Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle
Fitsum Feleke
Sahle
ab,
Michael
Giulbudagian
a,
Julian
Bergueiro
a,
Jürgen
Lademann†
*b and
Marcelo
Calderón†
*a
aInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Takustr. 3, 14195, Berlin, Germany. E-mail: marcelo.calderon@fu-berlin.de; Web: http://www.bcp.fu-berlin.de/chemie/calderon Fax: +49-30-838-459368; Tel: +49-30-83859368
bCenter of Experimental and Applied Cutaneous Physiology, Charité – Universitätsmedizin Berlin, Charitéplatz 1, 10117, Berlin, Germany. E-mail: info@ccp-berlin.org; Web: http://www.ccp-berlin.org/ Fax: +49-30-450518 918; Tel: +49-30-450518 235
First published on 18th November 2016
Abstract
Nanoparticles with a size of several hundred nanometers can effectively penetrate into the hair follicles and may serve as depots for controlled drug delivery. However, they can neither overcome the hair follicle barrier to reach the viable cells nor release the loaded drug adequately. On the other hand, small drug molecules cannot penetrate deep into the hair follicles. Thus, the most efficient way for drug delivery through the follicular route is to employ nanoparticles that can release the drug close to the target structure upon exposure to some external or internal stimuli. Accordingly, 100–700 nm sized thermoresponsive nanogels with a phase transition temperature of 32–37 °C were synthesized by the precipitation polymerization technique using N-isopropylacrylamide as a monomer, acrylated dendritic polyglycerol as a crosslinker, VA-044 as an initiator, and sodium dodecyl sulphate as a stabilizer. The follicular penetration of the indodicarbocyanine (IDCC) labeled nanogels into the hair follicles and the release of coumarin 6, which was loaded as a model drug, in the hair follicles were assessed ex vivo using porcine ear skin. Confocal laser scanning microscopy (CLSM) enabled independent tracking of the nanogels and the loaded dye, although it is not as precise and accurate as standard analytical methods. The results showed that, unlike smaller nanogels (<100 nm), medium and larger sized nanogels (300–500 nm) penetrated effectively into the hair follicles with penetration depths proportional to the nanogel size. The release of the loaded dye in the hair follicles increased significantly when the investigation on penetration was carried out above the cloud point temperature of the nanogels. The follicular penetration of the nanogels from the colloidal dispersion and a 2.5% hydroxyethyl cellulose gel was not significantly different.
1. Introduction
Nanoparticles have been extensively studied as advanced drug delivery devices due to their unique features, such as capability to protect therapeutic compounds, targeted delivery, increased bioavailability, versatility to control the release profile of loaded drugs and tunable surface properties.1,2 A number of studies also indicated that nanoparticles can enhance the penetration and permeation of drugs through the skin.3–5 However, the mechanism by which the nanoparticles enhance drug penetration through the stratum corneum is not well explained and the transfollicular pathway was mentioned as the major penetration pathway.6,7 Some papers also reported the penetration of nanoparticles into the deeper layers of a skin whose barrier is using microneedles or a razor blade,8,9 but not through the intact stratum corneum. A recent interesting report demonstrated that the use of a low frequency sonophoresis, which was utilized as a penetration enhancer, reduced the percutaneous penetration of drugs due to the partial plugging of the hair follicles.10On the other hand, studies indicated that nanoparticles with a size of several hundred nanometers penetrate effectively into the hair follicles and stay in the follicles for several days.11 This tendency of nanoparticles makes them good candidates for the sustained and extended delivery of drugs through the skin. The follicular route might also be used to target distinct cell populations such as stem cells (e.g. nestin expressing follicular bulge cells).12–14 However, the release of the encapsulated drug from the nanoparticles within the confined environment remains an issue11 and there must be a mechanism to trigger drug release to ensure the desired targeting and sustaining effect of nanoparticles intended for transfollicular drug delivery. As a result, the formulation of smart nanocarriers which respond to various stimuli such as temperature, pH, and ionic strength has recently been given due attention.
Thermoresponsive nanoparticles, such as thermoresponsive nanogels1,15,16 are mostly prepared by using thermoresponsive polymers that are capable of undergoing conformational changes from an extended/hydrophilic coil to a globular/hydrophobic state upon heating above a certain temperature known as the lower critical solution temperature (LCST).17 This behavior is assumed to be attributed to changes in intramolecular and intermolecular hydrogen bonding and hydrophobic interactions, and makes them attractive as smart tools in materials and biomedical sciences.18
Nanogels are loosely crosslinked polymeric chains that are arranged in a three-dimensional network. Thermoresponsive nanogels with great potential as drug delivery systems are commonly prepared from biocompatible hydrophilic thermoresponsive polymers such as poly(N-isopropylacrylamide) (PNIPAm), poly(glycidyl methyl ether-co-ethyl glycidyl ether), poly(N-vinylcaprolactam), poly(oligo(ethylene glycol)-methacrylate), and poly(N-dimethylacrylamide), which undergo reversible volume-phase transitions near the physiological temperature.15,18,19 Other promising nanogels based on oligo ethylene glycol as the thermoresponsive unit and dendritic polyglycerol (dPG) as a crosslinker are also reported.20–22
PNIPAm is a well-studied thermoresponsive polymer. Its LCST can be increased or decreased by using hydrophilic or hydrophobic comonomers and copolymers, respectively.18,19 Consequently, a number of PNIPAm-based thermoresponsive nanogels that were synthesized by using different crosslinkers and synthesis methods have been reported.5,16,19 dPG on the other hand is an efficient crosslinker which has good aqueous solubility, high biocompatibility and multi-functionality.5,23,24
The synthesis of 100–200 nm PNIPAm thermoresponsive nanogels prepared by using acrylated dendritic polyglycerol as a macro-crosslinker was reported by our group.5,16 These nanogels even showed promising results in enhancing the stability and permeation of proteins across the stratum corneum.5 Nonetheless, the nanogels are too small for optimal follicular penetration, while nanoparticles as big as 600 nm showed optimum penetration into the hair follicles.7,11 Therefore, the objective of this study was to synthesize and characterize thermoresponsive dPG-PNIPAm nanogels of different sizes using the precipitation polymerization method and evaluate their potential as follicular drug delivery devices.
2. Materials and methods
2.1. Materials
dPG (Nanopartica GmbH, Berlin, Germany), acryloyl chloride, triethylamine, dry dimethylformamide, N-isopropylacrylamide (NIPAm, 97%), sodium dodecyl sulphate (SDS, 98%), 2,2′-azobis[2-methyl-N-(2-hydroxyethyl)propionamide] (VA-086), 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (VA-044), 2-iminothiolane, coumarin 6, potassium persulfate, phosphotungstic acid (Sigma-Alrdrich, Schnelldorf, Germany), maleimide functionalized indodicarbocyanine (IDCC) dye (Lumiprobe GmbH, Hannover, Germany), adhesive solution (Marabu GmbH & Co. KG, Bietigheim-Bissingen, Germany), hydroxyethyl cellulose (HEC, Euro OTC Pharma GmbH, Bönen, Germany), cryo spray (Solidofix®-Cryo spray, Carl Roth GmbH + C0. KG., Karlsruhe, Germany), and tissue freezing medium (Leica Biosystems Nussloch GmbH, Nussloch, Germany) were used as received. Milli-Q water was used throughout.2.2. Methods
3. Results and discussion
3.1. Synthesis and characterization of dPG-PNIPAm nanogels
The degree of acrylation and molecular weight of dPG affect the nanogel size and various other nanogel characteristics.16,20 In our case, better results were obtained when 10 kDa dPG, with 9% of its hydroxyl groups acrylated, was used as a crosslinker and SDS was used as a stabilizer. The use of different initiators, namely potassium persulfate and the water soluble azo initiators VA-086 and VA-044, was considered and larger nanogels were obtained with the azo initiators. Similar results were also reported, where the azo initiator azobisisobutyronitrile gave significantly bigger styrene nanoparticles than potassium persulfate.27 VA-044 was chosen for the synthesis of the intended nanogels because it can be activated at a relatively lower temperature than VA-086, without the need for UV light irradiation.
NG-1 to NG-6 (Table 1) were synthesized to investigate the effects of the crosslinker percentage and initiator concentration on the particle size and Tcp. The particle size increased significantly with decreasing the percentage of the crosslinker (Fig. 1a). This can be attributed to the formation of a more compact and smaller nanogel due to the high degree of crosslinking.16 However, for further investigations, the crosslinker concentration was maintained at or above 20% as the percentage of the crosslinker affected the Tcp (Fig. 3b) and other properties of the nanogels.
 | ||
| Fig. 1 Effect of the (a) percentage of the crosslinker and (b) initiator concentration on the nanogel size (labels represent the corresponding PDI values). | ||
| Nanogel | NIPAm + dPG-Ac (mg mL−1) | Wt% of dPG-Ac in feed precursor | VA-044 (wt%) | Yield ± SD (wt%) |
|---|---|---|---|---|
| NG-1 | 40 | 33 | 1 | 83.9 (1.3) |
| NG-2 | 40 | 20 | 1 | 88.1 (1.9) |
| NG-3 | 40 | 10 | 1 | 87.3 (3.3) |
| NG-4 | 40 | 33 | 0.5 | 85.8 (1.3) |
| NG-5 | 40 | 33 | 2 | 82.9 (2.7) |
| NG-6 | 40 | 33 | 3 | 84.5 (2.1) |
| NG-7 | 20 | 20 | 2 | 90.3 (2.0) |
| NG-8 | 40 | 20 | 2 | 86.1 (1.3) |
| NG-9 | 46 | 20 | 2 | 86.4 (2.5) |
| NG-10 | 49 | 20 | 2 | 86.5 (2.5) |
| NG-11 | 52 | 20 | 2 | 85.1 (3.1) |
| NG-12 | 20 | 20 | 3 | 92.1 (1.5) |
| NG-13 | 40 | 20 | 3 | 87.4 (2.0) |
| NG-14 | 46 | 20 | 3 | 86.3 (1.3) |
| NG-15 | 49 | 20 | 3 | 87.6 (1.4) |
| NG-16 | 52 | 20 | 3 | 87.1 (1.1) |
Mostly, increasing the initiator concentration results in increased primary radicals which in turn results in an increased particle number and reduced particle size.29 Surprisingly, in this case, the particle size increased significantly with increasing initiator concentration (Fig. 1b). The results in Fig. 2a and b also showed a similar effect where significantly larger nanogels were obtained at 3% VA-044 than at 2%. Factors like multiple polymer branching due to chain transfer or self-destruction of the primary radicals might contribute to this. However, considering the magnitude of the change in particle size, the more plausible explanation would be an occurrence of the coagulation of primary particles to form bigger nanogels during the polymerization process. That is, the increased initiator concentration may increase the primary radicals and primary particle number, which further enhances the extent of particle coagulation. The same effect was observed when the water soluble initiators KPS and AIBA were used for the synthesis of styrene polymer nanoparticles using the emulsion polymerization technique.27 Thus, further investigations, including the determination of the change in the particle number as a function of monomer conversion, should be conducted for a clear understanding of the process.27
NG-8 (2% initiator) and NG-13 (3% initiator) were synthesized at 5 different concentrations of SDS (2, 3, 4, 5 and 6 mM) to investigate the effect of the surfactant concentration on the particle size (Fig. 2a) and Tcp (Fig. 3). The particle size decreased significantly with increasing concentration of the surfactant. This is expected as a higher concentration of the surfactant has a better stabilization effect by forming smaller pockets, in which the insoluble polymer is accommodated.30 However, at a higher concentration of the precursor a lower SDS concentration resulted in significantly higher polydispersity (results not shown), and further synthesis of the nanogels was carried out at 6 mM SDS.
