Electric field controlled CO2 capture and CO2/N2 separation on MoS2 monolayers†
Qiao
Sun
*a,
Gangqiang
Qin
a,
Yingying
Ma
b,
Weihua
Wang
c,
Ping
Li
c,
Aijun
Du
d and
Zhen
Li
*a
aCollaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, School for Radiological and Interdisciplinary Sciences, Soochow University, Suzhou 215123, P. R. China. E-mail: sunqiao@suda.edu.cn; zhenli@suda.edu.cn
bInstitute of Mining Technology, Inner Mongolia University of Technology, Hohhot 010051, P. R. China
cSchool of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong 273165, P. R. China
dSchool of Chemistry, Physics and Mechanical Engineering, Queensland University of Technology, Brisbane, QLD 4001, Australia
First published on 25th November 2016
Abstract
Developing new materials and technologies for efficient CO2 capture, particularly for separation of CO2 post-combustion, will significantly reduce the CO2 concentration and its impacts on the environment. A challenge for CO2 capture is to obtain high performance adsorbents with both high selectivity and easy regeneration. Here, CO2 capture/regeneration on MoS2 monolayers controlled by turning on/off external electric fields is comprehensively investigated through a density functional theory calculation. The calculated results indicate that CO2 forms a weak interaction with MoS2 monolayers in the absence of an electric field, but strongly interacts with MoS2 monolayers when an electric field of 0.004 a.u. is applied. Moreover, the adsorbed CO2 can be released from the surface of MoS2 without any energy barrier once the electric field is turned off. Compared with the adsorption of CO2, the interactions between N2 and MoS2 are not affected significantly by the external electric fields, which indicates that MoS2 monolayers can be used as a robust absorbent for controllable capture of CO2 by applying an electric field, especially to separate CO2 from the post-combustion gas mixture where CO2 and N2 are the main components.
Introduction
The rapid increase of CO2 in the atmosphere due to anthropogenic activities is one of the greatest global issues faced by mankind today.1–3 The development of effective materials and technologies for efficient CO2 capture and gas separation is a challenging and urgent task. Recently, solid materials have emerged as effective adsorbents for CO2 capture because of their large surfaces and high selectivity.4,5 However, high selectivity for CO2 capture usually means that the regeneration step is difficult and energy consuming. Therefore, searching for high-performance adsorbents with both high selectivity and easy regeneration is crucial for CO2 capture and reutilization.6It is well-known that external electric fields can affect many chemical reactions, especially the electron transfer reactions. The reaction rates can be boosted to several orders of magnitude by applying external electric fields. There is growing interest in applying external electric fields to modulate chemical reactions. For example, electric fields can dramatically enhance hydrogen storage on boron nitride (BN) nanomaterials.7 The recent investigations of CO2 capture and gas separation on boron containing nanomaterials,8–14 such as BN and pure boron, indicated that CO2 capture and release can be controlled by turning on/off the charge states or electric fields applied to these nanomaterials.8 With an electric field of 0.030 a.u., BN nanosheets were proven to be an effective adsorbent for CO2 capture.15 However, turning on/off the charge states or electric fields are energetic processes, due to the wide band gaps of BN nanostructures (their band gaps vary from 3.6 to 7.1 eV with the preparation methods and conditions), and the strong external electric fields applied to BN monolayers.8,15 Therefore, the main purpose of this study is to search for advanced materials for efficient CO2 capture/regeneration.
Recently, molybdenum disulfide (MoS2) as an emerging two-dimensional material has attracted considerable attention due to its exciting properties and great potential for diverse applications, such as energy conversion, piezotronics, phototransistors, catalysts, drug deliveries, lithium-ion batteries, gas sensors, adsorbents and separation.16–26 However, MoS2 cannot be used directly as an adsorbent for CO2 capture because of the weak interactions between MoS2 and CO2.23 Various technologies, such as introduction of dopants, defects, and strains, have been used to successfully increase the interactions between CO2 and MoS2.27–29 As mentioned previously, however, an ideal adsorbent for CO2 capture should be featured with high selectivity and easy regeneration. Could MoS2 monolayers serve as ideal adsorbents for CO2 capture and separation by engineering their interactions with CO2 by applying electric fields, and by well-understanding the impacts of electric fields on the mechanisms of CO2 capture, regeneration and separation?
To answer the above question, we carry out the following investigations: (i) the interactions of CO2 with MoS2 monolayers in the absence/presence of an electric field, (ii) the reaction mechanisms of CO2 adsorbed on MoS2 monolayers with/without the electric field, (iii) optimization of electric fields for CO2 capture, and (iv) comparing the adsorption of N2 on MoS2 in the presence/absence of electric fields, with that of CO2 to demonstrate whether CO2 could be selectively separated from the CO2/N2 gas mixture by MoS2 with the assistance of external electric fields.
Calculation methods
The first-principles density functional theory plus dispersion method implemented in the DMol3 package was used for all the calculations of the study.30,31 All the structures are fully optimized using the generalized gradient approximation,32 treated by the Perdew–Burke–Ernzerhof exchange–correlation potential (PBE) with long range dispersion correction via Grimme's scheme.33 The basis set for the system is an all-electron double numerical atomic orbital augmented by d-polarization functions (DNP). The optimized lattice constant of the MoS2 monolayer is 3.185 Å, and a 4 × 4 supercell of the MoS2 monolayer with a dimension of 12.74 × 12.74 × 20 Å3 is used for CO2 and N2 adsorption without and with different electric fields. The vacuum between the MoS2 monolayers is 20 Å which is large enough to avoid interactions between periodic images. Geometry optimizations were performed with convergence thresholds of 0.002 a.u. Å−1 on the gradient, 0.005 Å on the displacement, and 10−5 a.u. on the energy. The real-space global cut-off radius is set to be 4.10 Å. The self-consistent field (SCF) procedure was used with a convergence threshold of 10−6 a.u. on energy and electron density. The direct inversion of the iterative subspace technique developed by Pulay is used with a subspace size of 6 to speed up SCF convergence on these systems.34 The Brillouin zones are sampled by 6 × 6 × 1 k-points using the Monkhorst–Pack scheme. Electron distributions and transfer mechanisms are determined by the Mulliken method.35The adsorption energies of CO2 and N2 on MoS2 monolayers are calculated from eqn (1).
| Eads = (EMoS2 + Egas) − EMoS2-gas | (1) |
Results and discussion
CO2 adsorption on MoS2 monolayers without an electric field
The adsorption of CO2 on MoS2 monolayers without an external electric field at the PBE level with a long range dispersion correction was firstly investigated. The optimized configuration with the minimum energy for CO2 adsorbed on the surface of MoS2 monolayers is presented in Fig. 1. The configuration shows that the linear CO2 molecule is parallel to the MoS2 monolayer and quite far from the surface. The adsorbed CO2 molecule has little structural change compared to the free CO2 molecule, with an O–C–O angle of 179.7°. The S⋯C distance between CO2 and the MoS2 sheet in the configuration is long with a value of 3.334 Å, and the adsorption energy is 3.16 kcal mol−1. The charge transfer from the MoS2 monolayer to the adsorbed CO2 molecule is negligible with a value of 0.008e−. Their interactions are very weak and mainly attributed to the van der Waals forces between them. The above results indicate that CO2 is physisorbed on the MoS2 monolayer without an external electric field, which agrees well with the recent report23 where MoS2 monolayers can't be directly used as an efficient adsorbent for CO2 capture.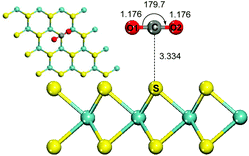 | ||
| Fig. 1 Top and side views of physisorbed CO2 on a single layer MoS2 surface without an electric field. Atom color code: yellow, sulfur; blue, molybdenum; gray, carbon; red, oxygen. | ||
CO2 adsorption on MoS2 monolayers with the electric fields from 0.0020 to 0.0050 a.u.
