Triterpenoids
Robert A.
Hill
* and
Joseph D.
Connolly
School of Chemistry, Glasgow University, Glasgow, G12 8QQ, UK. E-mail: bob.hill@glasgow.ac.uk
First published on 4th November 2016
Abstract
Covering: 2013. Previous review: Nat. Prod. Rep., 2015, 29, 1028–1065
This review covers the isolation and structure determination of triterpenoids reported during 2013 including squalene derivatives, lanostanes, holostanes, cycloartanes, cucurbitanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, quassinoids, lupanes, oleananes, friedelanes, ursanes, hopanes, serratanes, isomalabaricanes and saponins; 350 references are cited.
1. Introduction
The anticancer activities of triterpenoids and their saponins have been extensively reviewed.1–8 Other activities such as the antidiabetic properties9,10 and neuropharmacological effects11 of triterpenoids have also been covered. Surveys of triterpenoids from Albizia,12Aphanamixis,13Boswellia,14Calendula,15Clinopodium,16Lonicera,17Schefflera,18 and Toona19 species and Argania spinosa,20Olea europea,21 and Platycodon grandiflorum,22 have appeared. There is interest in the production of triterpenoids with cell and tissue cultures23 and enhancing saponin production by cell and gene engineering.24 The biological roles of triterpenoid saponins in plants have been reviewed.252. The squalene group
The synthesis of armatol A, from the red alga Chondria armata, has led to the revision of the relative and absolute configuration to 1.26 The configurations of the other compounds in this series, armatols B–F have also been revised and a biosynthetic pathway to the armatols from squalene has been proposed.3. The lanostane group
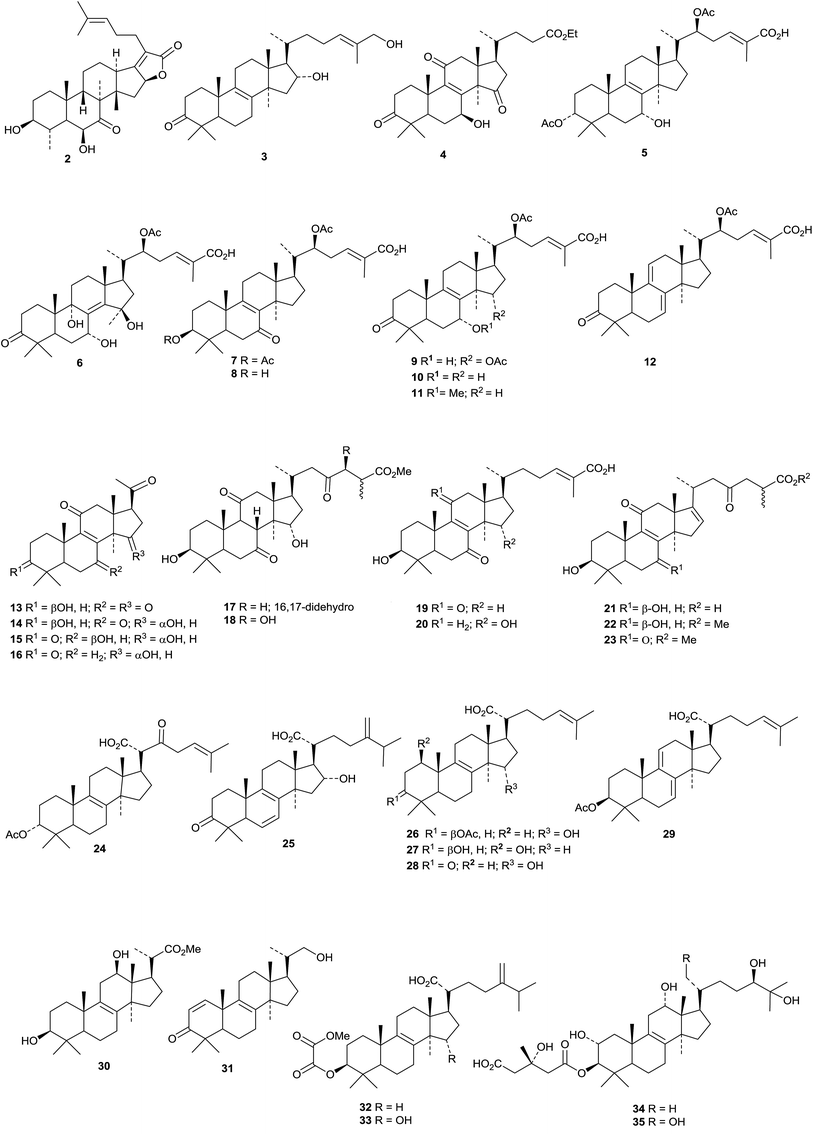
Streptoseolactone 2, a protostane derivative from the marine-derived actinomycete Streptomyces seoulensis, shows neuraminidase inhibition activity.27 The structures and biological activities of natural protostane and fusidane triterpenoids have been reviewed.28The following lanostane derivatives have been reported from Ganoderma species: 16α,26-dihydroxylanosta-8,24E-dien-3-one 3 from the fruiting bodies of Ganoderma hainanense,29 ethyl lucidenate A 4 (ref. 30) and the ganoderic acid 5 (ref. 31) from Ganoderma lucidum, ganorbiformins A 6–G 12 from cultures of Ganoderma orbiforme,32 lucidones D 13–G 16, ganoderesins A 17 and B 18 and 7-oxoganoderic acids Z219 and Z320 from Ganoderma resinaceum,33 the related compounds 21–23 from the fruiting bodies of Ganoderma tropicum34 and tsugaric acids D 24 and E 25 from Ganoderma tsugae.35 Three new lanostanes 26–28 have been isolated from the fungus Ceriporia lacerata (associated with Acanthaster planci).36 The lanostane 26 has also been isolated from another strain of Ceriporia lacerata (an endophytic fungus of Huperzia serrata) together with compound 29.37 Other lanostane derivatives include the two pentanorlanostanes curvalarols A 30 and B 31 from the soil fungus Curvularia borreriae,38 the oxalate esters 32 and 33 from the fungus Perenniporia maackiae,39 fasciculols J 34–M 37 from the mushroom Naematoloma fasciculare40 and terresterol 38 from the oomycete Saprolegnia terrestris.41 15-O-Acetylganolucidate A 39 and the tetraketo-acid 40 have been isolated from Antrodia camphorata together with the ethyl esters of the known lucidenic acids A and F.42
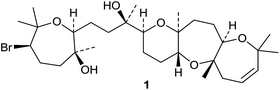
The structure 41, originally proposed for a lanostane from Wyethia mollis, has been revised to 42 on the basis of an X-ray crystallographic analysis.43 Other simple lanostanes include klainedoxalanostenone 43 from the stem bark of Klainedoxa gabonensis,44 moruslanosteryl acetate 44 from the stem bark of Morus alba,45 conyzagenins A 45 and B 46 from Conyza canadensis,46 the spiroacetals yunnanterpenes D 47 and E 48 from Cimicifuga yunnanensis47 and the unusual 3β-hydroxylanosta-8,17(20)-diene-22-carboxylic acid 49 from a crown gall induced on Eucalyptus tereticornis.48 Structure 50 has been proposed for turpetholanostenol from the roots of Operculina turpethum.49 Two other compounds, 51 and 52, from this source, have been assigned unlikely structures with a Δ5-7α,3α-hemiacetal. Further rearranged lanostane derivatives have been isolated from Abies species. These include compounds 53–58 from Abies sibirica and the unrearranged lanostanes 59,50 the methyl ester 60 of the known abiesonic acid and the E-isomer 61 of sibiric acid, together with the ring-A cleaved derivative 62, from the oleoresin of Abies balsamea.51 The friedolanostane (mariesane) derivatives garcihombronanes K 63 and L 64 have been found in the twigs of Garcinia hombroniana.52 Omphalocarpoidone 65 is a constituent of the wood of Tridesmostemon omphalocarpoides.53 Scillanostasides H–L are new glycosides from the bulbs of Scilla scilloides.54 Scillanostasides I and J have the new genin 66.
The 29-norlanostane derivatives 29-norpenasterone 67 and 68 have been isolated from a marine sponge of the genus Penares together with the lanostanes 69–72.55 The structure of the 29-nor compound 68 was confirmed by X-ray crystallographic analysis. Urabosides A and B and ulososide F are new glycosides from the marine sponge Ectyoplasia ferox.56 Urabosides A and B have the new 30-norlanostane genins 73 and 74, respectively. The genin 75 of ulososide F is known but its side chain stereochemistry has been revised. A glycosyl ester from Artemisia absinthium has the new genin 76.57
Several new sea cucumber holostane glycosides have been isolated. Typicosides A1, A2, B1, C1 and C2 are from Actinocucumis typica.58 Typicoside A1 has a known genin while the others have the new genins 77 (C1) and 78 (A2, B and C2). Cladolosides B1, B2, C, C1, C2 and D from Cladolabes schmeltzii have the new genins 79 (B2, C and D) and 80 (B1 and C1) while C2 has a known genin.59 Cucumariosides I1, I3 and I4 (ref. 60) and I2 (ref. 61) are from Eupentacta fraudatrix. Only cucumarioside I4 has a new genin 81 with a pentanor side chain. Turquetoside A from Staurocucumis turqueti62 and eleganoside A from Gelsemium elegans63 have known genins.
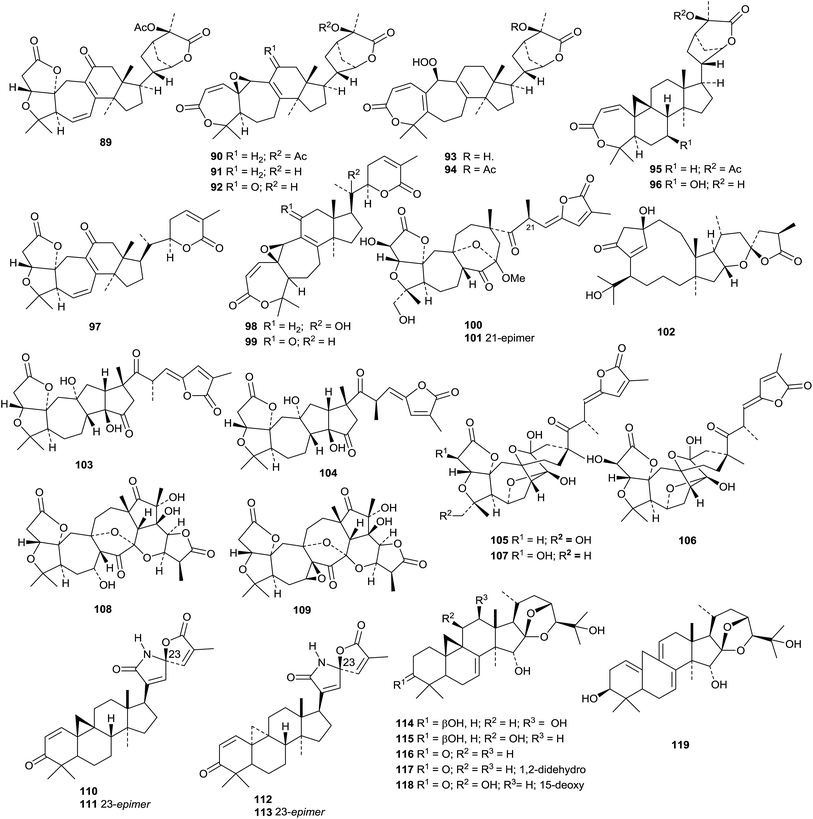
Several new skeletal types have been reported from Schisandra and related species. Lancolides A 82–D 85, from Schisandra lancifolia, are accompanied by preschisanartanin O 86.64 The structures of 82, 83, 85 and 86 were all confirmed by X-ray crystallographic analyses. The related schilancidilactones V 87 and W 88 are from the fruit of Schisandra wilsoniana.65 Schinchinenins A 89–H 96 and schinchinenlactones A 97–C 99 have been reported from the leaves and stems of Schisandra chinensis.66 The structure of schinchinenin A 89 was confirmed by X-ray crystallographic analysis. Further constituents from the stems of Schisandra chinensis include schisdilactones H 100 and I 101.67 Pseudolarenone 102, from Pseudolarix amabilis, has an unusual bicyclo[8.2.1]tridecane core.68 Other new Schisandra compounds include schisarisanlactones A 103 and B 104 from the fruit of Schisandra arisanensis69 and schicagenins D 105–F 107 and negleschidilactones A 108 and B 109 from the stems of Schisandra neglecta.70
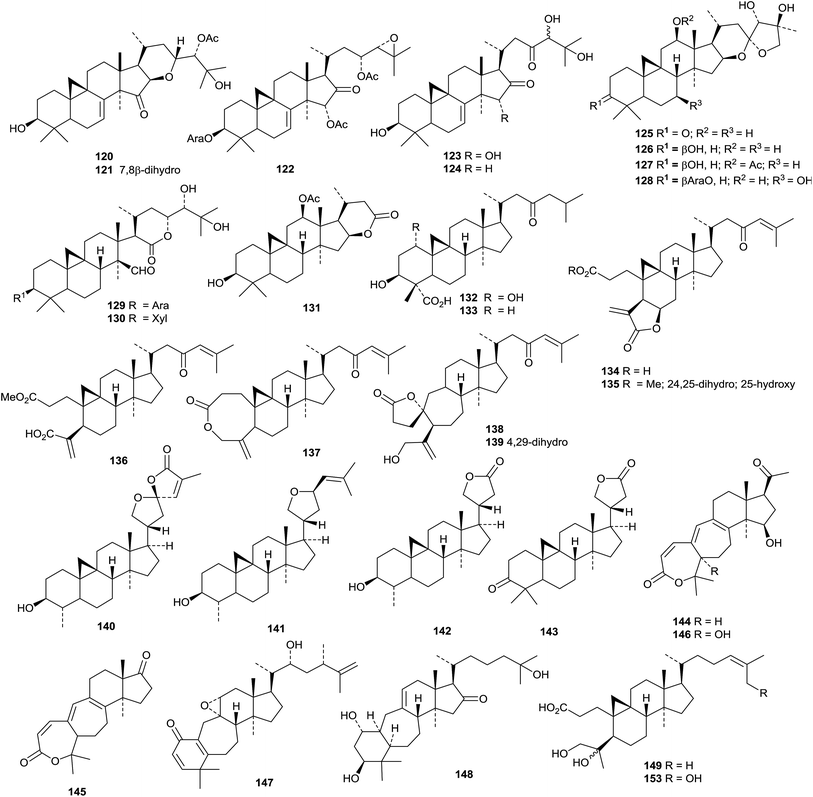
Kleinhospitines A 110–D 113, cycloartanes with spiro-nitrogen containing side chains, have been isolated from Kleinhovia hospita.71 The Chinese medicine Shengma (Cimicifuga dahurica) is a rich source of cimigenol derivatives and related cycloartanes 114–122.72 Only three of the new saponins, heracleifolinosides A–F, from Cimicifuga heracleifolia, have new genins 123 (A and C) and 124. (B).73 The aerial parts of Cimicifuga yunnanensis produce an interesting range of metabolites which include cycloartanes yunnanterpenes A 125–C 127 and F 128, the cleaved cycloartanes 15,16-secocimiterpenes A 129 and B 130 and the tetranor-derivative cimilactone C 131.47 The structures of yunnanterpene A 125 and 15,16-secocimiterpene A 129 were confirmed by X-ray crystallographic analyses. Carinatins A 132–H 139 are new compounds from the leaves and twigs of Gardenia carinata.74Aphanamixis grandifolia is the source of aphagrandinoids A 140–D 143.75
New 9,10-cleaved cycloartanes include cattienoids A 144–C 146 from the mushroom Tomophagus cattienensis,76 balansinone 147 from the leaves and twigs of Casearia balansae77 and compound 148 from the goat willow Salix caprea.78 Seven new cycloartane derivatives are reported from Gardenia gummifera resin with the trivial names gummiferartanes 1 149–5 153, 8 154 and 9 155.79
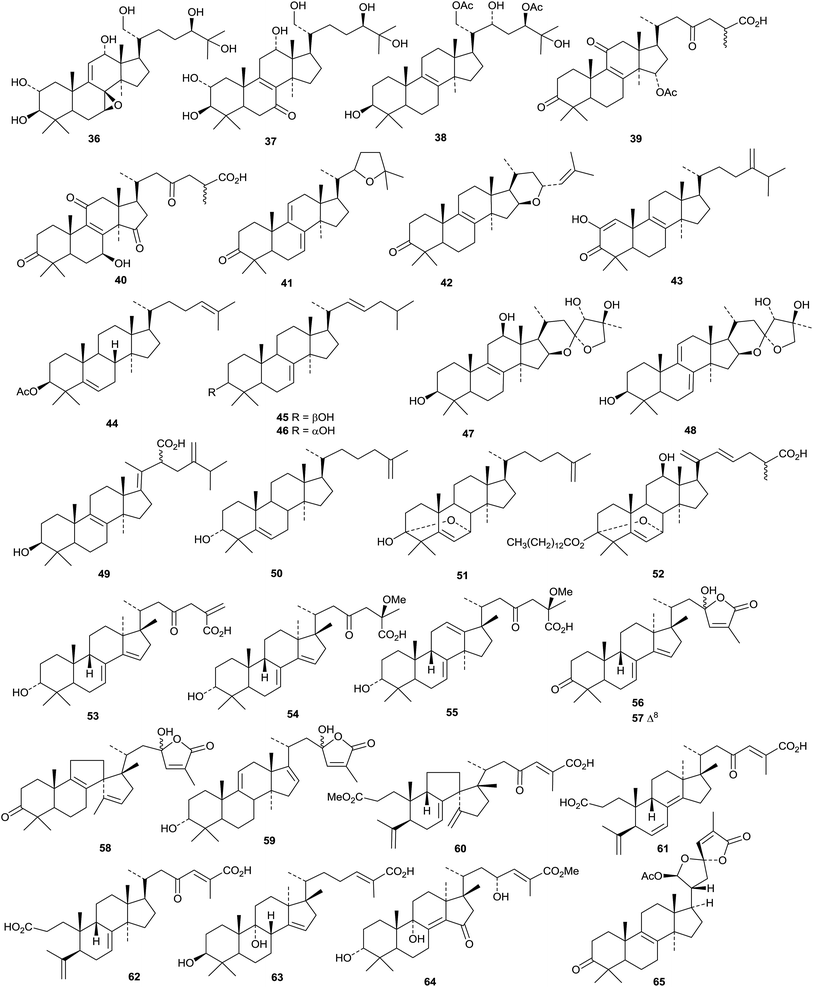
Other new cycloartanes reported this year include 3α-hydroxy-23-oxocycloart-25(27)-en-26-oic acid 156 from the oleoresin of Abies balsamea,51 perviridisinols A 157–C 159 from Aglaia perviridis,80 compounds 160 and 161 from Dasymaschalon dasymaschalum,81 12α,16β-dihydroxycycloartane-3,24-dione 162 from Curculigo orchioides whose structure was confirmed by X-ray crystallographic analysis,82 odoratanone A 163 from Aglaia odorata,63 the Z-coumaroyl ester of dihydrocycloeucalenol 164 from Nervilia fordii,83 the acetate 165 from the stems and leaves of Quercus variabilis,84 the 28,29-dinorcycloartane 166 and its 24,25-dihydro-derivative 167 from Marcetia latifolia,85 annonaretin A 168 from the leaves of Annona reticulata86 and esters 169 and 170 from Trichilia connaroides.87
Tareciliosides N–S are cycloartane saponins from Tarenna gracilipes.88 Tareciliosides N and S have the new genins 171 and 172, respectively. The others have known genins. Further saponins from Nervilia fordii include nervisides D–H, of which only G and H have a new genin 173.89 Agroastragaloside V is a saponin with a new genin 174 from Astragalus membranaceous.90 Cyclopassiflosides XII 175 and XIII 176, from Passiflora edulis, also have new genins.91 Cycloartane saponins with known genins include cycloasgenin C 3-O-β-D-xylopyranoside from Astragalus mucidus92 and cyclolehmanoside C from Astragalus lehmannianus93 and saponins from Astragalus halicacabus,94Beesia calthaefolia95 and Thalictrum fortunei.96
A review of the pharmacological activities of cucurbitane triterpenoids, particularly the anticancer activities of kuguacin J, from Momordica charantia, has been published.97 New cucurbitane derivatives include compounds 177–182 from Momordica charantia,98 the 27-nor-derivatives 183 and 184 from the fruit of Momordica charantia var. abbreviata99 and compounds 185 and 186 from the fruit pulp of Momordica charantia.100 The hydroperoxides 187 and 188 have been reported from the seeds of watermelon Citrullus lanatus.101 The stereochemistry at C-9 reported for 3,10-epoxides 189 and 190, from the dried fruit of Vitex negundo, seems unusual.102 Datiscosides I–O are cucurbitane glycosides with known genins from Datisca glomerata.103
4. The dammarane group
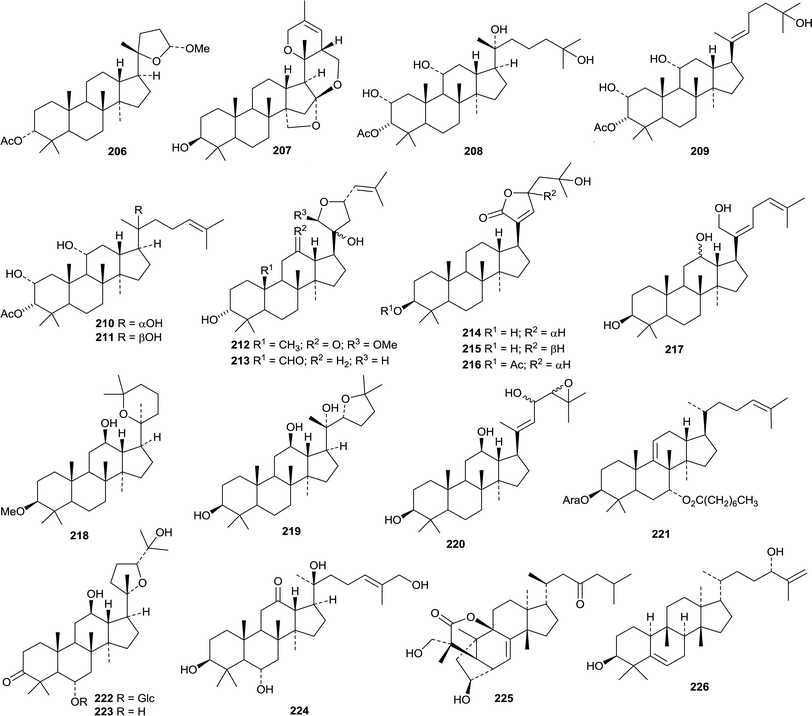
An interesting group of nordammaranes 191–197 has been isolated from Viburnum mongolicum.104 Three nordammaranes 198–200 have also been obtained from Dysoxylum hainanense.105 Other dammaranes include ixorene 201 from the leaves of Ixora coccinea,106 the ring A-cleaved derivative 202 from the leaves of Dysoxylum grande,107 3-acetylaglinin C 203 from the leaves of Aglaia odorata,108 mauritic acid 204 from the roots of Mauritia flexuosa,109 altissimanin C 205 from Ailanthus altissima110 and the 25,26,27-trinordammarane 206 from the dried fruit of Forsythia koreana.111Hodulosides XI and XII are new saponins from the seeds of Hovenia trichocarpa.112 They both have the new genin 20,26-epoxypseudojujubogenin 207. Several new saponins, combretasides A–G, have been found in Combretum inflatum.113 There are four new genins 208 (A and B), 209 (C and D), 210 (E and F) and 211 (G). Two new saponins from Gymnostemma pentaphyllum have the new genins 212 and 213.114 Hydrolysis of the total Gymnostemma pentaphyllum saponins afforded three new compounds, gypensapogenins E 214–G 216.115 The structure of gypensapogenin E 214 was confirmed by X-ray crystallographic analysis.
The saponins of Panax species continue to attract attention. Sanchirhinoside D, with the new genin 217, was obtained from Panax notoginseng.116 Hydrolysis of the saponin from the leaves and stem of Panax notoginseng afforded notoginsengaglycone MPD 218.117 Hydrolysis of the total ginsenosides of Panax ginseng gave the new compound 219 whose structure was confirmed by X-ray crystallographic analysis.118 Three ginsenosides Rh10, Rg11 and 12-O-glucoginsenoside Rh4 have been isolated from the heat-processed roots of Panax ginseng.119 Only ginsenoside Rg11 has a new genin 220. The arabinoside 221 was also isolated from the heat-processed roots of Panax ginseng.120 The leaves and stems of Panax quinquefolium afforded pseudoginsenoside RT6222 and its genin pseudoginsengenin R1223.121 Heptdamoside A is a dammarane saponin from Schefflera heptaphylla with the new genin 3β,6α,20S,26-tetrahydroxy-24E-dammaren-12-one 224.122
Dammarane saponins with known genins include chikusetsusaponins LM3–LM6 (ref. 123) and VIII124 from Panax japonicas, ginsenjilinol125 and 20R-ginsenoside Rf126 from Panax ginseng, jujubosides I–IV from Ziziphus jujuba,127 a different jujuboside I128 and jujuboside A2 (ref. 129) from Zizyphus jujuba var. spinosa, sanchirhinosides A1–A6 and B from Panax notoginseng,130 quinquefolosides Ld and Le from Panax quinquefolium,131 and unnamed saponins from Panax notoginseng.116,132
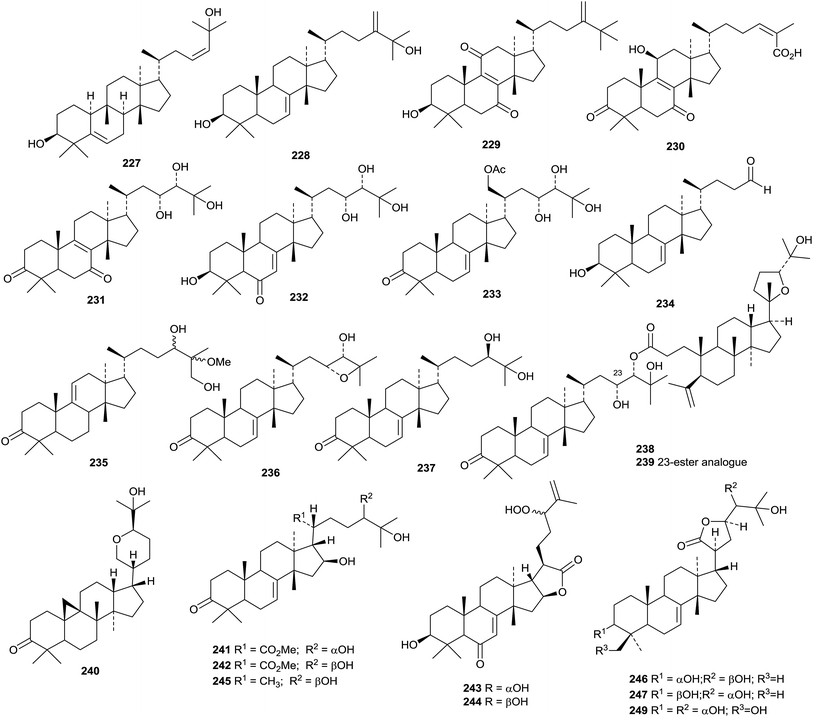
The unusual structure 225 of aphanamgrandiol A, a rearranged tirucallane derivative from Aphanamixis grandifolia, has been confirmed by X-ray crystallographic analysis.133 Two 19(10→9)-abeo derivatives, tiliacols A 226 and B 227, have been reported from the semi-mangrove plant Hibiscus tiliaceus.134 Aquilacallanes A 228 and B 229 are tirucallane derivatives from the leaves of Aquilaria sinensis.135 Aquilacallane B 229 has an extra methyl group at C-25. Other new tirucallanes include 230 from resin of Pistacia lentiscus,136 brumollisols A 231–C 233 from the stems of Brucea mollis,137 the trinor-derivative sikkimenoid F 234 from the aerial parts of Euphorbia sikkimensis138 and compound 235 from the roots of Salacia hainanensis.139 The bark of Ailanthus altissima (the “Tree of Heaven”) is the source of the tirucallane derivatives altissimanins A 236, B 237, D 238 and E 239.110 Altissimanins D 238 and E 239 are 24- and 23-esters of the known tirucallane piscidinol A with the secodammarane shoreic acid. Hirtinone 240, from Trichilia hirta is described as a cycloartane but is actually a 9,19-cycloeuphane derivative.140 Further compounds from the stem of Melia toosendan include the euphanes meliasenins S 241–W 245 and the tirucallane meliasenin X 246.141 The names meliasenins S and T have already been used. The 3,24-diepimer of meliasenin X, cochinchinoid K 247, together with 3-epimesendanin S 248 have been reported from Walsura cochinchinensis.142 The structure of cochinchinoid K 247 was confirmed by X-ray analysis. The related mesendanin M 249 has been found in Melia azedarach.143 The euphane 250 is a metabolite of the endophytic fungus Phomopsis chimonanthi isolated from Tamarix chinensis.144
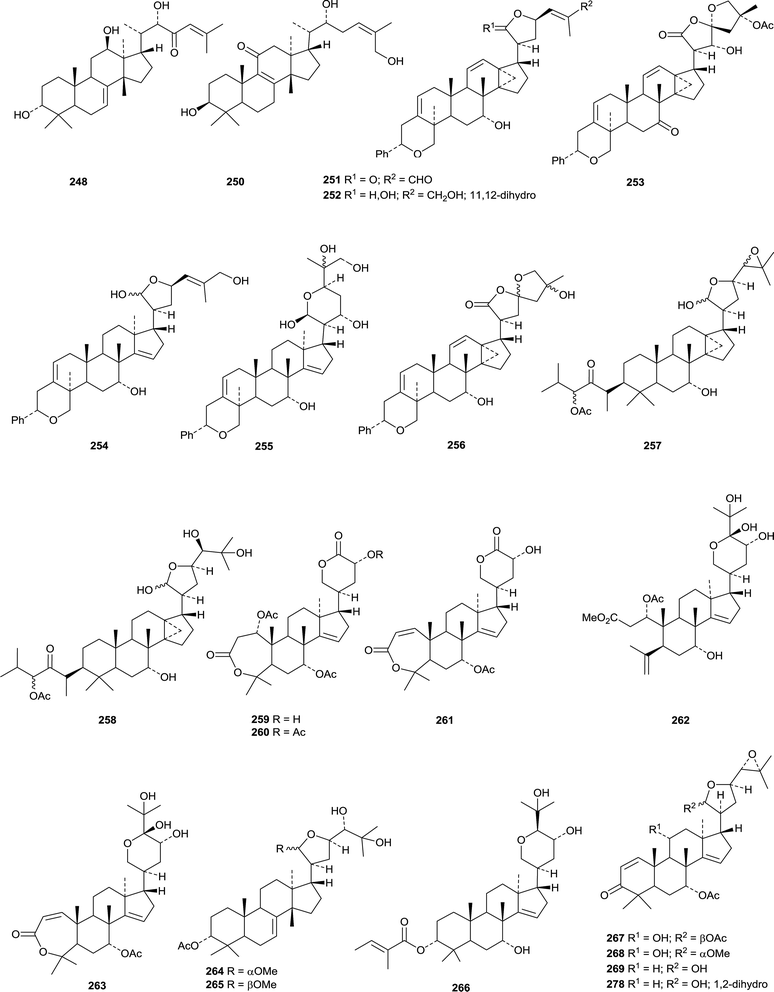
Dichapetalins N 251, O 252–R 255 and S 256 have been obtained from Dichapetalum mombuttense, Dichapetalum zenkeri and Dichapetalum leucosia, respectively.145 Dysoxylumglabretols A 257 and B 258 were isolated from Dysoxylum mollissimum as mixtures of 21-epimers.146 Dictamins A 259–C 261 are trinorapotirucallanes from Dictamnus dasycarpus.147 The structure of dictamin A 259 was confirmed by X-ray crystallographic analysis. The fruit of Aphanamixis polystachya is the source of the apotirucallanes polystanins A 262 and B 263 and the tirucallanes polystanins C 264 and D 265.148 3-Tigloylsapelin D 266 is a further apotirucallane constituent of the fruit of Melia azedarach.149
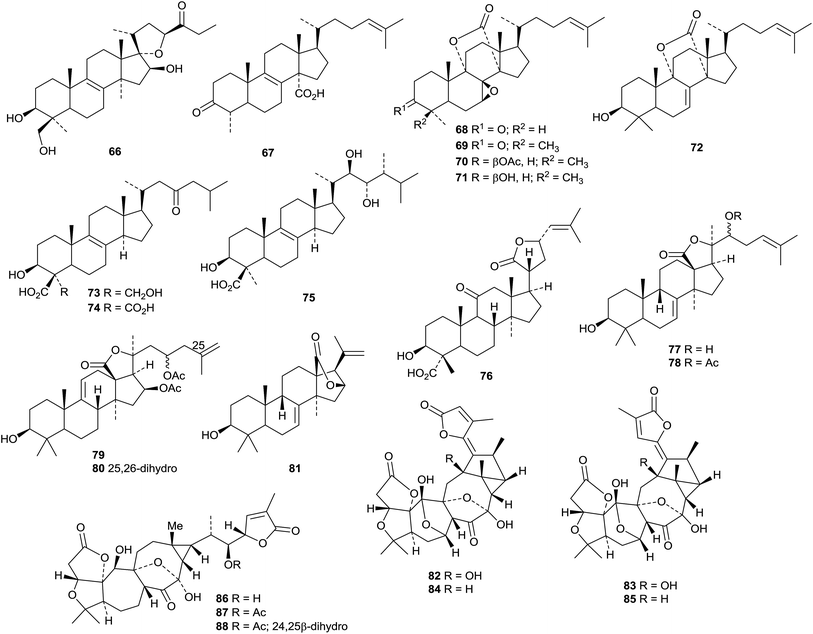
The twigs of Brucea javanica proved to be a rich source of apotirucallanes, yielding fourteen new compounds, brujavanones A 267–N 280.150 During this work the structure of bruceajavanin C was revised to its C-21 epimer 281. Other new compounds in this series include feroniellides C 282–E 284 from Feroniella lucida,151 lepidotrichilins A 285 and B 286 from Trichilia lepidota,152 polystanin E 287 from the fruit of Aphanamixis grandifolia,153288, 289 and dihydrosapelin E acetate 290 from Turraea pubescens154 and azadirahemiacetal 291 from Azadirachta indica.155
4.1 Tetranortriterpenoids
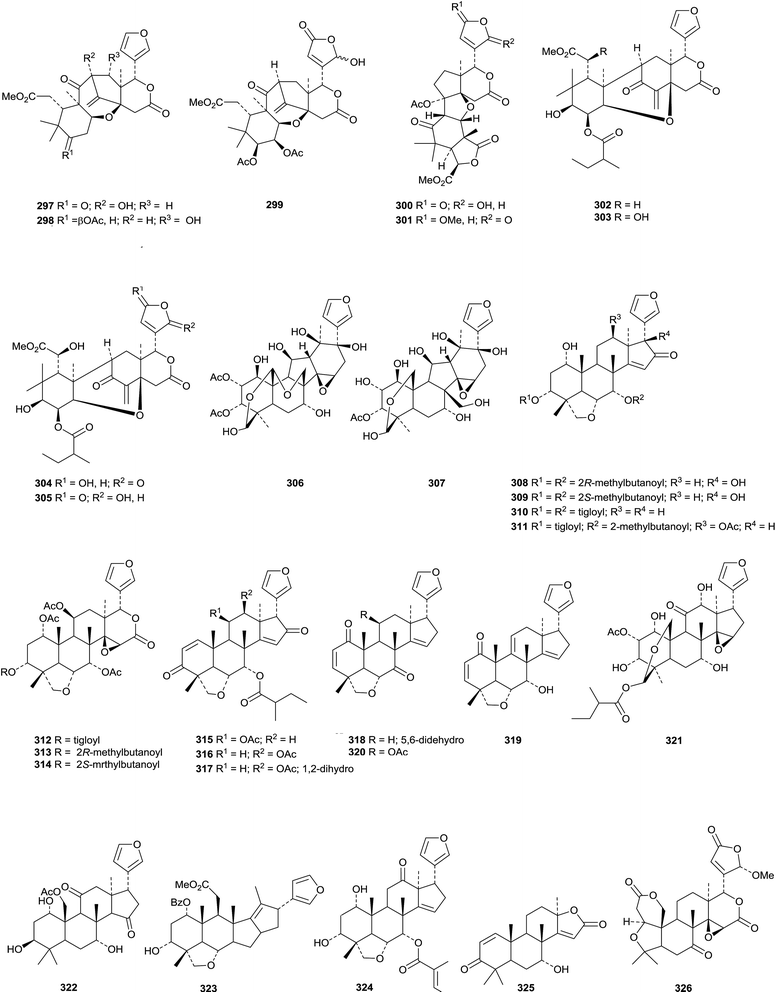
Interesting new tetranortriterpenoid skeletal types continue to appear. Thaixylomolin A 292 has a 6,7-cleaved skeleton while thaixylomolins B 293 and C 294 both incorporate a pyridine ring.156 They are constituents of Xylocarpus moluccensis. The structure of harperforatin 295, from Harrisonia perforata, was confirmed by X-ray crystallographic analysis.157 It was accompanied by harperfolide 296. New trijugin-related derivatives include cipatrijugins G 297 and H 298 from Cipadessa cinerascens,158 compound 299, also named cipatrijugin G, and also from Cipadessa cinerascens,159 and trijugins I 300 and J 301 from Trichilia connaroides.160Cipaferens A 302–D 305 are constituents of the leaves of Cipadessa baccifera.161 Phyllanthoids A 306 and B 307 are two C,D-rearranged derivatives from Phyllanthus cochinchinensis.162 The structure of phyllanthoid A 306 was confirmed by X-ray crystallographic analysis.
