen, Adv. Mater., 2014, 26, 4999 CrossRef CAS PubMed.
- F. Béguin, V. Presser, A. Balducci and E. Frackowiak, Adv. Mater., 2014, 26, 2219–2251 CrossRef PubMed.
- X. Liu, D. Wu, H. Wang and Q. Wang, Adv. Mater., 2014, 26, 4370 CrossRef CAS PubMed.
- Z. Niu, W. Zhou, X. Chen, J. Chen and S. Xie, Adv. Mater., 2015, 27, 6002 CrossRef CAS PubMed.
- X. Xiao, L. Yuan, J. Zhong, T. Ding, Y. Liu, Z. Cai, Y. Rong, H. Han, J. Zhou and Z. L. Wang, Adv. Mater., 2011, 23, 5440 CrossRef CAS PubMed.
- X. Lu, M. Yu, G. Wang, Y. Tong and Y. Li, Energy Environ. Sci., 2014, 7, 2160 Search PubMed.
- W. Si, C. Yan, Y. Chen, S. Oswald, L. Han and O. G. Schmidt, Energy Environ. Sci., 2013, 6, 3218 Search PubMed.
- Y. Fu, X. Cai, H. Wu, Z. Lv, S. Hou, M. Peng, X. Yu and D. Zou, Adv. Mater., 2012, 24, 5713 CrossRef CAS PubMed.
- X. Xiao, T. Li, P. Yang, Y. Gao, H. Jin, W. Ni, W. Zhan, X. Zhang, Y. Cao and J. Zhong, ACS Nano, 2012, 6, 9200 CrossRef CAS PubMed.
- Y. Shi, L. Pan, B. Liu, Y. Wang, Y. Cui, Z. Bao and G. Yu, J. Mater. Chem. A, 2014, 2, 6086 RSC.
- X. Ding, Y. Zhao, C. Hu, Y. Hu, Z. Dong, N. Chen, Z. Zhang and L. Qu, J. Mater. Chem. A, 2014, 2, 12355 RSC.
- B. Anothumakkool, R. Soni, S. N. Bhange and S. Kurungot, Energy Environ. Sci., 2015, 8, 1339 Search PubMed.
- Y. Xie, H. Du and C. Xia, Microporous Mesoporous Mater., 2015, 204, 163 CrossRef CAS.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025 CrossRef CAS PubMed.
- M. Zhong, Y. Song, Y. Li, C. Ma, X. Zhai, J. Shi, Q. Guo and L. Liu, J. Power Sources, 2012, 217, 6 CrossRef CAS.
- H. Gómez, M. K. Ram, F. Alvi, P. Villalba, E. Stefanakos and A. Kumar, J. Power Sources, 2011, 196, 4102 CrossRef.
- S. Xiong, Y. Shi, J. Chu, M. Gong, B. Wu and X. Wang, Electrochim. Acta, 2014, 127, 139 CrossRef CAS.
- Y. Meng, K. Wang, Y. Zhang and Z. Wei, Adv. Mater., 2013, 25, 6985 CrossRef CAS PubMed.
- K. Wang, H. Wu, Y. Meng and Z. Wei, Small, 2014, 10, 14 CrossRef CAS PubMed.
- Y. Xie, Y. Liu, Y. Zhao, Y. H. Tsang, S. P. Lau, H. Huang and Y. Chai, J. Mater. Chem. A, 2014, 2, 9142 RSC.
- K. Wang, P. Zhao, X. Zhou, H. Wu and Z. Wei, J. Mater. Chem., 2011, 21, 16373 RSC.
- K. Zhang, H. Hu, W. Yao and C. Ye, J. Mater. Chem. A, 2015, 3, 617 RSC.
- Z. Niu, P. Luan, Q. Shao, H. Dong, J. Li, J. Chen, D. Zhao, L. Cai, W. Zhou, X. Chen and S. Xie, Energy Environ. Sci., 2012, 5, 8726 Search PubMed.
- H. Mi, X. Zhang, X. Ye and S. Yang, J. Power Sources, 2008, 176, 403 CrossRef CAS.
- R. Xiao, S. I. Cho, R. Liu and S. B. Lee, J. Am. Chem. Soc., 2007, 129, 4483 CrossRef CAS PubMed.
- C. Xia, Y. Xie, Y. Wang, W. Wang, H. Du and F. Tian, J. Appl. Electrochem., 2013, 43, 1225 CrossRef CAS.
- H. Xia, J. Feng, H. Wang, M. O. Lai and L. Lu, J. Power Sources, 2010, 195, 4410 CrossRef CAS.
- X. Lu, G. Wang, T. Zhai, M. Yu, J. Gan, Y. Tong and Y. Li, Nano Lett., 2012, 12, 1690 CrossRef CAS PubMed.
- Z. L. Wang, X. J. He, S. H. Ye, Y. X. Tong and G. R. Li, ACS Appl. Mater. Interfaces, 2014, 6, 642 Search PubMed.
- Y. Yang and W. Yang, Polym. Adv. Technol., 2005, 16, 24 CrossRef CAS.
- M. Murdoch, G. Waterhouse, M. Nadeem, J. Metson, M. Keane, R. Howe, J. Llorca and H. Idriss, Nat. Chem., 2011, 3, 489 CrossRef CAS PubMed.
- T.-T. Yang, W.-T. Chen, Y.-J. Hsu, K.-H. Wei, T.-Y. Lin and T.-W. Lin, J. Phys. Chem. C, 2010, 114, 11414 CrossRef CAS.
- W. Chen, R. B. Rakhi, L. Hu, X. Xie, Y. Cui and H. N. Alshareef, Nano Lett., 2011, 11, 5165 CrossRef CAS PubMed.
- C. C. Hu and J. Y. Lin, Electrochim. Acta, 2002, 47, 4055–4067 CrossRef CAS.
- Y. Gao, Y. S. Zhou, M. Qian, H. M. Li, J. Redepenning, L. S. Fan, X. N. He, W. Xiong, X. Huang, M. Majhouri-Samani, L. Jiang and Y. F. Lu, RSC Adv., 2013, 3, 20613 RSC.
- C. Yang, J. Shen, C. Wang, H. Fei, H. Bao and G. Wang, J. Mater. Chem. A, 2014, 2, 1458 RSC.
- H. Hu, K. Zhang, S. Li, S. Ji and C. Ye, J. Mater. Chem. A, 2014, 2, 20916 RSC.
- L. Yuan, X. Xiao, T. Ding, J. Zhong, X. Zhang, Y. Shen, B. Hu, Y. Huang, J. Zhou and Z. L. Wang, Angew. Chem., Int. Ed., 2012, 51, 4934 CrossRef CAS PubMed.
- B. Yao, L. Yuan, X. Xiao, J. Zhang, Y. Qi, J. Zhou, J. Zhou, B. Hu and W. Chen, Nano Energy, 2013, 2, 1071 CrossRef CAS.
- J. Zhao, H. Lai, Z. Lyu, Y. Jiang, K. Xie, X. Wang, Q. Wu, L. Yang, Z. Jin, Y. Ma, J. Liu and Z. Hu, Adv. Mater., 2015, 27, 3541 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr07921k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |