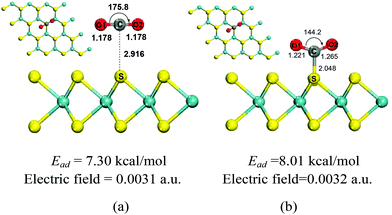
It has been known that external electric fields can enhance gas adsorption on solid surfaces, especially for the polarizable nanomaterials,7,24 which suggests that the adsorption of CO2 on the MoS2 surface could be improved by applying an electric field. In this part, CO2 adsorption on MoS2 monolayers with the applied electric fields in the range of 0.0020–0.0050 a.u. was investigated. The important structural parameters of optimized configurations (such as C–S bond length, charge transfer) and the adsorption energies obtained with the electric fields in the above range are shown in Fig. 2 and 3, and given in Table S1 in the ESI.† When the electric field is increased from 0.0020 a.u. to 0.0031 a.u., the distances between CO2 and MoS2 decrease slowly and the C⋯S distances are around 3.0 Å [Fig. 2(a) and Table S1†]. The charge transfer from the MoS2 monolayer to CO2 increases from 0.036 to 0.077e− [Fig. 2(b) and Table S1†]. The adsorption energies increase smoothly from 5.29 to 7.30 kcal mol−1 [Fig. 2(c) and Table S1†]. These results demonstrate that the CO2 molecules are physisorbed on the MoS2 monolayer when the electric field is below 0.0031 a.u. However, when the strength of electric fields increases to 0.0032 a.u., there is a chemical bond (i.e. C–S bond) formed between CO2 and MoS2, and the C–S bond of the optimized configuration is 2.048 Å [Fig. 3(b)].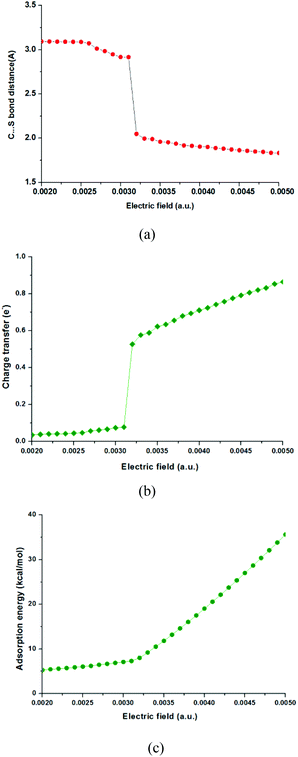 | ||
| Fig. 2 The distances of C–S bonds (Å), charge transfer (e−) and adsorption energies (kcal mol−1) of CO2 adsorbed on the surface of MoS2 monolayers with different electric fields. | ||
 | ||
| Fig. 3 Top and side views of chemisorbed CO2 on the MoS2 surface with different electric fields. Atom colors are the same as that in Fig. 1. | ||
The results also indicate the transition of the physisorbed configuration into the chemisorbed configuration, and the charge transfer from the MoS2 monolayer to CO2 dramatically increases from 0.077 to 0.527e−, despite the fact that the electric field is slightly increased from 0.0031 a.u. to 0.0032 a.u. [Fig. 2(b) and Table S1†]. Further increase of the electric field from 0.0032 a.u. to 0.0050 a.u. leads to further decrease of the C–S bond from 2.048 Å to 1.833 Å and increase of charge transfer from 0.527 to 0.865e−. The slope of adsorption energies increases dramatically with the increase of the electric fields [Fig. 2(c)], and the adsorption energies increase from 8.01 to 35.62 kcal mol−1. The ideal adsorption energy of CO2 on high performance adsorbents should be in the range of 40–80 kJ mol−1.36 According to this criterion, the MoS2 monolayer should be a good sorbent for CO2 capture if the applied electric field is in the range of 0.0035–0.0040, because the absorption energy in this range is 11.51–19.03 kcal mol−1 (49.37–79.54 kJ mol−1).
CO2 adsorption on MoS2 monolayers in the presence of an electric field with a strength of 0.0040 a.u.
The results in the previous section demonstrate that CO2 is chemisorbed on MoS2 monolayers in the presence of electric fields above 0.0031 a.u. To compare the adsorption of CO2 on BN monolayers, we investigated CO2 adsorption on the surface of MoS2 monolayers with an electric field of 0.0040 a.u. The direction of the applied electric field is shown in Fig. 4(a). The optimized structure of CO2 adsorbed on the MoS2 surface with a perpendicular external electric field (0.0040 a.u.) is displayed in Fig. 4(b), which illustrates a chemisorbed configuration formed between the MoS2 monolayer and CO2. In detail, a C–S bond has a length of 1.904 Å. The adsorbed CO2 molecule is drastically bent with an O–C–O angle of 138.6°, and the two double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are elongated to 1.237 Å. A Mulliken charge population analysis shows that there is 0.771e− charge transferred from the MoS2 sheet to the CO2 molecule. The Mulliken population analysis further demonstrates that the charge is mainly transferred from the S atom of the MoS2 monolayer to the C atom of adsorbed CO2, and the electron transfer is along the direction of the electric field. The CO2 molecule strongly interacts with the MoS2 surface with an adsorption energy of 19.03 kcal mol−1. The adsorption energy is similar to that of CO2 captured on BN monolayers by applying external electric fields.8,15 With an external electric field of 0.050 a.u., the adsorption energy of CO2 adsorbed on the BN monolayer where CO2 vertically interacts with B atoms is 19.26 kcal mol−1.8 In the case of CO2 strongly interacting with the N atoms of the BN sheet in the presence of an electric field of 0.030 a.u., the adsorption energy is 15.68 kcal mol−1 (0.68 eV).15 Compared with adsorption of CO2 on BN monolayers, the strength of the electric field applied to MoS2 monolayers is only around 10% of that applied to BN monolayers.8,15 The reason is that the MoS2 monolayer is more polarizable than BN sheets.