Ten limonoids, cochinchinoids A 308–J 317 have been reported from Walsura cochinchinensis.142 The structure of cochinchinoid A 308 was confirmed by X-ray analysis. Rubescins A 318–C 320 are constituents of Trichilia rubescens.163 The limonoids mesendanins K 321 and L 322 have been isolated from Melia azedarach.143 Extraction of the kernels of Azadirachta indica afforded the new compounds 323, 324 and azadiralactone 325.155
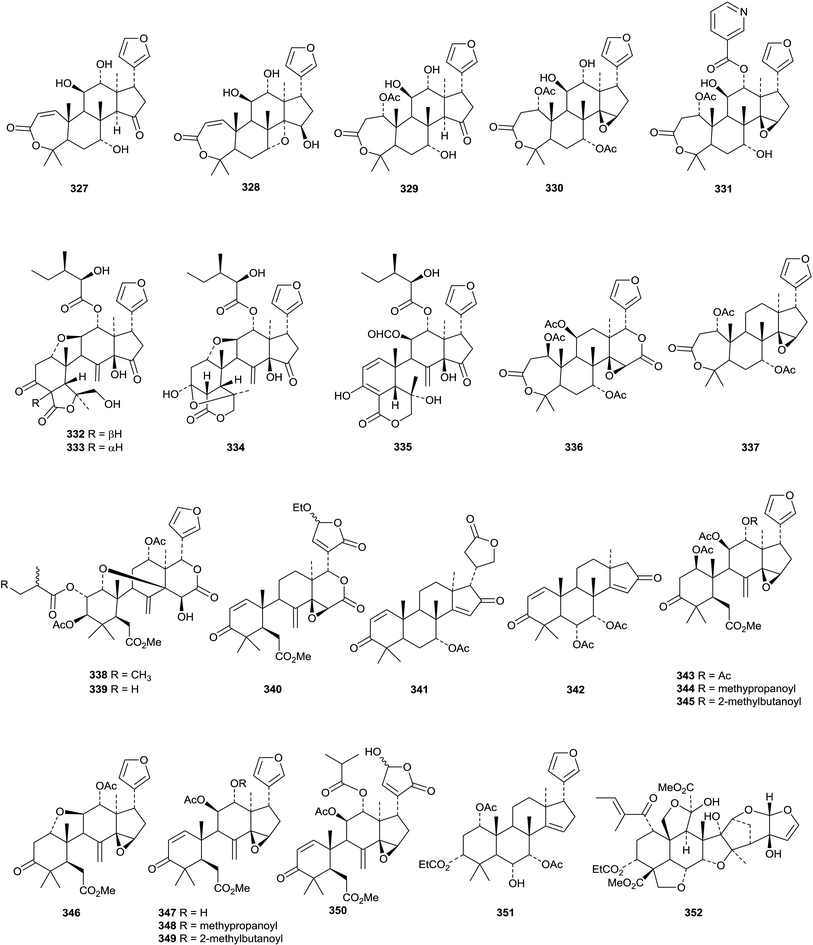
X-Ray crystallographic analysis established the 21S-configuration of evorubodinin 326 (21-O-methyllimonexic acid) from Euodia rutaecarpa var. bodinieri.164 The ring A cleaved limonoids aphanalides I 327–M 331 have been isolated from the fruit of Aphanamixis grandifolia.153 The structure of aphanalide I 327 was confirmed by X-ray crystallographic analysis. Other compounds from this source include the rearranged aphagranols A 332 and B 333 (ref. 165) and aphanamolides C 334 and D 335.166 Toonins A 336 and B 337 are new constituents of the roots of Toona sinensis.167Sandoricum koetjape is the source the cleaved limonoids sanjecumins A 338 and B 339.168 Andirolide S 340 is a related ring-cleaved limonoid from the flowers of Carapa guianensis together with the intact limonoid andirolide Q 341 and the defuro-derivative andirlide R 342.169
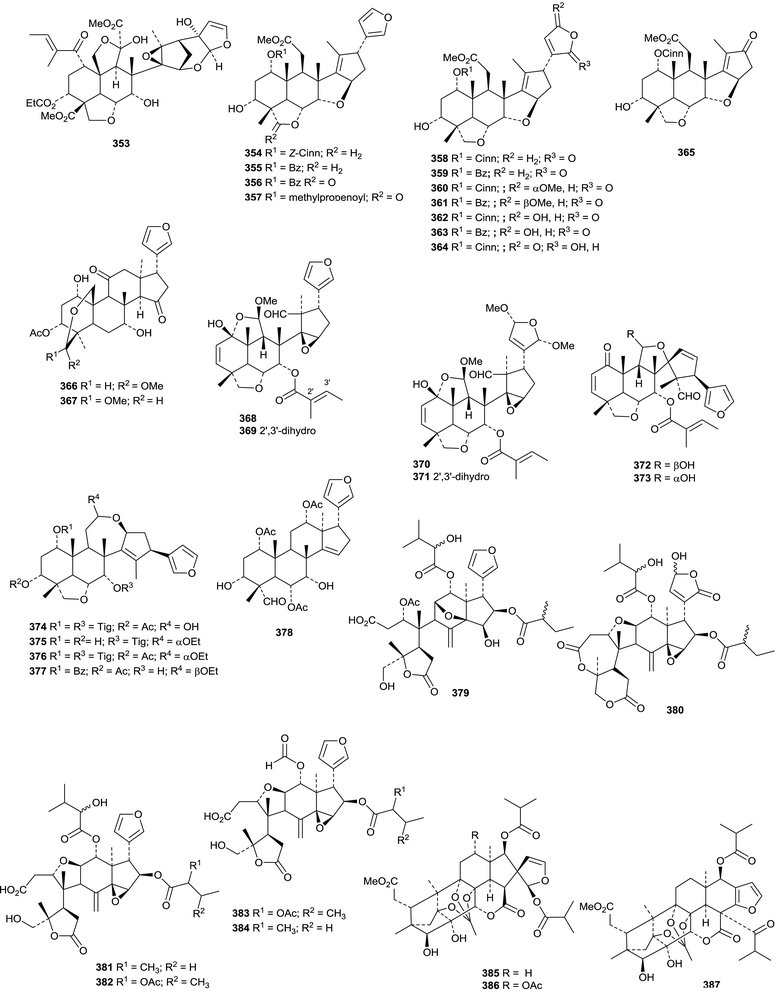
Various limonoids, turrapubins A 343–K 353 have been obtained from Turraea pubescens.154 More ring C-cleaved derivatives 354–365, accompanied by the intact limonoids 366 and 367, have been reported from the fruit of Melia azedarach.170 The name ohchininolide has been assigned to 358. Compound 355 has also been isolated from kernals of Azadirachta indica.155 Other ring C-cleaved constituents include walsogynes B 368–G 373 from Walsura chrysogyne171 and toosendalinin 374,172 isolated as an epimeric mixture, and the nimbolinin derivatives 375–377,173 together with compound 378, from the fruit of Melia toosendan. Dysoxylum mollissimum is the source of the AB-cleaved limonoids dysoxylumasins A 379–F 384.174
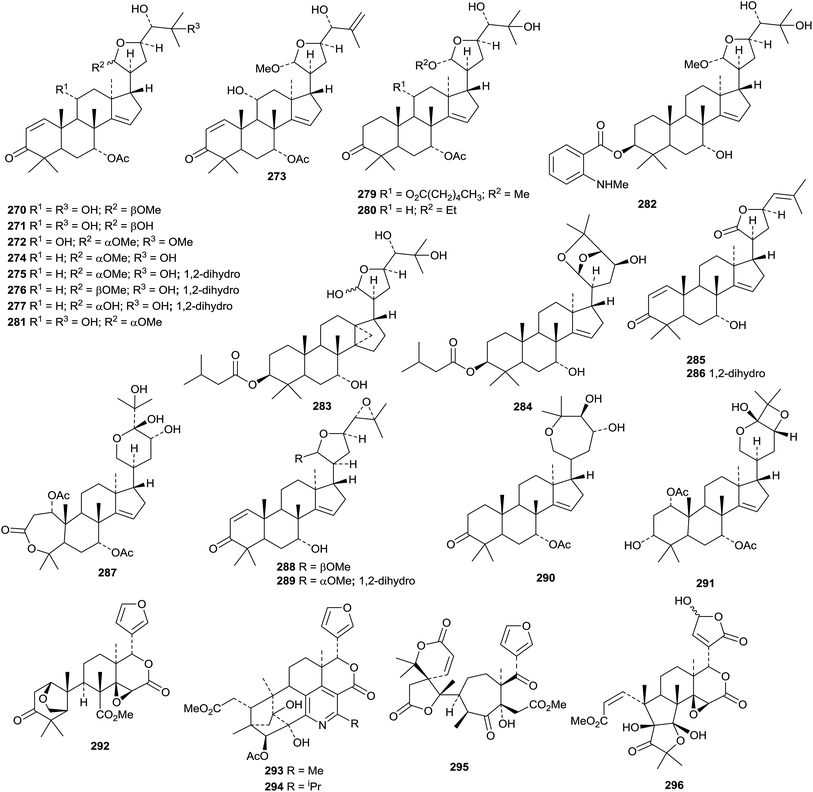
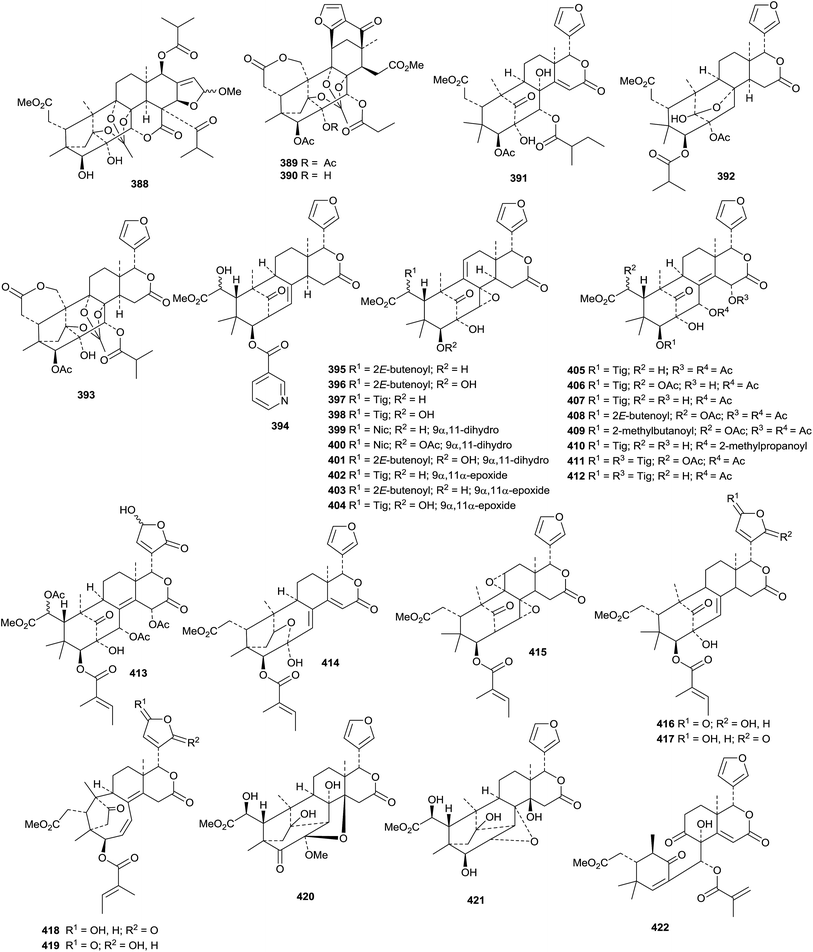
The phragmalin derivatives chukfuransins A 385–D 388 from Chukrasia tabularis,175 and guianolides A 389 and B 390, from Carapa guianensis,176 all have novel features involving cyclisations of the furan ring. The structures of chukfuranisins A 385 and C 387 and of guianolide A 389 were all confirmed by X-ray crystallographic analyses. Andirolides T 391–V 393 are further constituents of the flowers of Carapa guianensis.169 Twenty new mexicanolide derivatives, trichinenlides A 394–T 413, have been isolated from Trichilia sinensis.177 Secotrichagmalin A 414 and trichanolides A, B, C 415, D 416 and E 417 have been reported from Trichilia connaroides.160 Trichanolides A and B are identical to trichinenlides I 402 and D 397, respectively. The structure of trichanolide A was confirmed by X-ray crystallographic analysis.
1,2-Cleaved derivatives include trichilitons G 418 and H 419 from Trichilia connaroides87 and deacetyl-2α-methoxykhayanolide E 420 and kigelianolide 421 from Kigelia africana.178 The structure of deacetyl-2α-methoxykhayanolide E 420 was confirmed by X-ray analysis.