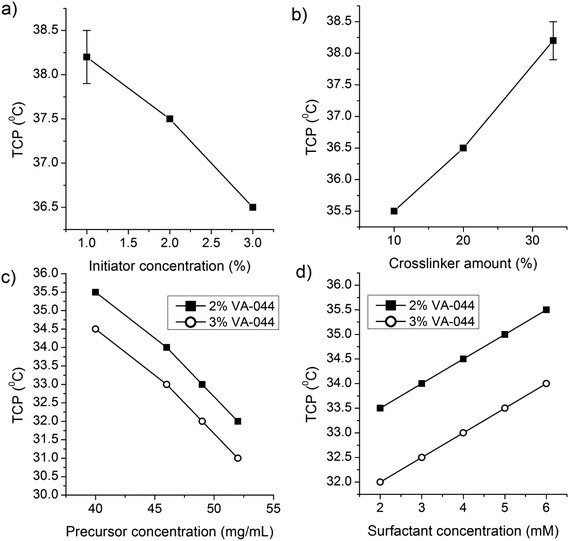 | ||
| Fig. 3 Effects of the (a) initiator, (b) crosslinker, (c) precursor, and (d) surfactant concentrations on the volume phase transition temperature of dPG-PNIPAm nanogels. | ||
NG-7 to NG-16 were synthesized at 50 and 68 °C to investigate the effect of the precursor concentration and reaction temperature on the particle size. The nanogel size increased significantly with increasing precursor concentration and reaction temperature (Fig. 2b). Above a precursor concentration of 40 mg mL−1, the increase in size was exponential and above 60 mg mL−1, the nanogels aggregated to form a gel at all levels of the initiator concentrations and reaction temperatures considered. In principle, increasing the initiation temperature should also increase the primary radicals and particle number and tends to result in a reduced particle size.29 Thus, the increase in particle size as a function of temperature is also a significant indicator of particle coagulation to form bigger nanogels. This is also supported by a recent finding by Liu et al.27 The nanogels also had acceptable PDI values with acceptable standard deviations (Fig. 2). Therefore, at a high temperature of the synthesis and precursor and initiator concentrations, nanogels of the desired sizes of 600–700 nm, with an acceptable level of polydispersity, were obtained. The yield was also relatively high (82.9–92.1) (Table 1).
Representative Tcp values for small and large nanogels are shown in Fig. 4a. Generally, at a given SDS concentration, factors that had a positive effect on the particle size had a negative effect on Tcp. Consequently, bigger nanogels resulted in lower Tcp and vice versa. The Tcp value of the different nanogels was plotted against their size (Fig. 4b) and the same relationship was observed.
The TEM images of NG-15 and NG-12 (Fig. 5a and c) were by about 3–5 factors smaller than the hydrodynamic diameters of the nanogels obtained by dynamic light scattering (NG-15: 576.8 ± 52.7 nm; PDI = 0.229 ± 0.065 and NG-12: 128.2 ± 3.5 nm; PDI = 0.157 ± 0.010). Water contributes to a significant proportion of the nanogels’ mass and shrinking of the nanogels in TEM can be attributed to the drying process during sample preparation.31,32 However, irrespective of the volume contraction, the difference in size between the bigger (Fig. 5a) and smaller (Fig. 5c) nanogels was apparent.
 | ||
| Fig. 5 Different microscopy images of selected nanogels obtained at two different magnifications: (a) TEM and (b) AFM images of NG-15, (c) TEM and (d) AFM images of NG-12. | ||
The AFM images of NG 15 and NG-12 are also shown in Fig. 5b and d, respectively, and the average, minimum and maximum sizes of the nanogels are given in Table 2. Compared to TEM, relatively larger nanogels were obtained with AFM, which can be attributed to the minimal degree of water removal with AFM compared to TEM. Interestingly, the minimum sized nanogels obtained with NG-15, which was prepared at a higher concentration of the initiator, were smaller than the minimum sized nanogels obtained with NG-12 and the results again substantiate that a high degree of agglomeration of the primary particles occurred at a higher concentration of the initiator and feed concentration. In addition, the average nanogel sizes obtained with AFM are still smaller than the hydrodynamic radius obtained by DLS and this can partly be attributed to the differences in the methods and partly to the partial drying of the sample during the preparation for the AFM analysis.
| Nanogel | Mean size (nm) | Minimum size (nm) | Maximum size (nm) |
|---|---|---|---|
| NG-12 | 118.3 | 68.7 | 172.3 |
| NG-15 | 165.6 | 33.7 | 564.0 |
Besides, both TEM and AFM images showed that the bigger the particle size the more irregular was the nanogel. It can also be taken as evidence that significant agglomeration of the primary particles occurred during the polymerization process to form bigger nanogels. The low zeta potential of the nanoparticles (Table 3) also substantiates the possibility of agglomeration of the primary nanoparticles.
| Formulation | Reaction temp. (°C) | Zeta potential (mV) in H2O | Zeta potential (mV) in phosphate buffer | ||
|---|---|---|---|---|---|
| 25 °C | 45 °C | 25 °C | 45 °C | ||
| NG-15 | 68 | 2.69 | −3.38 | −0.0493 | −0.248 |
| NG-15 | 50 | 1.65 | −4.42 | −0.609 | −0.995 |
| NG-12 | 68 | −0.661 | −9.76 | −1.89 | −4.45 |
| NG-12 | 50 | −1.28 | −9.72 | −2.56 | −4.03 |
3.2. Ex vivo follicular penetration study
The nanogels were labeled with IDCC (λmax excitation = 650 nm) prior to the experiment to enable their independent tracking from the loaded dye coumarin 6 (λmax excitation = 444 nm). Three different sizes of the labeled nanogels were synthesized and characterized (L-76, L-396 and L-508, Table 4) to investigate the effect of the nanogel size on the depth of follicular penetration. L-508 was also incorporated into a 2.5% HEC gel to assess the effect of the formulation of the nanogels into the final dosage form on the follicular penetration of the nanogels.
| No | Nanogel | NIPAM + 20% crosslinkera (mg mL−1) | VA-044 (wt%) | SDS (mM) | Size (±SD) (nm) | PDI (±SD) | T cp (±SD) (°C) |
|---|---|---|---|---|---|---|---|
a IDCC labeled dPG-Ac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dPG-Ac = 1 dPG-Ac = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3.
|
|||||||
| 1 | L-76 | 20 | 2 | 6 | 76.7 (3.4) | 0.255 (0.016) | 36.0 (0.0) |
| 2 | L-396 | 46 | 2 | 6 | 396.3 (3.7) | 0.316 (0.037) | 34.3 (0.3) |
| 3 | L-508 | 49 | 3 | 6 | 508.9 (39.2) | 0.332 (0.043) | 34.0 (0.0) |
The PDI values of the labeled nanogels were slightly higher than their non-labelled equivalents. This is assumed to occur due to the dye effect on the crosslinker hydrophilicity during the precipitation polymerization process. The TEM image of L-508 (Fig. 6) indicated particle shrinkage due to water loss.
Typical CLSM fluorescence emission images of the dye and the nanogel (L-508), which were taken independently but simultaneously, are shown in Fig. 7a and b, respectively. The light transmission image of the histological section was also obtained (Fig. 7c). Fig. 7d represents the superimposed images of the three and shows that the penetration depth of the nanogel (shown by the small arrow drawn perpendicular to the hair follicle) was different from the dye (shown by the line drawn parallel to the hair follicle). Fig. 7i to iv show the change in the fluorescence emission intensities of coumarin 6 and the nanogel as a function of the follicular penetration depth. Accordingly, the effect of various factors on the nanogel penetration depth and dye release was assessed.
The follicular penetration depth of the nanogels significantly increased with nanogel size. This is in line with the previous reports where optimal penetration of nanoparticles into the hair follicles was attained with particles sized at about 600 nm.11 However, L-76 (76 nm in diameter) failed to penetrate to any appreciable extent. In addition, the penetration depth of L-396 and L-508 was relatively shallow when compared with the previously reported solid nanoparticles of similar sizes.11 These phenomena can be attributed to the significant shrinking of the nanogels due to rapid water loss during application. The TEM and AFM images also confirmed the shrinking of the nanoparticles due to water loss. Therefore, unlike other compact nanoparticles, the penetration depth of nanogels could depend on the water evaporation kinetics and, to compensate for the volume contraction, it might be advantageous to synthesize even bigger nanogels for deeper follicular penetrations.
Apart from the results shown in Fig. 8, representative histological sections showing the effect of incubation temperature and time on nanogel and dye penetration are shown in Fig. 9 (green and red designate the dye and the nanogel and the parallel and perpendicular lines running along the hair follicle designate the depth of penetration of the dye and the nanogel, respectively). As can be seen from the two figures, there was no significant difference in nanogel penetration and dye release at RT and 35 °C after 1 h of incubation and the dye and the nanogel traveled almost the same distance showing the lack of significant dye release. In principle, the Tcp of the polymer was determined to be 34 °C and above this temperature a significant increase in dye release and diffusion deep into in the hair follicle was expected. However, Tcp is a temperature at which only 50% of the polymer responds to the change in temperature in a very dilute colloidal dispersion and the temperature at which all the particles respond is higher (Fig. 4a). Interestingly, unlike nanogel penetration, dye release and penetration increased significantly at 37 °C or when the incubation time was increased to 4 h. At 37 °C, increasing the incubation time to 4 h further increased the dye release and penetration significantly (2× the penetration at 35 °C and 1 h). This is attributed to the thermoresponsive nature of the nanogel. Thus, the temperature deep in the hair follicle is expected to be close to the body temperature of 37 °C, and a significant drug release is expected from the nanogels in vivo.
Looking at nanogel penetration from another perspective, above the Tcp, theoretically, the penetration depth of the nanogel into the hair follicle should decrease due to particle shrinking. However, interestingly, nanogel penetration at RT and 35 °C, and even at 37 °C when only incubated for 1 h, was not significantly different (Fig. 8). This is most likely attributed to the rapid penetration of the nanogels into the hair follicles, which need some seconds to few minutes,14,40 before undergoing any significant phase transition. This might also be attributed to the tendency of the nanogels to aggregate above their transition temperature to form bigger aggregates. Thus, a better understanding of the follicular penetration needs careful investigations of the nanogel penetration, shrinking and aggregation kinetics.
Generally, semisolid formulations are preferred for applications to the skin and one way of preparing nanogels into a semisolid dosage form is by formulating them into gels using gelling agents. Thus, the effect of the incorporation of the gelling agent HEC (2.5%) into the nanogels on their follicular penetration was investigated (Fig. 8), and no significant differences in nanogel penetration or dye release were observed.
The CLSM images also enabled the visualization of the distribution of the labeled nanogel and the loaded dye on the skin surface and showed that the nanogels and the loaded dye did not penetrate through the skin surface. Although CLSM clearly showed that the main penetration pathway for the nanogels is through the hair follicle, it is a semi-quantitative method and it is difficult to exactly quantify the amount of drug that penetrated at different depths of the skin. Thus, this method should be complemented with other sensitive, precise and accurate analytical methods if quantification of the amount of drug at different depths of the skin is required.
4. Conclusions
Various sizes of dPG-NIPAm based thermoresponsive nanogels that are as big as 600–700 nm were synthesized by the precipitation polymerization technique, by controlling the various synthetic conditions. The nanogels exhibited Tcp values of 32–37 °C, which are ideal for skin applications. Ex vivo follicular penetration investigations showed that the depth of nanogel penetration was proportional to their sizes. Temperature dependent dye release from the thermoresponsive nanogels in the hair follicle was also investigated ex vivo for the first time and there was a significant increase in dye release above the Tcp of the nanogels. Interestingly, the formulation of the nanogels into gels did not affect their follicular penetration.Abbreviations
| dPG-Ac | Acrylated dendritic polyglycerol |
| AFM | Atomic force microscopy |
| T cp | Cloud point temperature |
| CLSM | Confocal laser scanning microscope |
| dPG | Dendritic polyglycerol |
| HEC | Hydroxyethyl cellulose |
| NIPAm | N-Isopropylacrylamide |
| PNIPAm | Poly N-isopropylacrylamide |
| SDS | Sodium dodecyl sulphate |
| TEM | Transmission electron microscopy |
Acknowledgements
This work has been partly supported by the collaborative research center 1112 (http://www.sfb1112.de), Projects A04 and C05 of the DFG. We greatly acknowledge the financial support provided to Fitsum F. Sahle by the Alexander von Humboldt Foundation. Marcelo Calderón also acknowledges the Bundesministerium für Bildung und Forschung (BMBF) through the NanoMatFutur award (13N12561, Thermonanogele). Julian Bergueiro acknowledges the Dahlem Research Center for a Dahlem International Network PostDocs fellowship. We would also like to gratefully acknowledge Dr Alexa Patzelt for her consultation on some aspects of the work, Heike Richter for her technical support, and Dr Fanny Knorr for proofreading the manuscript.References
- A. Rahikkala, V. Aseyev, H. Tenhu, E. I. Kauppinen and J. Raula, Biomacromolecules, 2015, 16, 2750–2756 CrossRef CAS PubMed.