O bonds are elongated to 1.237 Å. A Mulliken charge population analysis shows that there is 0.771e− charge transferred from the MoS2 sheet to the CO2 molecule. The Mulliken population analysis further demonstrates that the charge is mainly transferred from the S atom of the MoS2 monolayer to the C atom of adsorbed CO2, and the electron transfer is along the direction of the electric field. The CO2 molecule strongly interacts with the MoS2 surface with an adsorption energy of 19.03 kcal mol−1. The adsorption energy is similar to that of CO2 captured on BN monolayers by applying external electric fields.8,15 With an external electric field of 0.050 a.u., the adsorption energy of CO2 adsorbed on the BN monolayer where CO2 vertically interacts with B atoms is 19.26 kcal mol−1.8 In the case of CO2 strongly interacting with the N atoms of the BN sheet in the presence of an electric field of 0.030 a.u., the adsorption energy is 15.68 kcal mol−1 (0.68 eV).15 Compared with adsorption of CO2 on BN monolayers, the strength of the electric field applied to MoS2 monolayers is only around 10% of that applied to BN monolayers.8,15 The reason is that the MoS2 monolayer is more polarizable than BN sheets.
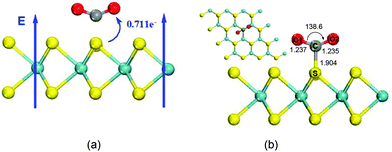 | ||
| Fig. 4 (a) Representation of the direction of the applied perpendicular electric field and the applied electric field is 0.0040 a.u. (b) Top and side views of adsorbed CO2 on a single layer MoS2 surface in the presence of an electric field with a strength of 0.0040 a.u. Atom colors are the same as that in Fig. 1. | ||
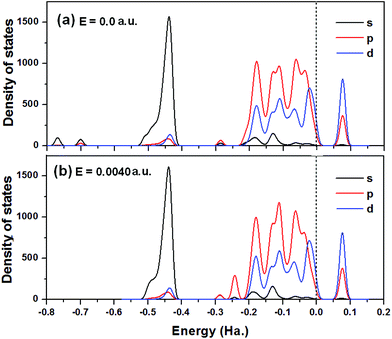
To investigate the electronic structure of MoS2 monolayers for CO2 capture with the effect of the electric field (0.0040 a.u.), the partial density states (PDOS), including s-, p-, and d-orbitals of atoms from the MoS2–CO2 system without and with an electric field are plotted in Fig. 5(a) and (b). Fig. 5(a) indicates that in the absence of an electric field, MoS2 exhibits the nature of a semiconductor. The corresponding energy band gap of 1.88 eV is slightly smaller than that of bare MoS2 (1.93 eV). Fig. 5(b) shows that when an electric field of 0.0040 a.u. is applied, the adsorption energy increases to 19.03 kcal mol−1, and the semiconductor properties of MoS2 are still retained with a similar band gap. The calculated result is consistent with the previous report that the bandgap of the MoS2 monolayer is insensitive to an external perpendicular field,37 which indicates that the MoS2 monolayer is electronically stable and would not be destroyed by the applied electric field. The PDOS shown in Fig. 5(b) also demonstrates that the p density of state at −0.25 a.u. contributes to the interactions of CO2 and MoS2 in the electric field of 0.0040 a.u. In addition, the applied electric field doesn't change its contribution to the density of state at the Fermi level.
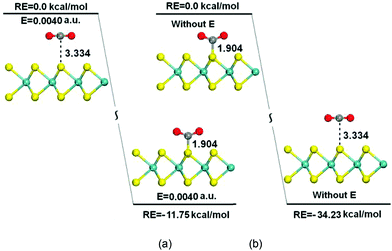
The mechanisms of CO2 capture/release on the MoS2 monolayer by turning on/off the electric field were also studied. Fig. 6(a) shows the turning of a physisorbed configuration obtained without an electric field into an optimized chemisorbed configuration when an electric field of 0.0040 a.u. is applied to the system. The interactions between CO2 and MoS2 drastically increase compared with the case of zero field, and the CO2 molecule is chemisorbed on the surface of MoS2 with a C–S distance of 1.904 Å, and the reaction is exothermic by about 11.75 kcal mol−1. The turning of the physisorbed configuration into the chemisorbed configuration is exothermic and without any energy barrier. When the electric field is turned off, the turning of the chemisorbed configuration into the physisorbed configuration is also studied. The results illustrate an increase of C⋯S bond distances from 1.904 Å to 3.334 Å. This transition also has no barrier and it is exothermic by 34.23 kcal mol−1 [Fig. 6(b)], which means that CO2 is easily released from the surface of MoS2 monolayers once the electric field is removed. The above results demonstrate that the capture and release of CO2 are overall controlled by the electric field applied to the system, and the energy costs of these processes are dependent on the applied electric field.
CO2/N2 separation on MoS2 monolayers
With population expansion and industrial development, the combustion of fossil fuels (coal, petroleum, and natural gas) increases and accounts for 86% of anthropogenic greenhouse gas emissions.3 Therefore, efficient separation of CO2 from the post-combustion mixture (the main containments are CO2 and N2) can reduce the greenhouse effect and mitigate climate change. In this part, we studied whether MoS2 monolayers can be used to separate CO2 from post-combustion gas (CO2/N2). Firstly, we investigated N2 adsorption on MoS2 monolayer without an electric field. The optimized configuration of N2 adsorbed on the surface of MoS2 without an electric field is shown in Fig. 7(a), where the distance between N2 and MoS2 is quite far with a value of 3.464 Å, and the adsorption energy is 2.28 kcal mol−1, which indicates that N2 is physisorbed on MoS2 and their interactions are weak. Similar weak interactions of CO2 and N2 adsorbed on MoS2 suggest that MoS2 can't be used to separate CO2 from the CO2/N2 gas mixture without an electric field. Then the adsorption of N2 on MoS2 monolayers with the electric fields ranging from 0.0020 a.u. to 0.0050 a.u. was carried out.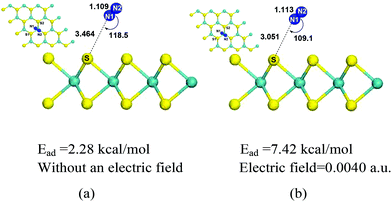 | ||
| Fig. 7 Top and side views of configurations of N2 adsorption on the MoS2 surface (a) without and (b) with an electric field at the strength of 0.0040 a.u. Atom colors are the same as that in Fig. 1. | ||
The optimized configuration of N2 adsorbed on the surface of the MoS2 monolayer with an electric field of 0.0040 is displayed in Fig. 7(b). The important structural information, such as bond lengths and bond angles, adsorption energies and electron transfers from MoS2 to N2, in the cases of N2 adsorbed on the MoS2 surface with different electric fields from 0.0020 a.u. to 0.0050 a.u. are shown in Fig. 8 and given in Table S2 in the ESI.†Fig. 7(b) shows that with the electric field of 0.0040 a.u., the distance between N2 and MoS2 is still far with a value of 3.051 Å, the adsorption energy is 7.42 kcal mol−1, and N2 is physisorbed on the MoS2 monolayer. This is in contrast to the adsorption of CO2 on the MoS2 monolayer under the same electric field (i.e. 0.0040 a.u.), where a chemisorbed configuration is formed. The drastic difference between the adsorptions of CO2/N2 on MoS2 monolayers with the same electric field (0.0040 a.u.) clearly demonstrates that the MoS2 monolayer can selectively capture CO2 from the CO2/N2 gas mixture with the assistance of an electric field. Table S2† shows that for the adsorption of N2 on the MoS2 surface with the electric field fields increasing from 0.0020 a.u. to 0.0050 a.u., the distances between N2 and MoS2 are in the range of 2.840–3.329 Å. Therefore, the interactions between N2 and MoS2 in all adsorbed configurations are relatively weak. Fig. 8 also clearly shows that the difference in adsorption energies of CO2 and N2 adsorbed on MoS2 monolayers increases with the electric fields. All the above results demonstrate that by applying an electric field (the optimized electric field is in the range of 0.0035–0.0040 a.u.), CO2 can be efficiently captured with MoS2 from the CO2/N2 gas mixture.