- J.-W. Yoo, N. Giri and C. H. Lee, Int. J. Pharm., 2011, 403, 262–267 CrossRef CAS PubMed.
- J. Shim, H. S. Kang, W. S. Park, S. H. Han, J. Kim and I. S. Chang, J. Controlled Release, 2004, 97, 477–484 CrossRef CAS PubMed.
- M. Schafer-Korting, W. Mehnert and H. C. Korting, Adv. Drug Delivery Rev., 2007, 59, 427–443 CrossRef PubMed.
- M. Witting, M. Molina, K. Obst, R. Plank, K. M. Eckl, H. C. Hennies, M. Calderon, W. Friess and S. Hedtrich, Nanomedicine, 2015, 11, 1179–1187 CrossRef CAS PubMed.
- T. Subongkot, N. Wonglertnirant, P. Songprakhon, T. Rojanarata, P. Opanasopit and T. Ngawhirunpat, Int. J. Pharm., 2013, 441, 151–161 CrossRef CAS PubMed.
- M. Schneider, F. Stracke, S. Hansen and U. F. Schaefer, Derm.-Endocrinol., 2009, 1, 197–206 CrossRef CAS.
- S. A. Coulman, A. Anstey, C. Gateley, A. Morrissey, P. McLoughlin, C. Allender and J. C. Birchall, Int. J. Pharm., 2009, 366, 190–200 CrossRef CAS PubMed.
- E. Kimura, Y. Kawano, H. Todo, Y. Ikarashi and K. Sugibayashi, Biol. Pharm. Bull., 2012, 35, 1476–1486 Search PubMed.
- W. Rangsimawong, P. Opanasopit, T. Rojanarata and T. Ngawhirunpat, Int. J. Nanomed., 2015, 10, 7413–7423 CAS.
- J. Lademann, F. Knorr, H. Richter, S. Jung, M. C. Meinke, E. Rühl, U. Alexiev, M. Calderon and A. Patzelt, J. Innovative Opt. Health Sci., 2015, 08, 1530004 CrossRef CAS.
- J. Lademann, N. Otberg, U. Jacobi, R. M. Hoffman and U. Blume-Peytavi, J. Invest. Dermatol. Symp. Proc., 2005, 10, 301–303 CrossRef PubMed.
- A. Vogt, N. Mandt, J. Lademann, H. Schaefer and U. Blume-Peytavi, J. Invest. Dermatol. Symp. Proc., 2005, 10, 252–255 CrossRef PubMed.
- C.-L. Fang, I. A. Aljuffali, Y.-C. Li and J.-Y. Fang, Ther. Delivery, 2014, 5, 991–1006 CrossRef CAS PubMed.
- J. Ramos, A. Imaz, J. Callejas-Fernandez, L. Barbosa-Barros, J. Estelrich, M. Quesada-Perez and J. Forcada, Soft Matter, 2011, 7, 5067–5082 RSC.
- J. C. Cuggino, C. I. Alvarez, M. C. Strumia, P. Welker, K. Licha, D. Steinhilber, R.-C. Mutihac and M. Calderon, Soft Matter, 2011, 7, 11259–11266 RSC.
- J. Bergueiro and M. Calderon, Macromol. Biosci., 2015, 15, 183–199 CrossRef CAS PubMed.
- I. Yildiz and B. S. Yildiz, J. Nanomater., 2015, 2015, 1–12 CrossRef.
- M. A. Ward and T. K. Georgiou, Polymers, 2011, 3, 1215–1242 CrossRef CAS.
- M. Asadian-Birjand, J. Bergueiro, F. Rancan, J. C. Cuggino, R. C. Mutihac, K. Achazi, J. Dernedde, U. Blume-Peytayi, A. Vogt and M. Calderon, Polym. Chem., 2015, 6, 5827–5831 RSC.
- F. Rancan, M. Asadian-Birjand, S. Dogan, C. Graf, L. Cuellar, S. Lommatzsch, U. Blume-Peytavi, M. Calderón and A. Vogt, J. Controlled Release, 2016, 228, 159–169 CrossRef CAS PubMed.
- M. Giulbudagian, F. Rancan, A. Klossek, K. Yamamoto, J. Jurisch, V. Colombo-Neto, P. Schrade, S. Bachmann, E. Rühl, U. Blume-Peytavi, A. Vogt and M. Calderón, J. Controlled Release, 2016, 243, 323–332 CrossRef CAS PubMed.
- M. Calderón, M. A. Quadir, S. K. Sharma and R. Haag, Adv. Mater., 2010, 22, 190–218 CrossRef PubMed.
- D. Wilms, S.-E. Stiriba and H. Frey, Acc. Chem. Res., 2010, 43, 129–141 CrossRef CAS PubMed.
- R. Haag, H. Tuerk and S. Mecking, German Patent DE10211664, 2003 Search PubMed.
- F. S. Mehrabadi, O. Hirsch, R. Zeisig, P. Posocco, E. Laurini, S. Pricl, R. Haag, W. Kemmner and M. Calderon, RSC Adv., 2015, 5, 78760–78770 RSC.
- B. Liu, Y. Wang, M. Zhang and H. Zhang, Polymers, 2016, 8, 55 CrossRef.
- T. Still, K. Chen, A. M. Alsayed, K. B. Aptowicz and A. G. Yodh, J. Colloid Interface Sci., 2013, 405, 96–102 CrossRef CAS PubMed.
- I. Capek and P. Potisk, Eur. Polym. J., 1995, 31, 1269–1277 CrossRef CAS.
- W. H. Blackburn and L. A. Lyon, Colloid Polym. Sci., 2008, 286, 563–569 Search PubMed.
- L.-W. Xia, R. Xie, X.-J. Ju, W. Wang, Q. Chen and L.-Y. Chu, Nat. Commun., 2013, 4, 2226 Search PubMed.
- K. Zielinska, H. Sun, R. A. Campbell, A. Zarbakhsh and M. Resmini, Nanoscale, 2016, 8, 4951–4960 RSC.
- J. Lademann, A. Patzelt, H. Richter, S. Schanzer, W. Sterry, A. Filbry, K. Bohnsack, F. Rippke and M. Meinke, Eur. J. Pharm. Biopharm., 2009, 72, 600–604 CrossRef CAS PubMed.
- G. A. Simon and H. I. Maibach, Skin Pharmacol. Appl. Skin Physiol., 2000, 13, 229–234 CrossRef CAS PubMed.
- I. Boudry, O. Blanck, C. Cruz, M. Blanck, V. Vallet, A. Bazire, A. Capt, D. Josse and G. Lallement, J. Appl. Toxicol., 2008, 28, 645–657 CrossRef CAS PubMed.
- F. P. Schmook, J. G. Meingassner and A. Billich, Int. J. Pharm., 2001, 215, 51–56 CrossRef CAS PubMed.
- A. M. Barbero and H. F. Frasch, Toxicol In Vitro, 2009, 23, 1–13 CrossRef CAS PubMed.
- E. C. Jung and H. I. Maibach, J. Appl. Toxicol., 2015, 35, 1–10 CrossRef CAS PubMed.
- B. Godin and E. Touitou, Adv. Drug Delivery Rev., 2007, 59, 1152–1161 CrossRef CAS PubMed.
- J. Lademann, F. Knorr, H. Richter, S. Jung, M. C. Meinke, E. Rühl, U. Alexiev, M. Calderon and A. Patzelt, J. Innovative Opt. Health Sci., 2015, 08, 1530004 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |