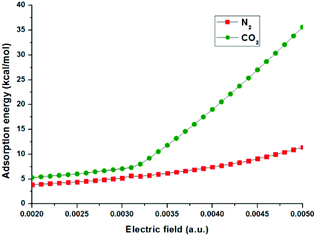 | ||
| Fig. 8 The adsorption energies (kcal mol−1) of CO2 and N2 adsorption on the MoS2 surface with different electric fields. | ||
In addition, the influences of H2O, NO, NO2 and SO2 on the adsorption of CO2 on MoS2 monolayers were also investigated with the effect of an external electric field, as they are the major containments of flue gas. The optimized configurations of H2O, NO, NO2 and SO2 adsorbed on MoS2 monolayers without an external electric field, and with external electric fields of 0.0020 a.u. and 0.0050 a.u. are listed in Fig. S1 and S2 in the ESI.† The adsorption energies of four gases, CO2 and N2 adsorbed on the surface of MoS2 by applying the electric fields in the range of 0.0020–0.0050 a.u. are shown in Fig. S2 and S4 in the ESI,† which clearly indicate that the adsorption of H2O, NO, NO2 and SO2 on MoS2 monolayers increases linearly with the increase of electric fields. Moreover, the adsorptions of four gases on MoS2 monolayers are stronger than that of CO2 with the same electric field. The above results suggest that with a relatively weak electric field (around 0.0020 a.u.), H2O, NO, NO2 and SO2 can be selectively separated from the gas mixture by MoS2 monolayers. By increasing the electric field to around 0.0035 a.u., CO2 can be selectively captured on MoS2 monolayers from the residual CO2/N2 gas mixture. The study also demonstrates selective capture of above gases by controlling the strength of electric fields applied to MoS2 monolayers.
Conclusions
In summary, the CO2 capture and regeneration on the MoS2 surface without and with electric fields were theoretically investigated. The results indicate that the adsorption of CO2 on MoS2 can be drastically enhanced by applying electric fields. By turning off the electric field, CO2 can be released from the MoS2 surface without any barrier. In contrast, the applied electric fields can only slightly affect the interaction strength between N2 and MoS2 compared with that of CO2 and MoS2, which means that with the applied electric fields, MoS2 can be employed to efficiently separate CO2 from the CO2/N2 gas mixture (the main containment of post combustion gas). The study demonstrates a versatile approach to CO2 capture, regeneration and gas separation by controlling the strength of electric fields applied to the sorbents, which provides interesting and complementary information to experiments in search for advanced materials and technologies for CO2 capture and gas separation.Acknowledgements
Q. Sun acknowledges support from Jiangsu province (Grant No. BK20151215). Z. Li greatly appreciates support from the National Nature Science Foundation of China (Grant No. 81471657), the 1000 Plan for Young Talents and Jiangsu Specially-Appointed Professorship. This work is also supported by the National Natural Science Foundation of China (Grant No. 21303009, 21577076, 21303093).Notes and references
- D. W. Keith, Science, 2009, 325, 1654 CrossRef CAS PubMed.
- J. Meyer, Nature, 2008, 455, 733 CrossRef CAS.
- N. Stern, Review on the Economics of Climate Change, Cambridge University Press, Cambridge, 2006 Search PubMed.
- J. Y. Wang, L. Huang, R. Y. Yang, Z. Zhang, J. W. Wu, Y. S. Gao, Q. Wang, D. O'Hare and Z. Y. Zhong, Energy Environ. Sci., 2014, 7, 3478 CAS.
- Y. L. Luo, B. Y. Li, W. Wang, K. B. Wu and B. Tan, Adv. Mater., 2012, 24, 5703 CrossRef CAS PubMed.
- T. M. McDonald, J. A. Mason, X. Q. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocella, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. W. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303 CrossRef CAS PubMed.
- J. Zhou, Q. Wang, Q. Sun, P. Jena and X. S. Chen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 2801 CrossRef CAS PubMed.
- Q. Sun, Z. Li, D. J. Searles, Y. Chen, G. Lu and A. Du, J. Am. Chem. Soc., 2013, 135, 8246 CrossRef CAS PubMed.
- Q. Sun, C. Sun, A. Du and Z. Li, J. Phys. Chem. C, 2014, 118, 30006 CAS.
- Q. Sun, M. Wang, Z. Li, A. Du and D. J. Searles, J. Phys. Chem. C, 2014, 118, 2170 CAS.
- Q. Sun, M. Wang, Z. Li, A. Du and D. J. Searles, Phys. Chem. Chem. Phys., 2014, 16, 12695 RSC.
- Q. Sun, M. Wang, Z. Li, P. Li, W. Wang, X. Tan and A. Du, Fuel, 2013, 109, 575 CrossRef CAS.
- Q. Sun, M. Wang, Z. Li, Y. Ma and A. Du, Chem. Phys. Lett., 2013, 575, 59 CrossRef CAS.
- W. Wang, X. Zhang, P. Li, Q. Sun, Z. Li, C. Ren and C. Guo, J. Phys. Chem. A, 2015, 119, 796 CrossRef CAS PubMed.
- H. Guo, W. Zhang, N. Lu, Z. Zhuo, X. C. Zeng, X. Wu and J. Yang, J. Phys. Chem. C, 2015, 119, 6912 CAS.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed.
- W. Wu, L. Wang, Y. Li, F. Zhang, L. Lin, S. Niu, D. Chenet, X. Zhang, Y. Hao, T. F. Heinz, J. Hone and Z. L. Wang, Nature, 2014, 514, 470 CrossRef CAS PubMed.
- G. Fiori, F. Bonaccorso, G. Iannaccone, T. Palacios, D. Neumaier, A. Seabaugh, S. K. Banerjee and L. Colombo, Nat. Nanotechnol., 2014, 9, 768 CrossRef CAS PubMed.
- Y.-C. Lin, D. O. Dumcencon, Y.-S. Huang and K. Suenaga, Nat. Nanotechnol., 2014, 9, 391 CrossRef CAS PubMed.
- Y.-C. Lin, D. O. Dumcenco, H.-P. Komsa, Y. Niimi, A. V. Krasheninnikov, Y.-S. Huang and K. Suenaga, Adv. Mater., 2014, 26, 2857 CrossRef CAS PubMed.
- W. Yin, L. Yan, J. Yu, G. Tian, L. Zhou, X. Zheng, X. Zhang, Y. Yong, J. Li, Z. Gu and Y. Zhao, ACS Nano, 2014, 8, 6922 CrossRef CAS PubMed.
- C. Zhu, X. Mu, P. A. van Aken, Y. Yu and J. Maier, Angew. Chem., Int. Ed., 2014, 53, 2152 CrossRef CAS PubMed.
- S. Zhao, J. Xue and W. Kang, Chem. Phys. Lett., 2014, 595, 35 CrossRef.
- Q. Yue, Z. Shao, S. Chang and J. Li, Nanoscale Res. Lett., 2013, 8, 425 CrossRef PubMed.
- M. Asadi, B. Kumar, A. Behranginia, B. A. Rosen, A. Baskin, N. Repnin, D. Pisasale, P. Phillips, W. Zhu, R. Haasch, R. F. Klie, P. Kral, J. Abiade and A. Salehi-Khojin, Nat. Commun., 2014, 5, 4470 CAS.
- A. Achari, S. Sahana and M. Eswaramoorthy, Energy Environ. Sci., 2016, 9, 1224 CAS.
- M. P. K. Sahoo, J. Wang, Y. J. Zhang, T. Shimada and T. Kitamura, J. Phys. Chem. C, 2016, 120, 14113 CAS.
- S. J. Ray, Sens. Actuators, B, 2016, 222, 492 CrossRef CAS.
- H. X. Li, M. Huang and G. Y. Cao, Phys. Chem. Chem. Phys., 2016, 18, 15110 RSC.
- B. Delley, J. Chem. Phys., 1990, 92, 508 CrossRef CAS.
- B. Delley, J. Chem. Phys., 2000, 113, 7756 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787 CrossRef CAS PubMed.
- P. Pulay, J. Comput. Chem., 1982, 3, 556 CrossRef CAS.
- R. S. Mulliken, J. Chem. Phys., 1955, 23, 1833 CrossRef CAS.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294 CrossRef CAS PubMed.
- Q. Yue, S. Chang, J. Kang, X. Zhang, Z. Shao, S. Qin and J. Li, J. Phys.: Condens. Matter, 2012, 24, 335501 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr07001a |
| This journal is © The Royal Society of Chemistry 2017 |