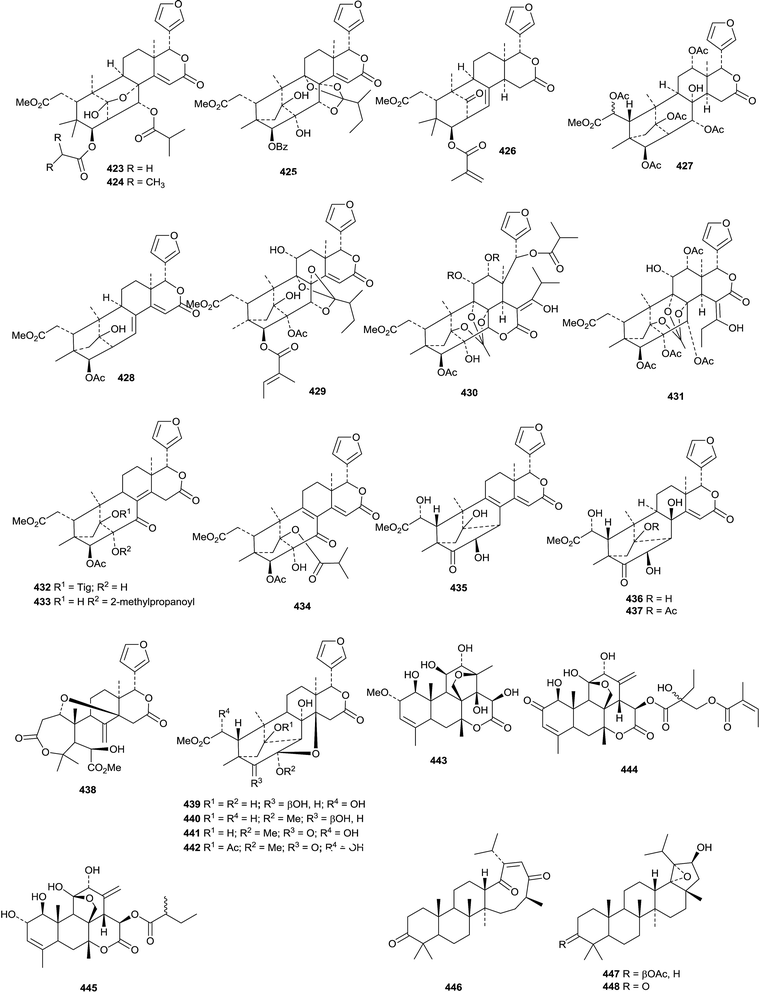
New derivatives from Xylocarpus granatum include xylomexicanins C 422 and D 423,179 xylogranins A 424 and B 425.180 and granatumins I 426, J 427 and K 428 (accompanied by granatumin H which is a known compound).181 Other related derivatives include 11α-hydroxyswietephragmin B 429 from Swietenia mahogani (accompanied by swietephragmins H and I which are known compounds),182 chukvelutilide G 430 and chukrasin F 431 from Chukrasia tabularis var. velutina,183 thaixylomolins D 432–F 434 from Xylocarpus moluccensis184 Khayaseneganins A 435–H 442 are further constituents of Khaya senegalensis.185
4.2 Quassinoids
There is little activity in the quassinoid field. Bruceine M 443 has been isolated from the fruit of Brucea javanica186 and altissinols A 444 and B 445 from Ailanthus altissima.187 Altissinol B 445 is not a new compound and was identified as 13,18-didehydroexcelsin in 1980.1885. The lupane group
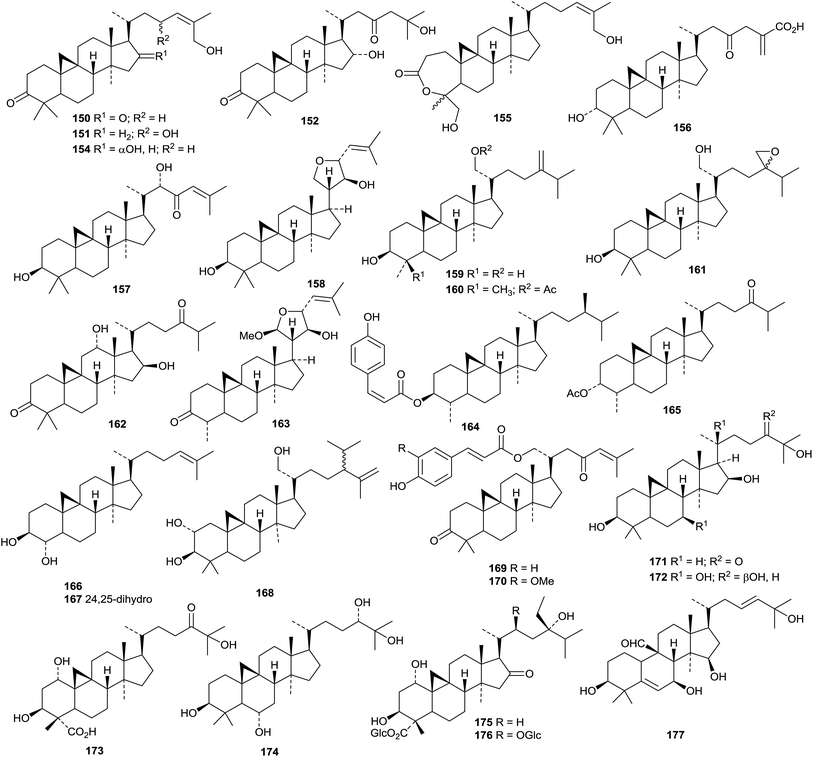
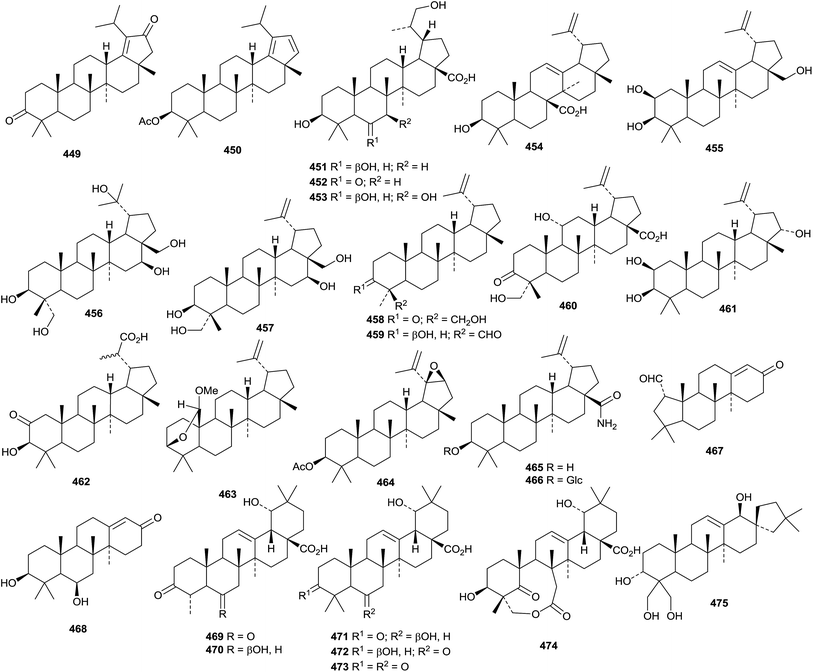
A review presenting spectral data of natural lupane triterpenoids has been published with a view to helping analysis of new structures.189 The anti-HIV activity of betulinic acid and its derivatives have been covered in a short review.190 Officinatrione 446, with an unusual 17,18-secolupane skeleton, was isolated from Taraxacum officinale where it occurs with four intact lupanes 447–450.191 The structure of officinatrione 446 was confirmed by X-ray analysis. The structures of three compounds from Saprosma merrillii were originally assigned as ursane derivatives.192 However it was later established that they were the lupane derivatives 451–453 by the X-ray analysis of 453.193 Moruslupenoic acid B 454, from the stem bark of Morus alba, is 3β-hydroxy-12,20(29)-lupadien-26-oic acid where it co-occurs with the well-known 3β-hydroxy-20(29)-lupen-28-oic acid which has been named as moruslupenoic acid A.45 Actinidin A 455, from Actinidia deliciosa, has been identified as 12,20(29)-lupadiene-2β,3β,28-triol.194 Other simple lupane derivatives include lupane-3β,16β,20,23,28-pentol 456 (ref. 195) and 20(29)-lupene-3β,16β,23,28-tetrol 457 (ref. 196) from Gymnema sylvestre, 24-hydroxy-20(29)-lupen-3-one 458 and 3β-hydroxy-20(29)-lupen-24-al 459 from Ilex cornuta, 11α,23-dihydroxy-3-oxo-20(29)-lupen-28-oic acid 460 from Acanthopanax trifoliatus,197 20(29)-lupene-2β,3β,22α-triol 461 and 3β-hydroxy-2-oxo-29-lupanoic acid 462 from the roots of Salacia hainanensis,139 the acetal 463 from Siphonodon celastrineus,198 the 19,21-epoxide 464 from Abelmoschus esculentus199 and the decanoyl ester of lupeol from Cadaba farinosa.200Betulinic amide 465 and its 3-O-β-D-glucopyranoside 466 have been isolated from Rhododendron lepidotum.201 New lupane saponins with known genins include the 28-O-β-D-glucopyranosyl ester of platanic acid from Tetracera scandens,202 lonimacranthoside A1 from Lonicera macranthoides203 and ryobusaponins E–G from Clethra barbinervis204 and unnamed saponins from Lonicera macranthoides205 and Pulsatilla chinensis.206
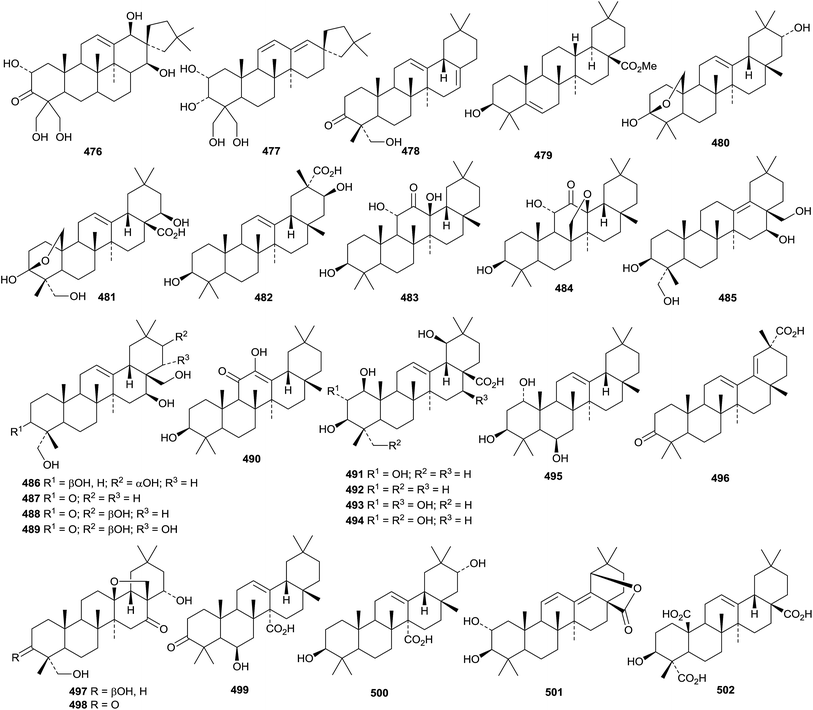
6. The oleanane group
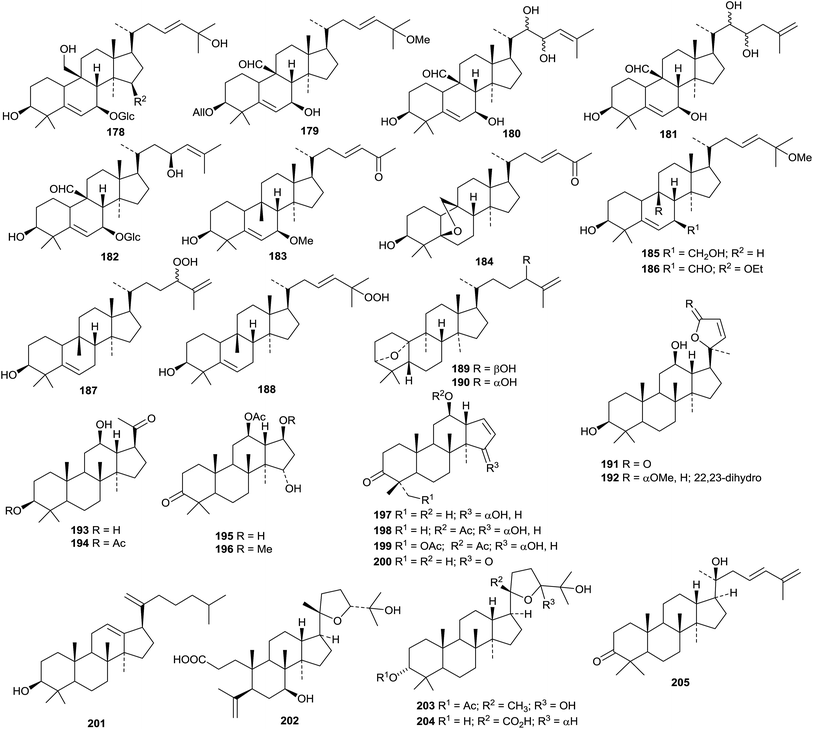
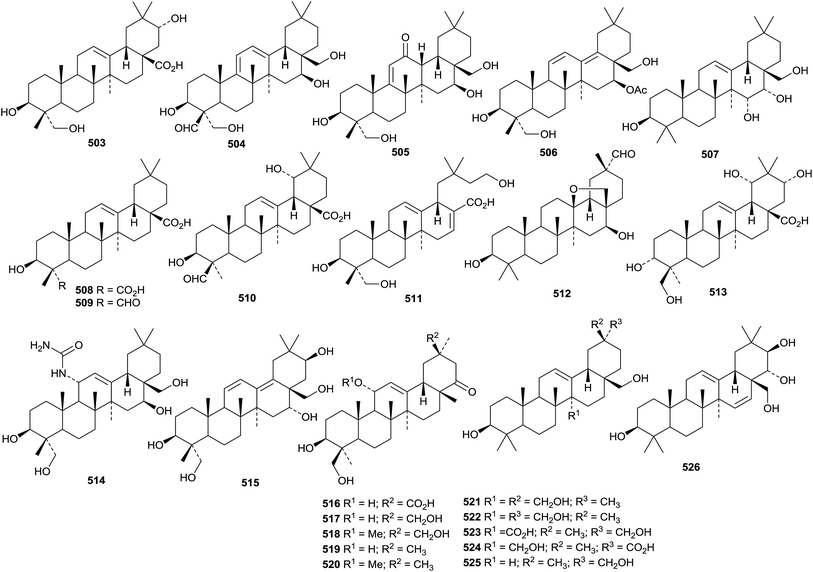
Teydealdehyde 467, from Nepeta teydea,207 has a contracted ring A and lacks ring E and the related laevigatone A 468 has been found in Uncaria laevigata where it occurs with the 24-noroleananes 469 and 470 and the intact oleanane derivatives 471–473.208 The oleanane 471 has also been isolated from Uncaria sessilifructus and named as uncarilic acid together with the 5,6-seco derivative secouncarilic acid 474.209 The 19(18→17)-abeo-28-noroleanane derivatives stewartiisins A 475–C 477 have been isolated from Phlomis stewartii.210 Sambucasan A 478 is a 28-nor-12,16-oleanadiene from Sambucus williamsii.211 Alstoprenyol 479, from Alstonia scholaris, has been reported to have the unusual 18α-configuration.212 Xyloketal 480 is a hemiacetal derivative from Cassine xylocarpa213 and a related hemiacetal 481 has been found in Lantana montevidensis.214 3β,21β-Dihydroxy-12-oleanen-29-oic acid 482, from Maytenus royleanus, has been named ficusonic acid.215 Japanese fermented tea (Camellia sinensis leaves) is the source of two oleanan-12-one derivatives 483 and 484.216 Other new simple oleanane derivatives include 13(18)-oleanene-3β,16β,23,28-tetrol 485 and four related 12-oleanene derivatives 486–489 from Gymnema sylvestre,196 3β,12-dihydroxy-12-oleanen-11-one 490 from Siphonodon celastrineus,198 1β,2α,3β,19β-tetrahydroxy-12-oleanen-28-oic acid 491 and the related compounds 492–494 from Euphorbia sieboldiana,217 12-oleanene-1α,3β,6β-triol 495 from Vernicia fordii,218 3-oxo-12,18-oleanadien-29-oic acid 496 from Limnophila indica,219 the 13,28-epoxides 497 and 498 from Lysimachia parvifolia,220 the 12-oleanen-27-oic acids 499 and 500 from Chrysosplenium carnosum221 and 2α,3β-dihydroxy-11,13(18)-oleanadien-28,19β-olide 501 from Rhaphiolepis indica var. tashiroi.222Celosins I and II, from Celosia argentea, are saponins with the new genin 3β-hydroxy-12-oleanene-23,25,28-trioic acid 502.223 Other oleanane saponins with new genins include clematangoside B from Clematis tangutica with 3β,21α,23-trihydroxy-12-oleanen-28-oic acid 503,224 clinoposapanins A–C from Clinopodium chinense with the genins 504–506, respectively,225 gordonsaponins B and E from Gordonia kwangensis with the genin 12-oleanene-3β,15α,16α,28-tetrol 507,226 heptoleosides A and B from Schefflera heptaphylla with the genins 508 and 509 respectively,122 ilexpublesnins L and M from Ilex pubescens with 3β,19α-hydroxy-12-oleanene-24,28-dioic acid 510,227 pittangretoside I from Pittosporum angustifolium with the 17,22-seco genin 511,228 psychotrianoside B from a Psychotria sp. with 13β,28-epoxy-3β,16β-dihydroxy-12-oleanen-29-al 512,229 ryobunin C from Clethra barbinervis with 3α,19α,21α,24-tetrahydroxy-12-oleanen-28-oic acid 513,230 saikosaponin W and 21β-hydroxysaikosaponin b2 from Bupleurum chinense with the genins 514 and 515 respectively,231 sarosiensins I–IV, VI and VII from Trifolium medium with the genins 516–520,232 saponins from Genista ulicina with the genins 521–525,233 and saponins from Xanthoceras sorbifolia with the genin 12,15-oleanadiene-3β,21β,22α,28-tetrol 526.234
New oleanane saponins with known genins that have been assigned trivial names are listed in Table 1
| Trivial name | Plant species | Reference |
|---|---|---|
| 2′′- and 6′′-O-acetylrandianins | Nematostylis anthophylla | 235 |
| 6′′-Acetylsaikosaponins b1, b3 and e | Bupleurum chinense | 231 |
| Achyranthosides B and D | Achyranthes bidentata | 236 |
| Aesculiosides C1–C15 | Aesculus californica | 237 |
| Assamicoside A | Glochidion assamicum | 238 |
| Caraganins A and B | Caragana microphylla | 239 |
| Chiococcasaponins III–V | Chiococca alba | 240 |
| Clematangosides A, C, D | Clematis tangutica | 224 |
| Clematochinenosides H–J | Clematis chinensis | 241 |
| Congmuyenosides C–E | Aralia elata | 242 |
| Cowpeasaponins I and II | Vigna sinensis | 243 |
| Cylindroside A | Cylindrokelupha dalatensis | 244 |
| Eryngiosides M and N | Eryngium yuccifolium | 245 |
| Gordonsaponins A, C, D, F–K | Gordonia kwangsiensis | 226 |
| Heptoleosides C and D | Schefflera heptaphylla | 122 |
| Kakkasaponins II and III | Pueraria thomsonii | 246 |
| Longicarposides A–I | Gordonia longicarpa | 247 |
| Mandshunosides C–E | Clematis mandshurica | 248 |
| Magnosides A and B | Cybianthus magnus | 249 |
| Neanoside A | Neanotis wightiana | 250 |
| Nebulosides A and B | Gypsophila arrostii var. nebulosa | 251 |
| Nummularoside | Lysimchia nummularia | 252 |
| Paritrisides A–F | Paris polyphylla var. yunnanensis | 253 |
| Patrinovilosides A and B | Petrinia villosa | 254 |
| Phaseoloidesides A–D | Entada phaseoloides | 255 |
| Piptadeniaoside | Piptadeniastrum africanum | 256 |
| Pittangretosides A–H | Pittosporum angustifolium | 228 |
| Platycodons A and B | Platycodon grandiflorum | 257 |
| Psychotrianosides A, C–G | Psychotria sp. | 229 |
| Pulsatilloside F | Pulsatilla koreana | 258 |
| Ryobusaponins A–D | Clethra barbinervis | 204 |
| Sarosiensin V | Trifolium medium | 232 |
| Sibiricasaponin E | Polygala sibirica | 259 |
| Soysaponins M1–M3 | Glycine max | 260 |
| Thyrsiloside A | Lysimachia thyrsiflora | 252 |
| Tomentosides A–C | Anemone tomentosa | 261 |
| Virgaureasaponins 4–6 | Solidago virgaurea | 262 |
| Xanthohuskisides A and B | Xanthoceras sorbifolia | 263 |
The sources of new oleanane saponins with known genins that have not been assigned trivial names are listed in Table 2
| Plant species | Reference |
|---|---|
| Anabasis setifera | 264 |
| Anemone raddeana | 265 |
| Anemone taipaiensis | 266 and 267 |
| Aralia elata | 268 |
| Ardisia gigantifolia | 269 |
| Astragalus tauricolus | 270 |
| Benincasa hispida | 271 |
| Calliandra pulcherrima | 272 |
| Clematis lasiandra | 273 |
| Clematis tangutica | 274 |
| Gypsophila trichotoma | 275 and 276 |
| Ilex cornuta | 277 |
| Lonicera macranthoides | 205 |
| Lysimachia parvifolia | 220 |
| Pittospermum verticillatum ssp. verticillatum | 278 |
| Pulsatilla chinensis | 279 |
| Ricinus communis | 280 |
| Sapindus mukorossi | 281 and 282 |
| Saponaria officinalis | 283 |
| Sesbania vesicaria | 284 |
| Swartzia apetala var. glabra | 285 |
| Swertia yunnanensis | 286 |
| Trifolium hybridum | 287 |
| Xanthoceras sorbifolia | 234 and 288 |
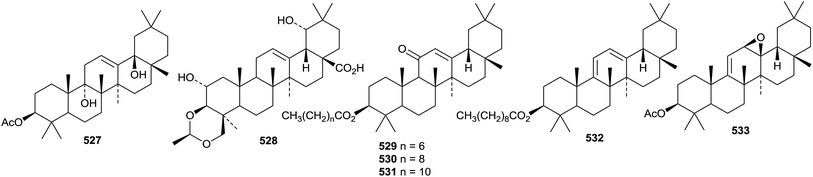
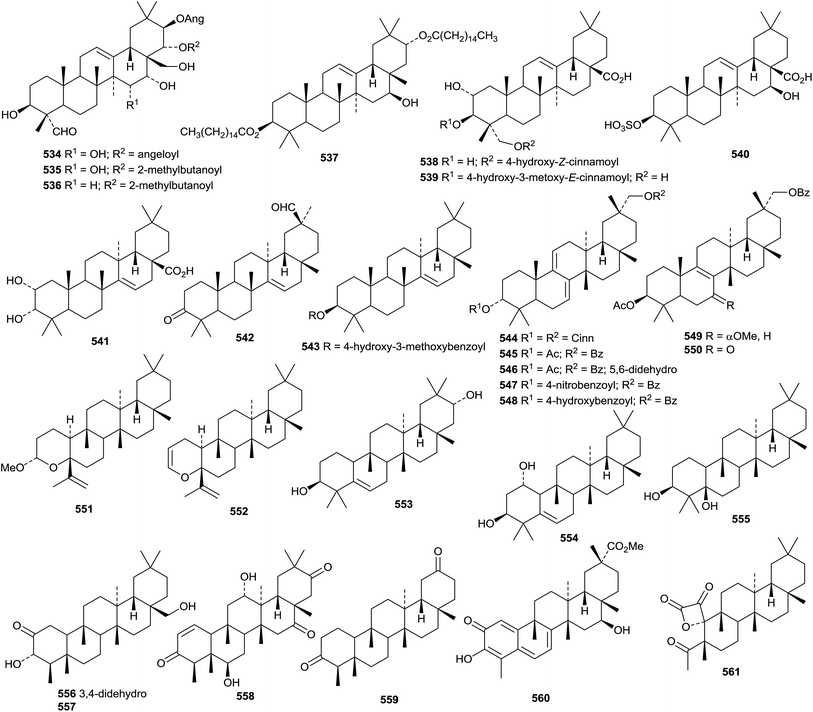
New oleanane esters include the tetraacetate of 12-oleanene-3β,16β,23,28-tetrol and three esters of 12-oleanene-3β,16β,21β,22α,23,28-hexol from Gymnema sylvestre,195 and the 3-acetate of 12-oleanene-3β,9α,17β-triol 527 from Abelmoschus exculentus.199 An acetaldehyde acetal 528 has been identified as a constituent of Terminalia brownii.289 Several oleanane esters 529–533 have been isolated from Dorstenia arifolia.290 Further new oleanane esters include the angeloyl esters 534–536 from Camellia oleifera,291 the dipalmitoyl ester 537 from Chrysanthemum macrocarpum,292 the cis-coumaroyl ester 538 and the trans-feruloyl ester 539 from Rhodomyrtus tomentosa293 and the 3-sulfate of 540 3β,16β-dihydroxy-12-oleanen-28-oic acid from Schefflera elegantissima.294
Zenkeric acid 541, a constituent of Hypodaphnis zenkeri, has been identified as 2α,3α-dihydroxy-14-taraxeren-28-oic acid.295 3-Oxo14-taraxeren-30-al 542 has been found in Vitex trifolia var. simplicifolia.296 Microcisin 543, the vanilloyl ester of taraxerol, has been isolated from the roots of Microcos tomentosa.297 The dicinnamoyl ester 544 of karounidiol is a constituent of fruits of Benicasa hispida.271 Three multiflorane esters 545–547, including the p-nitrobenzoyl ester 547, have been found in seeds of zucchini (Cucurbita pepo).298 Related esters 548–550 have been isolated from pumpkin seeds (Cucurbita maxima).299 Two related 3,4-secoglutinane derivatives torreyanoxane 551 and euphorbiane 552 have been isolated from Torreya nucifera300 and Euphorbia tirucalli,300 respectively. The structure of euphorbiane 552 was confirmed by X-ray analysis. Simple glutinane derivatives include 5-glutinene-3β,21α-diol 553 from Celastrus vulcanicola,213 5-glutinene-1α,3β-diol 554 from Vernicia fordii218 and glutinane-3β,5β-diol 555 from Rhaphiolepis indica var. tashiroi.222
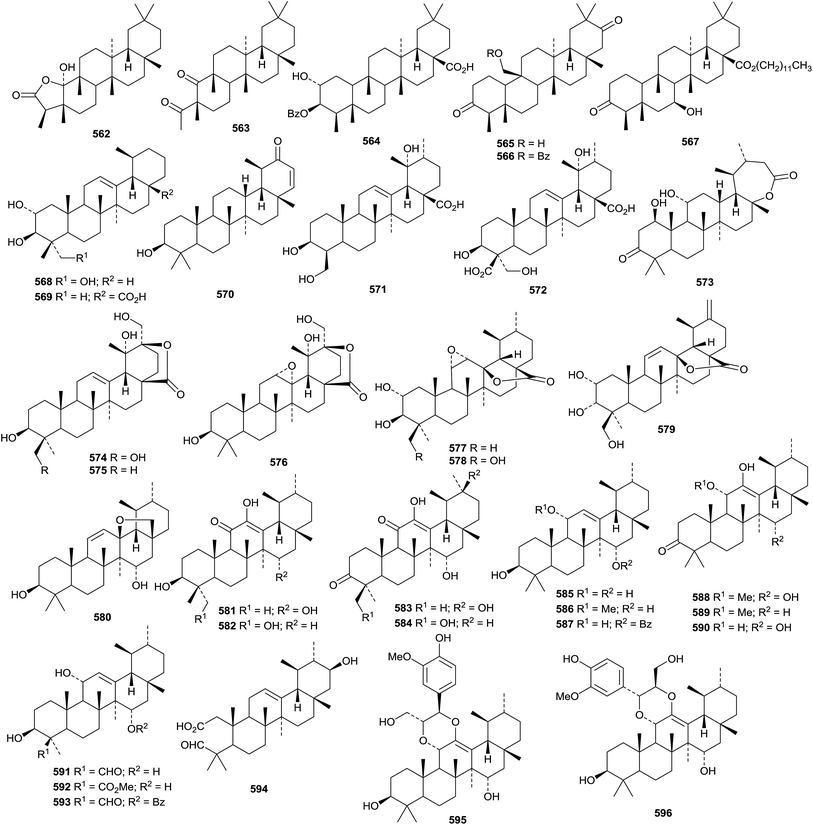
A review of naturally occurring friedelanes, isolated between 1977 and March 2011 has been published.301 Ovalifolones A 556 and B 557, from Garcinia ovalifolia,302 and maytensifolone 558, from Maytenus distichophylla,303 are new friedelane derivatives. Phyllaembicone A 559, isolated from the roots of Phyllanthus emblica, is 29,30-dinorfriedelane-3,20-dione.304 16β-Hydroxypristimerin 560 is a 24-norfriedlane quinine methide from Maytenus salicifolia.305 Three ring-A cleaved norfriedelane derivatives named as norfriedelins A 561–C 563 have been identified in Malpighia emarginata.306 The structure of norfriedelin A 561, containing an interesting α-keto-β-lactone, was confirmed by X-ray analysis. The 3-benzoyl ester 564 of pluricostatic acid has been found in Trigonostemon xyphophylloides307 and 25-hydroxyfriedelane-3,21-dione 565 and its 25-benzoate 566 have been isolated from Siphonodon celastrineus.198 The dodecyl ester 567 of 7β-hydroxy-3-oxofriedelan-28-oic acid has been found in Pouzolzia indica.308
7. The ursane group
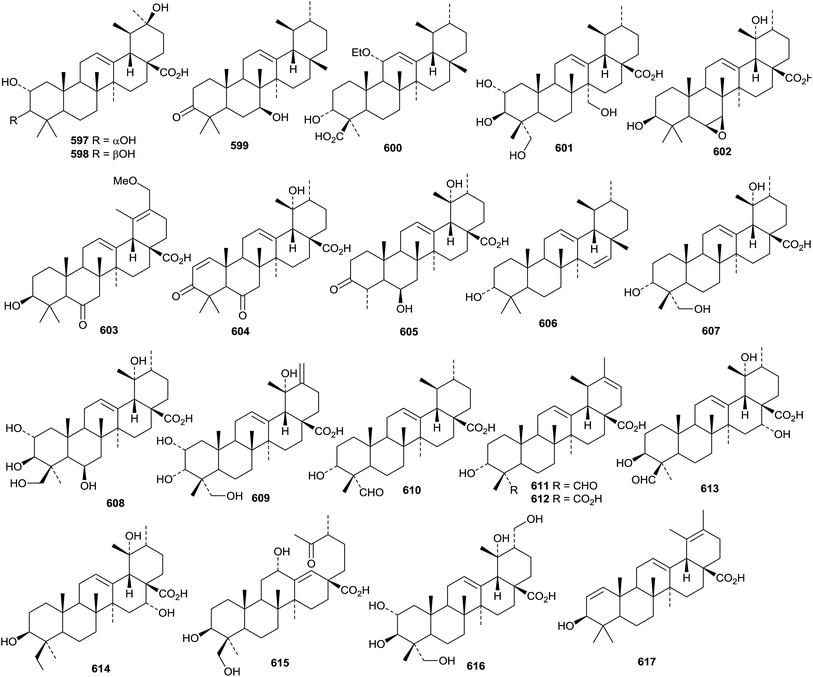
The sources and pharmacological properties of ursolic acid have been reviewed.309–311 The 30-nor ursane derivatives rubrajaleelol 568 and rubrajeelic acid 569 have been identified in Plumeria rubra.312 A further 30-nor derivative 3β-hydroxy-30-nor-21-ursen-20-one 570 has been found in Eupatorium fortunei.313 A 24-nor ursane derivative 571 together with 3β,19α-dihydroxy-12-ursene-24,28-dioic acid 572 have been isolated from Emmenopterys henryi.314 Urmiensolide 573, from Salvia urmiensis, is the first example of an E-ring ε-lactone.315 The structure of urmiensolide 573 was confirmed by X-ray analysis. Gluinosalactones A 574, B 575 and C 576 are 28,20-ursanolides isolated from the leaves of Rehmannia glutinosa.316 Three 28,13-ursanolides 577–579 have been identified in Isodon excisoides.317Seventeen ursane derivatives 580–596 have been isolated from Siphonodon celastrineus including the 2,3-seco aldehyde derivative 594 and two phenylpropanoid adducts 595 and 596.198 Fulgic acids A 597 and B 598, from Potentilla fulgens, have been identified as 2α,3α,20β-trihydroxy-12-ursen-28-oic acid and the 3β-epimer, respectively.318 6β-Hydroxy-12-ursen-3-one 599 a constituent of Boswellia sacra, has been named nizwanone and it is accompanied by 11α-ethoxy-β-boswellic acid 600 that is likely to be an isolation artefact.319 Other new simple ursane derivatives include 2α,3β,23,27-tetrahydroxy-12-ursen-28-oic acid 601 from Actinidia deliciosa,194 four derivatives 602–605 from Uncaria laevigata,208 12,15-ursadien-3α-ol 606 from Croton bonplandianum,320 4-epi-barbinervic acid 607 from Verbena officinalis,321 2α,3β,6β,19α,24-pentahydroxy-12-ursen-28-oic acid 608 from Ludwigia hyssopifolia322 and 2α,3α,19α,23-tetrahydroxy-12,20(30)-ursadien-28-oic acid 609 from Callicarpa nudiflora.323
New ursane saponins with new genins include hepturosides A–C from Schefflera heptaphylla with the genins 610–612 respectively,122 ilexpublesnins C and D from Ilex pubescens with 3β,19α-dihydroxy-24-oxo-12-ursen-28-oic acid 613,227 ryobunins A and B from Clethra barbinervis with 3α,16α,19α,24-tetrahydroxy-12-ursen-28-oic acid 614 and the 18,19-seco derivative 615 respectively,230 a saponin from Potentilla multicaulis with the new genin 2α,3β,19α,23,30-pentahydroxy-12-ursen-28-oic acid 616324 and a saponin from Asparagus racemosus with 3β-hydroxy-1,12,19-ursatrien-28-oic acid 617.325
Ursane saponins with known genins include actinidicoside from Premna fulva,326 asprellanosides C–E from Ilex asprella,327 heptursoside D from Schefflera heptaphylla,122 ilexgenin A2 (ref. 328) and ilexoside P329 from Ilex pubescens, monepalosides M and N from Morina nepalensis var. alba,330 sibiricasaponins A–D from Polygala sibirica259 and ilexpuplesnins E and G–K from Ilex pubescens.227 Unnamed saponins with known genins have been isolated from Callicarpa nudiflora,323Ilex asprella331 and Ilex cornuta.277
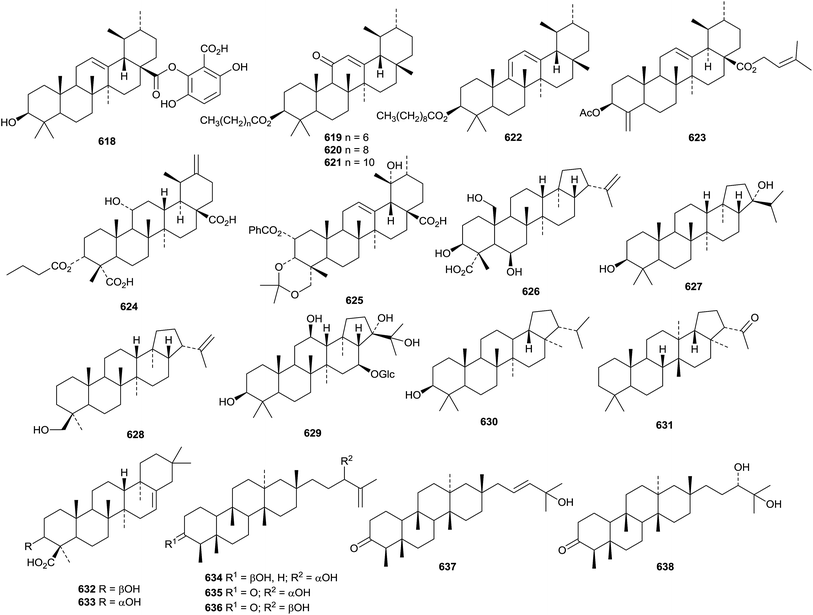
Prunol 618, from Prunus cerasoides, has been identified as an ester of ursolic acid with 2,3,6-trihydroxybenzoic acid.332 Other new ursane esters include the 28-formate of 12-ursene-3β,28-diol and its 3-acetate derivative from Ilex cornuta,333 the 6-acetate of ursane-3β,6β,19α-triol from Astilbe chinensis,334 the 30-cis-p-coumaroyl ester of 3β,30-dihydroxy-12-ursen-28-oic acid from Teucrium viscidum,335 the 2-cis-p-coumaroyl ester and the 2-trans-p-coumaroyl ester of 2α,3β,19α-trihydroxy-12-ursen-28-oic acid from Prinsepia utilis,336 and four long-chain ursane esters 619–622 from Dorstenia arifolia. Alstoprenylene 623, from Alstonia scholaris, has been reported to be a 24-norursane with the unusual 18α-stereochemistry.212 Clerodendrumic acid 624, from Clerodendrum glabrum, has also be reported with this stereochemistry.337 The acetonide 625 is likely to be an artefact from chromatography of the extract of Rhododendron hainanense.338
Ilexpublesnin F is a taraxastane saponin with a known genin from Ilex pubescens.227 The 3-acetate of 12,20(30)-taraxastadiene-3β,11α,21α-triol has been isolated from Echinops galalensis.339
8. The hopane group
The structure of sonhafouonic acid 626, from Zehneria scabra camerunensis, was confirmed by X-ray analysis as 3β,6β,25-trihydroxy-20(29)-hopen-23-oic acid.340 Hopane-3β,21α-diol 627 has been isolated from the leaves of Carissa carandas and has been named carandinol.341Humata tyermanni is the source of 20(29)-hopen-24-ol 628.342 The new hopane saponin glinuside C 629, from Glinus oppositifolius, has a new genin.343 Dryocrassol xylopyranoside, with a known hopane genin, has been isolated from Alsophila spinulosa.344 The migrated hopane derivatives capillirol B 630 (neophopane-3β-ol) and capillirone 631 (30-norfernan-22-one) have been identified in Adiantum capillus-veneris.3459. Miscellaneous compounds
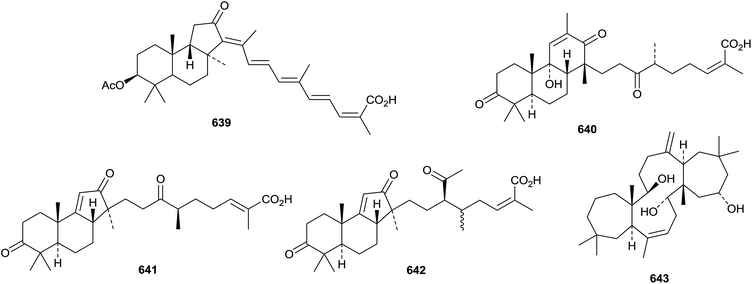
Two chiratane triterpenoids, kouitchenoids A 632 and B 633 have been isolated from Swertia kouitchensis.346Aster tataricus is the source of the five shionane triterpenoids astataticusol A 634 and astataticusones A 635–D 638.347 Stellettin N 639, from a sponge of the genus Stelletta, is an isomalabaricane triterpenoid that shows potent protein-tyrosine phosphatase 1B inhibition.348Three tricyclic triterpenoids with unusual skeletons, kadcotriones A 640–C 642, have been isolated from Kadsura coccinea.349 Biosynthetic pathways to the kadcotriones, from a common lanostane precursor, have been proposed. The biosynthesis of volvalerenol A 643, from the roots of Valeriana hardwickii, is thought to involve head-to-tail linkages of two units of farnesyl diphosphate.350
10. References
- Y.-X. Li, S. W. A. Himaya and S.-K. Kim, Molecules, 2013, 18, 7886–7909 CrossRef CAS PubMed.
- X. Tian, H. Tang, H. Lin, G. Cheng, S. Wang and X. Zhang, Mini-Rev. Med. Chem., 2013, 13, 1709–1724 CrossRef CAS PubMed.
- M. R. Loizzo, F. Menichini and R. Tundis, Stud. Nat. Prod. Chem., 2013, 40, 1–31 CAS.
- K. Yasukawa, Horiz. Cancer Res., 2013, 51, 89–113 CAS.
- A. V. Avtonomova and L. M. Krasnopolskaya, Mikol. Fitopatol., 2013, 47, 3–11 CAS.
- S. Keypour, H. Riahi and H. Rafati, Faslnamah-i Giyahan-i Daruyi, 2013, 12, 13–24 Search PubMed.
- B. Boh, Recent Pat. Anti-Cancer Drug Discovery, 2013, 8, 255–287 CrossRef CAS PubMed.
- G.-S. Wu, J.-J. Guo, J.-L. Bao, X.-W. Li, X.-P. Chen, J.-J. Lu and Y.-T. Wang, Expert Opin. Invest. Drugs, 2013, 22, 981–992 CrossRef CAS PubMed.
- J. M. Castellano, A. Guinda, T. Delgado, M. Rada and J. A. Cayuela, Diabetes, 2013, 62, 1791–1799 CrossRef CAS PubMed.
- A. Alqahtani, K. Hamid, A. Kam, K. H. Wong, Z. Abdelhak, V. Razmovski-Naumovski, K. Chan, K. M. Li, P. W. Groundwater and G. Q. Li, Curr. Med. Chem., 2013, 20, 908–931 CAS.
- S. K. Parmar, T. P. Sharma, V. B. Airao, R. Bhatt, R. Aghara, S. Chavda, S. O. Rabadiya and A. P. Gangwal, Phytopharmacology, 2013, 4, 354–372 CAS.
- K. Kokila, S. D. Priyadharshini and V. Sujatha, Int. J. Pharm. Pharm. Sci., 2013, 5, 70–73 CAS.
- G.-W. Wang, H.-Z. Jin and W.-D. Zhang, Phytochem. Rev., 2013, 12, 915–942 CrossRef CAS.
- Y. Zhang, Z. Ning, C. Lu, S. Zhao, J. Wang, B. Liu, X. Xu and Y. Liu, Chem. Cent. J., 2013, 7, 153 CrossRef PubMed.
- D. Arora, A. Rani and A. Sharma, Pharmacogn. Rev., 2013, 7, 179–187 CrossRef PubMed.
- S.-N. Wang, S.-C. Yu, X.-D. Xu, S.-J. She and S.-H. Fan, Bopuxue Zazhi, 2013, 30, 447–460 CAS.
- Y. Chen, Q.-z. Wang and X. Feng, Zhongcaoyao, 2013, 44, 1679–1686 CAS.
- Y. Wang, R.-y. Chen and D.-q. Yu, Zhongguo Zhongyao Zazhi, 2013, 38, 2254–2263 CAS.
- F. Zhang, H.-y. Wang, L. Wang, C.-l. Luo and J. Cen, Xiandai Shengwuyixue Jinzhan, 2013, 13, 5387–5392 CAS.
- I. Chafchaouni-Moussaoui, Z. Charrouf and D. Guillaume, Nat. Prod. Commun., 2013, 8, 43–46 CAS.
- C. Sanchez-Quesada, A. Lopez-Biedma, F. Warleta, M. Campos, G. Beltran and J. J. Gaforio, J. Agric. Food Chem., 2013, 61, 12173–12182 CrossRef CAS PubMed.
- Q. Liu, W. Li, Y.-n. Zheng and H.-f. Wu, Jilin Nongye Daxue Xuebao, 2013, 35, 221–228 CAS.
- M. Malinowska, E. Sikora and J. Ogonowski, Acta Biochim. Pol., 2013, 60, 731–735 Search PubMed.
- F.-y. Sun, Y. Hu, Y. Zhao and X.-l. You, Zhongcaoyao, 2013, 44, 3422–3428 CAS.
- A. Faizal and D. Geelen, Phytochem. Rev., 2013, 12, 877–893 CrossRef CAS.
- B. S. Underwood, J. Tanuwidjaja, S.-S. Ng and T. F. Jamison, Tetrahedron, 2013, 69, 5205–5220 CrossRef CAS PubMed.
- R. H. Jiao, H. Xu, J. T. Cui, H. M. Ge and R. X. Tan, J. Appl. Microbiol., 2013, 114, 1046–1053 CrossRef CAS PubMed.
- M. Zhao, T. Godecke, J. Gunn, J.-A. Duan and C.-T. Che, Molecules, 2013, 18, 4054–4080 CrossRef CAS PubMed.
- Q.-Y. Ma, Y. Luo, S.-Z. Huang, Z.-K. Guo, H.-F. Dai and Y.-X. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 1214–1219 CrossRef CAS PubMed.
- P. Li, Y.-P. Deng, X.-X. Wei and J.-H. Xu, Nat. Prod. Res., 2013, 27, 17–22 CrossRef CAS PubMed.
- Y.-B. Li, R.-M. Liu and J.-J. Zhong, Fitoterapia, 2013, 84, 115–122 CrossRef CAS PubMed.
- M. Isaka, P. Chinthanom, S. Kongthong, K. Srichomthong and R. Choeyklin, Phytochemistry, 2013, 87, 133–139 CrossRef CAS PubMed.
- X.-R. Peng, J.-Q. Liu, Z.-H. Han, X.-X. Yuan, H.-R. Luo and M.-H. Qiu, Food Chem., 2013, 141, 920–926 CrossRef CAS PubMed.
- L.-L. Hu, Q.-Y. Ma, S.-Z. Huang, Z.-K. Guo, H.-X. Ma, J.-C. Guo, H.-F. Dai and Y.-X. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 357–362 CrossRef CAS PubMed.
- K.-W. Lin, Y.-T. Chen, S.-C. Yang, B.-L. Wei, C.-F. Hung and C.-N. Lin, Fitoterapia, 2013, 89, 231–238 CrossRef CAS PubMed.
- Y. Zhao, S.-Q. Li, H.-J. Li and W.-J. Lan, Chem. Nat. Compd., 2013, 49, 653–656 CrossRef CAS.
- Y.-M. Ying, W.-G. Shan, L.-W. Zhang, Y. Chen and Z.-J. Zhan, Helv. Chim. Acta, 2013, 96, 2092–2097 CrossRef CAS.
- C.-Y. Xue, J.-S. Li, H. Qi, F.-Y. Xi, W.-S. Xiang, J.-D. Wang and X.-J. Wang, J. Antibiot., 2013, 66, 735–737 CrossRef CAS PubMed.
- H. Guo, J. Si, Z.-H. Li, T. Feng, Z.-J. Dong, Y.-C. Dai and J.-K. Liu, J. Asian Nat. Prod. Res., 2013, 15, 253–257 CrossRef CAS PubMed.
- K. H. Kim, E. Moon, S. U. Choi, S. Y. Kim and K. R. Lee, J. Nat. Prod., 2013, 76, 845–851 CrossRef CAS PubMed.
- H. Kikuchi, Y. Sato, S. Kurata, Y. Katou and Y. Oshima, Tetrahedron, 2013, 69, 3536–3542 CrossRef CAS.
- C.-C. Liaw, Y.-C. Chen, G.-J. Huang, Y.-C. Tsai, S.-C. Chien, J.-H. Wu, S.-Y. Wang, L. K. Chao, P.-J. Sung, H.-C. Huang and Y.-H. Kuo, J. Nat. Prod., 2013, 76, 489–494 CrossRef CAS PubMed.
- C. T. Smith, B. Noll, T. G. Waddell and K. S. Knight, Nat. Prod. Commun., 2013, 8, 299–300 CAS.
- E. R. S. Nkanwen, A. S. Gojayev, H. K. Wabo, J. J. K. Bankeu, M. C. Iqbal, A. A. Guliyev and P. Tane, Fitoterapia, 2013, 86, 108–114 CrossRef CAS PubMed.
- A. Ali and M. Ali, Nat. Prod. Res., 2013, 27, 524–531 CrossRef CAS PubMed.
- J. A. Banday, S. Farooq, M. A. Qurishi, S. Koul and T. K. Razdan, Nat. Prod. Res., 2013, 27, 975–981 CrossRef CAS PubMed.
- Y. Nian, H. Zhu, W.-R. Tang, Y. Luo, J. Du and M.-H. Qiu, J. Nat. Prod., 2013, 76, 896–902 CrossRef CAS PubMed.
- M. M. Salama, S. M. Ezzat, R. S. El Dine, A. M. El-Sayed and A. A. Sleem, Z. Naturforsch., C: J. Biosci., 2013, 68, 461–470 CrossRef CAS.
- M. Shuaib, M. Ali and K. J. Naquvi, J. Nat. Prod. Plant Resour., 2013, 3, 12–19 CAS.
- M. Handa, T. Murata, K. Kobayashi, E. Selenge, T. Miyase, J. Batkhuu and F. Yoshizaki, Phytochemistry, 2013, 86, 168–175 CrossRef CAS PubMed.
- S. Lavoie, C. Gauthier, J. Legault, S. Mercier, V. Mshvildadze and A. Pichette, Beilstein J. Org. Chem., 2013, 9, 1333–1339 CrossRef CAS PubMed.
- S. Klaiklay, Y. Sukpondma, V. Rukachaisirikul and S. Phongpaichit, Phytochemistry, 2013, 85, 161–166 CrossRef CAS PubMed.
- C. G. Fru, L. P. Sandjo, V. Kuete, J. C. Liermann, D. Schollmeyer, S. O. Yeboah, R. Mapitse, B. M. Abegaz, B. T. Ngadjui and T. Opatz, Phytochem. Lett., 2013, 6, 676–680 CrossRef CAS.
- M. Ono, T. Ochiai, S. Yasuda, Y. Nishida, T. Tanaka, M. Okawa, J. Kinjo, H. Yoshimitsu and T. Nohara, Chem. Pharm. Bull., 2013, 61, 592–598 CrossRef CAS PubMed.
- S. A. Kolesnikova, E. G. Lyakhova, A. I. Kalinovsky, M. A. Pushilin, S. S. Afiyatullov, E. A. Yurchenko, S. A. Dyshlovoy, C. V. Minh and V. A. Stonik, J. Nat. Prod., 2013, 76, 1746–1752 CrossRef CAS PubMed.
- J. Colorado, D. Munoz, D. Marquez, M. E. Marquez, J. Lopez, O. P. Thomas and A. Martinez, Molecules, 2013, 18, 2598–2610 CrossRef CAS PubMed.
- J. Ahamad, K. J. Naquvi, M. Ali and S. R. Mir, Nat. Prod. J., 2013, 3, 260–267 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas, V. I. Kalinin, P. Jayasandhya, G. C. Rajan and K. P. Padmakumar, Nat. Prod. Commun., 2013, 8, 301–310 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko, I. Y. Dolmatov, V. I. Kalinin and V. A. Stonik, Nat. Prod. Commun., 2013, 8, 1527–1534 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas and V. I. Kalinin, Nat. Prod. Commun., 2013, 8, 1053–1058 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. S. Menchinskaya, D. L. Aminin and V. I. Kalinin, Nat. Prod. Res., 2013, 27, 1776–1783 CrossRef CAS PubMed.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjashchenko, P. S. Dmitrenok, V. I. Kalinin, S. Taboada and C. Avila, Biochem. Syst. Ecol., 2013, 51, 45–49 CrossRef CAS.
- B. Liu, L. Yang, Y.-K. Xu, S.-G. Liao, H.-R. Luo and Z. Na, Nat. Prod. Commun., 2013, 8, 1373–1376 CAS.
- Y.-M. Shi, X.-B. Wang, X.-N. Li, X. Luo, Z.-Y. Shen, Y.-P. Wang, W.-L. Xiao and H.-D. Sun, Org. Lett., 2013, 15, 5068–5071 CrossRef CAS PubMed.
- C.-Q. Liang, J. Hu, Y.-M. Shi, S.-Z. Shang, X. Du, R. Zhan, W.-G. Wang, W.-Y. Xiong, W.-L. Xiao, H.-B. Zhang and H.-D. Sun, Helv. Chim. Acta, 2013, 96, 1376–1385 CrossRef CAS.
- Q.-Y. Song, K. Jiang, Q.-Q. Zhao, K. Gao, X.-J. Jin and X.-J. Yao, Org. Biomol. Chem., 2013, 11, 1251–1258 CAS.
- L.-F. Li, H.-Q. Liu, R. Zhang, J. Zeng and L.-H. Wu, J. Asian Nat. Prod. Res., 2013, 15, 1168–1172 CrossRef CAS PubMed.
- B. Li, D.-Y. Kong, Y.-H. Shen, K.-L. Fu, R.-C. Yue, Z.-Z. Han, H. Yuan, Q.-X. Liu, L. Shan, H.-L. Li, X.-W. Yang and W.-D. Zhang, Chem. Commun., 2013, 49, 1187–1189 RSC.
- I. W. Lo, Y.-C. Lin, T.-C. Liao, S.-Y. Chen, P.-H. Lin, C.-T. Chien, S.-y. Chang and Y.-C. Shen, Food Chem., 2013, 136, 1095–1099 CrossRef CAS PubMed.
- X.-M. Gao, Y.-Q. Li, L.-D. Shu, Y.-Q. Shen, L.-Y. Yang, L.-M. Yang, Y.-T. Zheng, H.-D. Sun, W.-L. Xiao and Q.-F. Hu, Bull. Korean Chem. Soc., 2013, 34, 827–830 CrossRef CAS.
- C.-X. Zhou, L. Zou, L.-S. Gan and Y.-L. Cao, Org. Lett., 2013, 15, 2734–2737 CrossRef CAS PubMed.
- Y. Nian, H.-Y. Wang, L. Zhou, J. Su, Y. Li and M.-H. Qiu, Planta Med., 2013, 79, 60–69 CAS.
- Y.-R. Liu, Z.-J. Wu, C.-T. Li, F.-M. Xi, L.-N. Sun and W.-S. Chen, Planta Med., 2013, 79, 301–307 CrossRef CAS PubMed.
- N. Kongkum, P. Tuchinda, M. Pohmakotr, V. Reutrakul, P. Piyachaturawat, S. Jariyawat, K. Suksen, R. Akkarawongsapat, J. Kasisit and C. Napaswad, J. Nat. Prod., 2013, 76, 530–537 CrossRef CAS PubMed.
- X.-Y. Wang, G.-H. Tang, C.-M. Yuan, Y. Zhang, T. Zou, C. Yu, Q. Zhao, X.-J. Hao and H.-P. He, Fitoterapia, 2013, 85, 64–68 CrossRef CAS PubMed.
- B. T. T. Hien, L. T. P. Hoa, L. X. Tham and D. N. Quang, Fitoterapia, 2013, 91, 125–127 CrossRef CAS PubMed.
- B. Wang, X.-L. Wang, S.-Q. Wang, T. Shen, Y.-Q. Liu, H. Yuan, H.-X. Lou and X.-N. Wang, J. Nat. Prod., 2013, 76, 1573–1579 CrossRef CAS PubMed.
- M. A. Tantry, S. Shah, M. Y. Dar, M. M. Mir, K. Ghazanfar, F. A. Sheikh, M. A. Khuroo and S. Akbar, Nat. Prod. Res., 2013, 27, 171–175 CrossRef CAS PubMed.
- S. Gandhe, S. Lakavath, S. Palatheeya, W. Schuehly, K. Amancha, R. K. R. Nallamaddi, A. Palepu, Y. Thakur, V. R. A. R. Belvotagi, R. K. Bobbala, V. Narasimha Appa Rao Achanta and O. Kunert, Chem. Biodiversity, 2013, 10, 1613–1622 CAS.
- L. Pan, U. M. Acuna, J. Li, N. Jena, T. N. Ninh, C. M. Pannell, H. Chai, J. R. Fuchs, E. J. Carcache de Blanco, D. D. Soejarto and A. D. Kinghorn, J. Nat. Prod., 2013, 76, 394–404 CrossRef CAS PubMed.
- U. Prawat, O. Chairerk, R. Lenthas, A.-W. Salae and P. Tuntiwachwuttikul, Phytochem. Lett., 2013, 6, 286–290 CrossRef CAS.
- W. Jiao, X. Chen, H. Wang, R. Lu and H. Shao, Fitoterapia, 2013, 84, 1–5 CrossRef CAS PubMed.
- J.-M. Chen, L.-B. Wei, C.-L. Lu and G.-X. Zhou, J. Asian Nat. Prod. Res., 2013, 15, 1088–1093 CrossRef PubMed.
- L.-Y. Jia, J. Wang, C.-N. Lv, T.-Y. Xu, B.-Z. Jia and J.-C. Lu, J. Asian Nat. Prod. Res., 2013, 15, 1050–1054 CrossRef CAS PubMed.
- T. C. C. Leite, F. H. A. Leite, I. J. C. Vieira, R. Braz Filho and A. Branco, Nat. Prod. Commun., 2013, 8, 1049–1052 CAS.
- T. D. Thang, P.-C. Kuo, G.-J. Huang, N. H. Hung, B.-S. Huang, M.-L. Yang, N. X. Luong and T.-S. Wu, Molecules, 2013, 18, 4477–4486 CrossRef CAS PubMed.
- H.-Y. Wang, J.-S. Wang, Y. Zhang, J. Luo, M.-H. Yang, X.-B. Wang and L.-Y. Kong, Chem. Pharm. Bull., 2013, 61, 1075–1080 CrossRef CAS PubMed.
- Z. Zhao, K. Matsunami, H. Otsuka, T. Shinzato and Y. Takeda, J. Nat. Med., 2013, 67, 503–511 CrossRef CAS PubMed.
- L.-B. Wei, H.-E. Yuan, J.-M. Chen, Q.-Q. Wang, H. Wang, W.-C. Ye and G.-X. Zhou, Helv. Chim. Acta, 2013, 96, 150–157 CrossRef CAS.
- D.-Y. Lee, H.-J. Noh, J. Choi, K.-H. Lee, M.-H. Lee, J.-H. Lee, Y. Hong, S.-E. Lee, S.-Y. Kim and G.-S. Kim, Molecules, 2013, 18, 3725–3732 CrossRef CAS PubMed.
- C. Wang, F.-Q. Xu, J.-H. Shang, H. Xiao, W.-W. Fan, F.-W. Dong, J.-M. Hu and J. Zhou, J. Ethnopharmacol., 2013, 148, 812–817 CrossRef CAS PubMed.
- T. K. Naubeev, A. A. Zhanibekov, K. K. Uteniyazov, K. M. Bobakulov and N. D. Abdullaev, Chem. Nat. Compd., 2014, 49, 1048–1049 CrossRef CAS.
- A. A. Zhanibekov, T. K. Naubeev, K. K. Uteniyazov, K. M. Bobakulov and N. D. Abdullaev, Chem. Nat. Compd., 2013, 49, 475–477 CrossRef CAS.
- B.-J. Djimtombaye, O. Alankus-Caliskan, D. Guelcemal, I. A. Khan, H. Anil and E. Bedir, Chem. Biodiversity, 2013, 10, 1328–1334 CAS.
- H.-J. Li, L.-H. Mu, X.-Z. Dong, X.-Y. Ge and P. Liu, Nat. Prod. Res., 2013, 27, 1987–1993 CrossRef CAS PubMed.
- X.-T. Zhang, L. Wang, S.-W. Ma, Q.-W. Zhang, Y. Liu, L.-H. Zhang and W.-C. Ye, J. Nat. Med., 2013, 67, 375–380 CrossRef CAS PubMed.
- P. Limtrakul, P. Pitchakarn and S. Suzuki, Carcinogenesis, 2013, 275–296 CAS.
- B.-H. Cheng, J.-C. Chen, J.-Q. Liu, L. Zhou and M.-H. Qiu, Helv. Chim. Acta, 2013, 96, 1111–1120 CrossRef CAS.
- L.-Y. Liu, Z.-H. Li, Z.-H. Ding, Z.-J. Dong, G.-T. Li, Y. Li and J.-K. Liu, J. Nat. Prod., 2013, 76, 79–84 CrossRef CAS PubMed.
- Y.-W. Liao, C.-R. Chen, J.-J. Chuu, H.-C. Huang, J.-L. Hsu, T.-C. Huang, Y.-H. Kuo and C.-I. Chang, J. Chin. Chem. Soc., 2013, 60, 526–530 CrossRef CAS.
- T. Kikuchi, R. Okada, Y. Harada, K. Ikushima, T. Yamakawa, T. Yamada and R. Tanaka, Nat. Prod. Commun., 2013, 8, 1367–1369 CAS.
- D. Huang, S. Qing, G. Zeng, Y. Wang, H. Guo, J. Tan and Y. Zhou, Fitoterapia, 2013, 86, 144–148 CrossRef CAS PubMed.
- R. Graziose, M. H. Grace, T. Rathinasabapathy, P. Rojas-Silva, C. Dekock, A. Poulev, M. A. Lila, P. Smith and I. Raskin, Phytochemistry, 2013, 87, 78–85 CrossRef CAS PubMed.
- X. Wang and W. Wang, Molecules, 2013, 18, 1405–1417 CrossRef CAS PubMed.
- P. Cao, G. Liang, X. Gao, X. Wang and Z. Li, Arch. Pharmacal Res., 2013, 36, 322–326 CrossRef CAS PubMed.
- A. Ikram, M. A. Versiani, S. Shamshad, S. K. Ahmed, S. T. Ali and S. Faizi, Rec. Nat. Prod., 2013, 7, 302–306 CAS.
- L. K. Wah, F. Abas, G. A. Cordell, H. Ito and I. S. Ismail, Steroids, 2013, 78, 210–219 CrossRef CAS PubMed.
- D.-X. Wang and S.-M. Yang, Z. Naturforsch., C: J. Biosci., 2013, 68, 82–86 CrossRef CAS.
- H. H. F. Koolen, E. R. Soares, F. M. A. da Silva, A. A. de Oliveira, A. Q. L. de Souza, L. S. de Medeiros, E. Rodrigues-Filho, B. C. Cavalcanti, C. O. Pessoa, M. O. Moraes, M. J. Salvador and A. D. L. de Souza, Nat. Prod. Res., 2013, 27, 2118–2125 CrossRef CAS PubMed.
- Z.-L. Hong, J. Xiong, S.-B. Wu, J.-J. Zhu, J.-L. Hong, Y. Zhao, G. Xia and J.-F. Hu, Phytochemistry, 2013, 86, 159–167 CrossRef CAS PubMed.
- U. W. Hawas, A. M. Gamal-Eldeen, S. K. El-Desouky, Y.-K. Kim, A. Huefner and R. Saf, Z. Naturforsch., C: J. Biosci., 2013, 68, 29–38 CrossRef CAS.
- Y. Zhou, J. Yang, L. Peng, Y. Li and W. Chen, Fitoterapia, 2013, 87, 65–68 CrossRef CAS PubMed.
- R. B. Williams, V. L. Norman, M. G. Goering, M. O'Neil-Johnson, G. R. Eldridge and C. M. Starks, J. Nat. Prod., 2013, 76, 1592–1597 CrossRef CAS PubMed.
- F. Yang, H. Shi, X. Zhang, H. Yang, Q. Zhou and L. Yu, Food Chem., 2013, 141, 3606–3613 CrossRef CAS PubMed.
- X.-S. Zhang, X.-L. Bi, X. Wan, J.-Q. Cao, X.-C. Xia, Y.-P. Diao and Y.-Q. Zhao, Bioorg. Med. Chem. Lett., 2013, 23, 297–300 CrossRef CAS PubMed.
- K. J. Sakah, T. Wang, L. Liu, Y. Chen, L. Han and Y. Zhang, Acta Pharm. Sin. B, 2013, 3, 381–384 CrossRef.
- J.-Q. Cao, P. Fu and Y.-Q. Zhao, Zhongcaoyao, 2013, 44, 137–140 CAS.
- Q.-X. Guan, D.-Y. Sun, J.-H. Liu, W. Li, Q. Meng, C. Geng and J.-Y. Yin, Chin. Chem. Lett., 2013, 24, 524–526 CrossRef CAS.
- J.-G. Cho, D.-Y. Lee, S. Shrestha, S.-K. Lee, H.-M. Kang, S.-H. Son, D.-C. Yang and N.-I. Baek, Chem. Nat. Compd., 2013, 49, 882–887 CrossRef CAS.
- I.-M. Chung, M. Ali, Y.-P. Hong and A. Ahmad, Asian J. Chem., 2013, 25, 4667–4669 CAS.
- J.-P. Liu, X. Tian, H.-Y. Liu, Q.-H. Zhang, D. Lu, P.-Y. Li and C.-F. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 974–978 CrossRef CAS PubMed.
- C. Wu, Y.-H. Duan, M.-M. Li, W. Tang, X. Wu, G.-C. Wang, W.-C. Ye, G.-X. Zhou and Y.-L. Li, Planta Med., 2013, 79, 1348–1355 CrossRef CAS PubMed.
- K. Yoshizaki, H. P. Devkota and S. Yahara, Chem. Pharm. Bull., 2013, 61, 273–278 CrossRef CAS PubMed.
- K. Yoshizaki, H. P. Devkota, H. Fujino and S. Yahara, Chem. Pharm. Bull., 2013, 61, 344–350 CrossRef CAS PubMed.
- H.-P. Wang, X.-B. Yang, X.-W. Yang, J.-X. Liu, W. Xu, Y.-B. Zhang, L.-X. Zhang and Y.-P. Wang, J. Asian Nat. Prod. Res., 2013, 15, 579–587 CrossRef CAS PubMed.
- S. M. Lee, S. C. Kim, J. Oh, J. H. Kim and M. K. Na, Phytochem. Lett., 2013, 6, 620–624 CrossRef CAS.
- Y. Wang, B. Ding, D. Luo, L.-Y. Chen, Y.-L. Hou, Y. Dai and X.-S. Yao, Fitoterapia, 2013, 90, 185–191 CrossRef CAS PubMed.
- Y. Wu, J. Zhang, D. Wang, J.-G. Liu and Y. Hu, Chem. Nat. Compd., 2013, 49, 677–681 CrossRef CAS.
- S. Y. Lee, J. S. Kim, J.-H. Lee, Y. S. Kim and S. S. Kang, Bull. Korean Chem. Soc., 2013, 34, 657–660 CrossRef CAS.
- Y. Zhang, L.-F. Han, K. J. Sakah, Z.-Z. Wu, L.-L. Liu, K. Agyemang, X.-M. Gao and T. Wang, Molecules, 2013, 18, 10352–10366 CrossRef CAS PubMed.
- Z. Xiang, J. Lv, Z. Zhou, Y. Li, D. Dou and J. Zhao, Nat. Prod. Res., 2013, 27, 1271–1276 CrossRef CAS PubMed.
- H.-Z. Fu, R.-J. Zhong, D.-M. Zhang and D. Wang, J. Asian Nat. Prod. Res., 2013, 15, 1139–1143 CrossRef CAS PubMed.
- Q. Zeng, B. Guan, J. Ren, C.-H. Wang, X.-r. Cheng, J.-J. Qin, S.-K. Yan, H. Z. Jin and W. D. Zhang, Fitoterapia, 2013, 86, 217–221 CrossRef CAS PubMed.
- C.-L. Cheng, Z.-Z. Wang, P.-L. Li, X.-W. Zhang, R.-C. Wu, H.-Y. Zhu, X.-L. Tang and G.-Q. Li, Chin. Chem. Lett., 2013, 24, 1080–1082 CrossRef CAS.
- J. T. Cheng, Y. Q. Han, J. He, W. X. De, L. B. Dong, L. Y. Peng, Y. Li and Q. S. Zhao, Arch. Pharmacal Res., 2013, 36, 1084–1089 CrossRef CAS PubMed.
- J.-B. Gao, G.-Y. Li, J. Huang, H.-Y. Wang, K. Zhang and J.-H. Wang, J. Asian Nat. Prod. Res., 2013, 15, 400–403 CrossRef CAS PubMed.
- H. Chen, S.-G. Ma, Z.-F. Fang, J. Bai, S.-S. Yu, X.-G. Chen, Q. Hou, S.-P. Yuan and X. Chen, Chem. Biodiversity, 2013, 10, 695–702 CAS.
- D.-S. Yang, W.-B. Peng, Z.-L. Li, X. Wang, J.-G. Wei, K.-C. Liu, Y.-P. Yang and X.-L. Li, Nat. Prod. Bioprospect., 2013, 3, 112–116 CrossRef CAS.
- Z.-h. Guo, J. Huang, G.-s. Wan, X.-l. Huo and H.-Y. Gao, J. Nat. Med., 2013, 67, 844–849 CrossRef CAS PubMed.
- I. J. C. Vieira, O. d. A. Azevedo, J. Jorgeanede Souza, R. Braz-Filho, M. d. S. Goncalves and M. Francisco de Araujo, Molecules, 2013, 18, 2589–2597 CrossRef CAS PubMed.
- Y. Zhang, C.-P. Tang, C.-Q. Ke, S. Yao and Y. Ye, Chem. Biodiversity, 2013, 10, 1630–1637 CAS.
- M.-L. Han, Y. Shen, G.-C. Wang, Y. Leng, H. Zhang and J.-M. Yue, J. Nat. Prod., 2013, 76, 1319–1327 CrossRef CAS PubMed.
- C.-M. Yuan, Y. Zhang, G.-H. Tang, Y. Li, H.-P. He, S.-F. Li, L. Hou, X.-Y. Li, Y.-T. Di, S.-L. Li, H.-M. Hua and X.-J. Hao, Planta Med., 2013, 79, 163–168 CAS.
- Y. Zhang, F. Hao, N. Liu, Y. Xu, A. Jia, Z. Yang, X. Xia and C. Liu, J. Antibiot., 2013, 66, 679–682 CrossRef CAS PubMed.
- C. Long, Y. Aussagues, N. Molinier, L. Marcourt, L. Vendier, A. Samson, V. Poughon, P. B. Chalo Mutiso, F. Ausseil, F. Sautel, P. B. Arimondo and G. Massiot, Phytochemistry, 2013, 94, 184–191 CrossRef CAS PubMed.
- C. Y. Ragasa, O. B. Torres, L. O. Bernardo, E. H. Mandia, M.-J. Don and C.-C. Shen, Phytochem. Lett., 2013, 6, 514–518 CrossRef CAS.
- H.-Y. Chen, Z.-Y. Hu, C.-P. Tang, R. J. Quinn, Y. Feng, S. Yao and Y. Ye, Tetrahedron Lett., 2013, 54, 4150–4153 CrossRef CAS.
- Y. Zhang, J.-S. Wang, X.-B. Wang, Y.-C. Gu and L.-Y. Kong, Chem. Pharm. Bull., 2013, 61, 75–81 CrossRef CAS PubMed.
- Q. Jin, C. Lee, J. W. Lee, M.-S. Lee, M. K. Lee and B. Y. Hwang, Nat. Prod. Sci., 2013, 19, 342–346 CAS.
- S.-H. Dong, J. Liu, Y.-Z. Ge, L. Dong, C.-H. Xu, J. Ding and J.-M. Yue, Phytochemistry, 2013, 85, 175–184 CrossRef CAS PubMed.
- P. Phuwapraisirisan, S. Sombund, S. Tip-pyang and P. Siripong, Nat. Prod. Res., 2013, 27, 753–760 CrossRef CAS PubMed.
- W. d. S. Terra, I. J. C. Vieira, R. Braz-Filho, W. R. de Freitas, M. M. Kanashiro and M. C. M. Torres, Molecules, 2013, 18, 12180–12191 CrossRef CAS PubMed.
- Y. Zhang, J.-S. Wang, D.-D. Wei, Y.-C. Gu, X.-B. Wang and L.-Y. Kong, J. Nat. Prod., 2013, 76, 1191–1195 CrossRef CAS PubMed.
- C.-M. Yuan, G.-H. Tang, Y. Zhang, X.-Y. Wang, M.-M. Cao, F. Guo, Y. Li, Y.-T. Di, S.-L. Li, H.-M. Hua, H.-P. He and X.-J. Hao, J. Nat. Prod., 2013, 76, 1166–1174 CrossRef CAS PubMed.
- H.-W. Wang, J.-Q. Liu, J.-X. Chen, Y.-F. Yang, Y.-X. Yan, Z.-R. Li and M.-H. Qiu, Nat. Prod. Bioprospect., 2013, 3, 33–37 CrossRef CAS.
- J. Li, M.-Y. Li, T. Bruhn, F. Z. Katele, Q. Xiao, P. Pedpradab, J. Wu and G. Bringmann, Org. Lett., 2013, 15, 3682–3685 CrossRef CAS PubMed.
- S. Choodej, D. Sommit and K. Pudhom, Bioorg. Med. Chem. Lett., 2013, 23, 3896–3900 CrossRef CAS PubMed.
- Z.-G. Zhang, Y.-T. Cheng, G.-L. Hu and G.-N. Li, Helv. Chim. Acta, 2013, 96, 2228–2232 CrossRef CAS.
- C.-S. Jiang, Y. Li, Z.-Z. Wang, X.-Y. Huang, W. Xiao and Y.-W. Guo, Nat. Prod. Bioprospect., 2013, 3, 267–270 CrossRef CAS.
- H.-Y. Wang, J.-S. Wang, S.-M. Shan, X.-B. Wang, J. Luo, M.-H. Yang and L.-Y. Kong, Planta Med., 2013, 79, 1767–1774 CrossRef CAS PubMed.
- B. Siva, G. Suresh, B. Poornima, A. Venkanna, K. Suresh Babu, K. Rajendra Prasad, L. Prasanna Anjaneya Reddy, A. S. Sreedhar and C. Venkata Rao, Tetrahedron Lett., 2013, 54, 2934–2937 CrossRef CAS.
- J.-Q. Zhao, Y.-M. Wang, H.-P. He, S.-H. Li, X.-N. Li, C.-R. Yang, D. Wang, H.-T. Zhu, M. Xu and Y.-J. Zhang, Org. Lett., 2013, 15, 2414–2417 CrossRef CAS PubMed.
- A. T. Tontsa, P. Mkounga, F. N. Njayou, J. Manautou, M. Kirk, P. G. Hultin and A. E. Nkengfack, Chem. Pharm. Bull., 2013, 61, 1178–1183 CrossRef CAS.
- X.-B. Yang, P. Qian, X.-W. Yang, J.-X. Liu, N.-B. Gong and Y. Lv, J. Asian Nat. Prod. Res., 2013, 15, 1130–1138 CrossRef CAS PubMed.
- X.-B. Wang, Y. Zhang, J.-S. Wang, Y.-C. Gu and L.-Y. Kong, Tetrahedron Lett., 2013, 54, 6023–6028 CrossRef CAS.
- Y. Zhang, J.-S. Wang, Y.-C. Gu and L.-Y. Kong, Phytochem. Lett., 2013, 6, 539–543 CrossRef CAS.
- X.-J. Dong, Y.-F. Zhu, G.-H. Bao, F.-L. Hu and G.-W. Qin, Molecules, 2013, 18, 2840–2850 CrossRef CAS PubMed.
- Y. Nagakura, A. E. Nugroho, Y. Hirasawa, T. Hosoya, A. Rahman, I. Kusumawati, N. C. Zaini and H. Morita, J. Nat. Med., 2013, 67, 381–385 CrossRef CAS PubMed.
- A. Sakamoto, Y. Tanaka, T. Inoue, T. Kikuchi, T. Kajimoto, O. Muraoka, T. Yamada and R. Tanaka, Fitoterapia, 2013, 90, 20–29 CrossRef CAS PubMed.
- T. Akihisa, X. Pan, Y. Nakamura, T. Kikuchi, N. Takahashi, M. Matsumoto, E. Ogihara, M. Fukatsu, K. Koike and H. Tokuda, Phytochemistry, 2013, 89, 59–70 CrossRef CAS PubMed.
- A. E. Nugroho, M. Okuda, Y. Yamamoto, Y. Hirasawa, C.-P. Wong, T. Kaneda, O. Shirota, A. H. A. Hadi and H. Morita, Tetrahedron, 2013, 69, 4139–4145 CrossRef CAS.
- S. Su, L. Shen, Y. Zhang, J. Liu, J. Cai, L. Hao, Y. Feng and S. Yang, Phytochem. Lett., 2013, 6, 418–424 CrossRef CAS.
- Q. Zhang, J.-K. Li, R. Ge, J.-Y. Liang, Q.-S. Li and Z.-D. Min, Fitoterapia, 2013, 90, 192–198 CrossRef CAS PubMed.
- J. Xu, G. Ni, S. Yang and J. Yue, Chin. J. Chem., 2013, 31, 72–78 CrossRef CAS.
- K. Hu, J.-Q. Liu, X.-n. Li, J.-C. Chen, W.-M. Zhang, Y. Li, L.-q. Li, L.-l. Guo, W.-g. Ma and M.-H. Qiu, Org. Lett., 2013, 15, 3902–3905 CrossRef CAS PubMed.
- T. Inoue, Y. Matsui, T. Kikuchi, Y. In, T. Yamada, O. Muraoka, S. Matsunaga and R. Tanaka, Org. Lett., 2013, 15, 3018–3021 CrossRef CAS PubMed.
- J.-B. Xu, Y. Lin, S.-H. Dong, F. Wang and J.-M. Yue, J. Nat. Prod., 2013, 76, 1872–1880 CrossRef CAS PubMed.
- B. Jabeen, N. Riaz, M. Saleem, M. A. Naveed, M. Ahmed, M. N. Tahir, G. Pescitelli, M. Ashraf, S. A. Ejaz, I. Ahmed and A. Jabbar, Z. Naturforsch., B: J. Chem. Sci., 2013, 68, 1041–1048 CAS.
- Y.-B. Wu, Z.-Y. Ni, C.-H. Huo, J. Su, M. Dong, F. Sauriol, Q.-W. Shi, Y.-C. Gu and H. Kiyota, Biosci., Biotechnol., Biochem., 2013, 77, 736–740 CrossRef CAS PubMed.
- K. Toume, K. Kamiya, M. A. Arai, N. Mori, S. K. Sadhu, F. Ahmed and M. Ishibashi, Org. Lett., 2013, 15, 6106–6109 CrossRef CAS PubMed.
- H. Chen, J. Zhang, M.-Y. Li, T. Satyanandamurty and J. Wu, Chem. Biodiversity, 2013, 10, 612–620 CAS.
- S. A. M. Abdelgaleil, M. Doe and M. Nakatani, Phytochemistry, 2013, 96, 312–317 CrossRef CAS PubMed.
- J. Luo, Y. Li, J.-S. Wang and L.-Y. Kong, Nat. Prod. Res., 2013, 27, 597–602 CrossRef CAS PubMed.
- J. Li, M.-Y. Li, Q. Xiao, P. Pedpradab and J. Wu, Phytochem. Lett., 2013, 6, 482–485 CrossRef CAS.
- C.-M. Yuan, Y. Zhang, G.-H. Tang, Y.-T. Di, M.-M. Cao, X.-Y. Wang, G.-Y. Zuo, S.-L. Li, H.-M. Hua, H.-P. He and X.-J. Hao, J. Nat. Prod., 2013, 76, 327–333 CrossRef CAS PubMed.
- Z. Su, J. Hao, Z. Xu, R. Huang, N. Zhang and S. Qiu, Nat. Prod. Res., 2013, 27, 2016–2021 CrossRef CAS PubMed.
- Y. Wang, W.-J. Wang, C. Su, D.-M. Zhang, L.-P. Xu, R.-R. He, L. Wang, J. Zhang, X.-Q. Zhang and W.-C. Ye, Bioorg. Med. Chem. Lett., 2013, 23, 654–657 CrossRef CAS PubMed.
- S. A. Khan and K. M. Shamsuddin, Phytochemistry, 1980, 19, 2484–2485 CrossRef CAS.
- X.-q. Liu, Q.-p. Zou and C.-z. Lu, Hunan Zhongyiyao Daxue Xuebao, 2013, 33, 10–32 CAS.
- Z. Li, J. Xu, W. Xie and X. Wu, Yaoxue Jinzhan, 2013, 37, 368–375 CAS.
- D. Saeki, T. Yamada, Y. In, T. Kajimoto, R. Tanaka, Y. Iizuka, T. Nakane, A. Takano and K. Masuda, Tetrahedron, 2013, 69, 1583–1589 CrossRef CAS.
- D. Zhang, W. Chen, W. Chen, X. Song, C. Han, Y. Wang and G. Chen, Molecules, 2013, 18, 14496–14504 CrossRef PubMed.
- J.-Y. Zhang, W.-H. Chen, D.-S. Zhang, G.-Y. Chen, X.-M. Zhou, X.-P. Song and C.-R. Han, Molecules, 2014, 19, 18618–18619 CrossRef CAS.
- Y.-d. Xu and L. Yin, Zhongcaoyao, 2013, 44, 935–937 CAS.
- A. Zarrelli, A. Ladhari, R. Haouala, G. Di Fabio, L. Previtera and M. Della Greca, Helv. Chim. Acta, 2013, 96, 2200–2206 CrossRef CAS.
- A. Zarrelli, M. Della Greca, A. Ladhari, R. Haouala and L. Previtera, Helv. Chim. Acta, 2013, 96, 1036–1045 CrossRef CAS.
- T. T. Nguyen, D. D. Thien, L. T. H. Nhung, T. V. Chien and T. V. Sung, Chem. Nat. Compd., 2013, 49, 274–276 CrossRef CAS.
- W. Kaweetripob, C. Mahidol, H. Prawat and S. Ruchirawat, Phytochemistry, 2013, 96, 404–417 CrossRef CAS PubMed.
- Y. Zhou, X. Jia, J. Shi, Y. Xu, L. Jing and L. Jia, Helv. Chim. Acta, 2013, 96, 533–537 CrossRef CAS.
- N. M. Al-Musayeib, G. A. Mohamed, S. R. M. Ibrahim and S. A. Ross, Med. Chem. Res., 2013, 22, 5297–5302 CrossRef CAS.
- Shakeel-u-Rehman, S. N. Sofi, M. A. Khuroo, S. C. Taneja, K. A. Bhat and R. Vishwakarma, Nat. Prod. Res., 2013, 27, 2033–2038 CrossRef CAS PubMed.
- M. T. T. Nguyen and N. T. Nguyen, Nat. Prod. Res., 2013, 27, 61–67 CrossRef CAS PubMed.
- Y. Chen, Y. Zhao, M. Wang, Q. Wang, Y. Shan, F. Guan and X. Feng, Chem. Nat. Compd., 2014, 49, 1087–1090 CrossRef CAS.
- T. Murata, A. Suzuki, N. Mafune, E. Sato, T. Miyase and F. Yoshizaki, Chem. Pharm. Bull., 2013, 61, 134–143 CrossRef CAS PubMed.
- J. Liu, J. Zhang, F. Wang, Y.-f. Zou and X.-f. Chen, Carbohydr. Res., 2013, 370, 76–81 CrossRef CAS PubMed.
- Q.-m. Xu, Z. Shu, W.-f. Zhu, Y.-l. Liu, X.-r. Li and S.-l. Yang, Planta Med., 2013, 79, 506–512 CrossRef CAS PubMed.
- B. M. Fraga, C. E. Diaz and L. J. Amador, Tetrahedron Lett., 2013, 54, 4337–4338 CrossRef CAS.
- Z.-W. Wang, J.-S. Wang, J. Luo and L.-Y. Kong, Fitoterapia, 2013, 90, 30–37 CrossRef CAS PubMed.
- M.-J. Zhang, B. Liu, S.-G. Liao, Y.-K. Xu, D.-Q. Feng, K.-L. Ji and Y. Li, Molecules, 2013, 18, 9727–9734 CrossRef CAS PubMed.
- B. Jabeen, N. Riaz, M. Saleem, M. A. Naveed, M. Ashraf, U. Alam, H. M. Rafiq, R. B. Tareen and A. Jabbar, Phytochemistry, 2013, 96, 443–448 CrossRef CAS PubMed.
- M.-m. Xu, Y.-h. Duan, Y. Dai, Z.-z. Wang, W. Xiao and X.-s. Yao, Zhongcaoyao, 2013, 44, 2639–2641 CAS.
- N. Sultana, Z. S. Saify, M. Saleem and M. Kamal, Nat. Prod. Res., 2013, 27, 1277–1286 CrossRef CAS PubMed.
- M. J. Nunez, A. E. Ardiles, M. L. Martinez, D. Torres-Romero, I. A. Jimenez and I. L. Bazzocchi, Phytochem. Lett., 2013, 6, 148–151 CrossRef CAS.
- M. A. Makboul, A. A. Attia, S. F. Farag, N. M. Mohamed, S. A. Ross, Y. Takaya and M. Niwa, Nat. Prod. Res., 2013, 27, 2046–2052 CrossRef CAS PubMed.
- A. U. Din, G. Uddin, N. Hussain and M. I. Choudary, J. Braz. Chem. Soc., 2013, 24, 663–668 CAS.
- Y.-L. Huang, S. Nagai, T. Tanaka, Y. Matsuo, Y. Saito and I. Kouno, Molecules, 2013, 18, 4868–4875 CrossRef CAS PubMed.
- B.-B. Zhang, Q. Jiang, Z.-X. Liao, C. Liu and S.-J. Liu, Helv. Chim. Acta, 2013, 96, 1281–1289 CrossRef CAS.
- Y.-H. Pei, O.-K. Kwon, J.-S. Lee, H.-J. Cha, K.-S. Ahn, S.-R. Oh, H.-K. Lee and Y.-W. Chin, Chem. Pharm. Bull., 2013, 61, 674–677 CrossRef CAS PubMed.
- G. Brahmachari, N. C. Mandal, R. Roy, R. Ghosh, S. Barman, S. Sarkar, S. K. Jash and S. Mondal, Fitoterapia, 2013, 90, 104–111 CrossRef CAS PubMed.
- Z. He, F. Liang, J. Lu and Y. Pan, Eur. J. Med. Chem., 2013, 67, 390–397 CrossRef CAS PubMed.
- M.-y. Lu, Z.-x. Liao, L.-j. Ji and H.-f. Sun, Fitoterapia, 2013, 85, 119–124 CrossRef CAS PubMed.
- C.-H. Lin, H.-S. Chang, H.-R. Liao, I.-S. Chen and I.-L. Tsai, Int. J. Mol. Sci., 2013, 14, 8890–8898 CrossRef PubMed.
- Q. B. Wu, Y. Wang, L. Liang, Q. Jiang, M. L. Guo and J. J. Zhang, Nat. Prod. Res., 2013, 27, 1353–1360 CrossRef CAS PubMed.
- W. Zhang, M.-N. Yao, H.-F. Tang, X.-R. Tian, M.-C. Wang, L.-J. Ji and M.-M. Xi, Planta Med., 2013, 79, 673–679 CrossRef CAS PubMed.
- S. Wang, G. Ma, M. Zhong, S. Yu, X. Xu, Y. Hu, Y. Zhang, H. Wei and J. Yang, Fitoterapia, 2013, 90, 14–19 CrossRef CAS PubMed.
- H.-Z. Fu, C.-J. Li, J.-Z. Yang, X.-G. Chen and D.-M. Zhang, Phytochemistry, 2013, 85, 167–174 CrossRef CAS PubMed.
- Y. Zhou, X.-Y. Chai, K.-W. Zeng, J.-Y. Zhang, N. Li, Y. Jiang and P.-F. Tu, Planta Med., 2013, 79, 70–77 CAS.
- C. Baecker, K. Jenett-Siems, K. Siems, M. Wurster, A. Bodtke, C. Chamseddin, M. Cruesemann and U. Lindequist, Planta Med., 2013, 79, 1461–1469 CrossRef CAS PubMed.
- C.-X. Zhang, D.-M. Zhang, M.-F. Chen, S.-Y. Guan, J.-H. Yao, X.-X. He, L.-F. Lei, Y. Zhong, Z.-F. Wang and W.-C. Ye, Planta Med., 2013, 79, 978–986 CrossRef CAS PubMed.
- S. Sugimoto, K. Matsunami and H. Otsuka, Chem. Pharm. Bull., 2013, 61, 581–586 CrossRef CAS PubMed.
- J.-Q. Yu, A.-J. Deng, L.-Q. Wu, Z.-H. Zhang, Y. Liu, W.-J. Wang and H.-L. Qin, Fitoterapia, 2013, 85, 101–108 CrossRef CAS PubMed.
- S. Pawelec, D. Jedrejek, M. Kowalczyk, L. Pecio, M. Masullo, S. Piacente, F. A. Macias, A. M. Simonet, W. Oleszek and A. Stochmal, J. Agric. Food Chem., 2013, 61, 9789–9796 CrossRef CAS PubMed.
- N. Boutaghane, L. Voutquenne-Nazabadioko, D. Harakat, A. Simon and Z. Kabouche, Phytochemistry, 2013, 93, 176–181 CrossRef CAS PubMed.
- Y.-Y. Li, Z. Xiang, H. Cui, H. Xiao, T.-G. Kang, D.-Q. Dou and H.-X. Kuang, Nat. Prod. Res., 2013, 27, 208–214 CrossRef CAS PubMed.
- Y. Dai, L. Harinantenaina, P. J. Brodie, C. Birkinshaw, R. Randrianaivo, W. Applequist, M. Ratsimbason, V. E. Rasamison, Y. Shen, K. TenDyke and D. G. I. Kingston, Chem. Biodiversity, 2013, 10, 233–240 CAS.
- T. Hoshino, Y. Narukawa, Y. Haishima, Y. Goda and F. Kiuchi, J. Nat. Med., 2013, 67, 386–389 CrossRef CAS PubMed.
- W. Yuan, P. Wang, Z. Su, V. S. Wang and S. Li, Phytochemistry, 2013, 90, 95–105 CrossRef CAS PubMed.
- J.-M. Tian, X.-Y. Fu, Q. Zhang, H.-P. He, J.-M. Gao and X.-J. Hao, Biochem. Syst. Ecol., 2013, 48, 288–292 CrossRef CAS.
- C.-J. Zheng, G.-L. Jin, J.-P. Zou, Y.-P. Jiang, P.-X. Sun and L.-P. Qin, J. Nat. Med., 2013, 67, 190–195 CrossRef CAS PubMed.
- R. M. Borges, S. S. Valenca, A. A. Lopes, N. d. S. Barbi and A. J. R. da Silva, Phytochem. Lett., 2013, 6, 96–100 CrossRef CAS.
- Q. Fu, K. Zan, M.-B. Zhao, S.-X. Zhou, S.-P. Shi, Y. Jiang and P.-F. Tu, J. Asian Nat. Prod. Res., 2013, 15, 610–618 CrossRef CAS PubMed.
- H.-X. Kuang, Z.-B. Wang, Q.-H. Wang, B.-Y. Yang, H.-B. Xiao, Y. Okada and T. Okuyama, Chem. Biodiversity, 2013, 10, 703–710 CAS.
- E. J. Cui, J. G. Cho, I. S. Chung, J. Y. Kim, S. G. Hong and N. I. Baek, Bull. Korean Chem. Soc., 2013, 34, 2499–2502 CrossRef CAS.
- W.-B. Zhou, J. Liu, S. Xing, Y. Zhang, G.-L. Xiong, Q.-L. Luo, B.-P. Ma, M.-H. Yang and P. Liu, J. Asian Nat. Prod. Res., 2013, 15, 1277–1283 CrossRef CAS PubMed.
- P. Wang, W. Yuan, G. Deng, Z. Su and S. Li, Phytochem. Lett., 2013, 6, 306–309 CrossRef CAS.
- J. Lu, J.-H. Sun, Y. Tan, Y. Kano and D. Yuan, J. Asian Nat. Prod. Res., 2013, 15, 1065–1072 CrossRef CAS PubMed.
- J. Tang, L. Yu, H. Fu, C. Li, J. Yang, W. Zhou, X. Chen and D. Zhang, Planta Med., 2013, 79, 353–360 CrossRef CAS PubMed.
- L. Li, M.-L. Gou and Y.-X. He, Phytochem. Lett., 2013, 6, 570–574 CrossRef CAS.
- M. Haddad, A.-C. Lelamer, L. M. Y. Banuls, P. Vasquez, M. Carraz, A. Vaisberg, D. Castillo, M. Sauvain, R. Rojas and R. Kiss, Phytochem. Lett., 2013, 6, 128–134 CrossRef CAS.
- N. Das, P. S. Ghosh, M. C. Das and B. Dinda, Phytochem. Lett., 2013, 6, 270–273 CrossRef CAS.
- I. Arslan, A. Celik and M. F. Melzig, Bioorg. Med. Chem., 2013, 21, 1279–1283 CrossRef CAS PubMed.
- I. Podolak, P. Zmudzki, P. Koczurkiewicz, M. Michalik, P. Zajdel and A. Galanty, Nat. Prod. Commun., 2013, 8, 1691–1696 CAS.
- X. Wu, L. Wang, G.-C. Wang, H. Wang, Y. Dai, X.-X. Yang, W.-C. Ye and Y.-L. Li, Carbohydr. Res., 2013, 368, 1–7 CrossRef CAS PubMed.
- J. Y. Lee, J. S. Kim, Y. S. Kim and S. S. Kang, Chem. Pharm. Bull., 2013, 61, 971–978 CrossRef CAS PubMed.
- H. Xiong, Z. Mei, G. Yang, S. Mo, X. Yang, P. Zhang and J. Wu, Helv. Chim. Acta, 2013, 96, 1579–1589 CrossRef CAS.
- O. P. Note, A. L. Tapondjou, A.-C. Mitaine-offer, T. Miyamoto, D. E. Pegnyemb and M.-A. Lacaille-Dubois, Phytochem. Lett., 2013, 6, 505–510 CrossRef CAS.
- G. Ma, W. Guo, L. Zhao, Q. Zheng, Z. Sun, J. Wei, J. Yang and X. Xu, Chem. Pharm. Bull., 2013, 61, 101–104 CrossRef CAS PubMed.
- W. Li, Y. Ding, Y. N. Sun, X. T. Yan, S. Y. Yang, C. W. Choi, J. Y. Cha, Y. M. Lee and Y. H. Kim, Chem. Pharm. Bull., 2013, 61, 471–476 CrossRef CAS PubMed.
- Y.-L. Song, K.-W. Zeng, T.-X. Shi, Y. Jiang and P.-F. Tu, Fitoterapia, 2013, 84, 295–301 CrossRef CAS PubMed.
- M. A. Tantry and I. A. Khan, Fitoterapia, 2013, 87, 49–56 CrossRef CAS PubMed.
- Y. Wang, W. Kang, L.-j. Hong, W.-l. Hai, X.-y. Wang, H.-f. Tang and X.-r. Tian, J. Nat. Med., 2013, 67, 70–77 CrossRef CAS PubMed.
- L. Laurencon, E. Sarrazin, M. Chevalier, I. Precheur, G. Herbette and X. Fernandez, Phytochemistry, 2013, 86, 103–111 CrossRef CAS PubMed.
- Z.-L. Li, D.-Y. Li, X.-M. He and H.-M. Hua, Nat. Prod. Res., 2013, 27, 232–237 CrossRef CAS PubMed.
- A. M. Abdou, H. M. Abdallah, M. A. Mohamed, G. A. Fawzy and A. B. Abdel-Naim, Arch. Pharmacal Res., 2013, 36, 715–722 CrossRef CAS PubMed.
- F.-Z. Ren, X.-X. Zhang, S.-H. Chen, L.-H. Li and X.-X. Cheng, Chem. Nat. Compd., 2013, 49, 271–273 CrossRef CAS.
- X.-Y. Wang, H. Gao, W. Zhang, Y. Li, G. Cheng, X.-L. Sun and H.-F. Tang, Bioorg. Med. Chem. Lett., 2013, 23, 5714–5720 CrossRef CAS PubMed.
- X. Wang, W. Zhang, K. Gao, Y. Lu, H. Tang and X. Sun, Fitoterapia, 2013, 89, 224–230 CrossRef CAS PubMed.
- Z.-Q. Ma, Y. Zhang, C.-K. Cai, Q. Li and J. Ni, J. Asian Nat. Prod. Res., 2013, 15, 849–854 CrossRef CAS PubMed.
- L.-H. Mu, X.-W. Huang, D.-H. Guo, X.-Z. Dong and P. Liu, J. Asian Nat. Prod. Res., 2013, 15, 1123–1129 CrossRef CAS PubMed.
- D. Gulcemal, M. Masullo, A. Napolitano, T. Karayildirim, E. Bedir, O. Alankus-Caliskan and S. Piacente, Phytochemistry, 2013, 86, 184–194 CrossRef CAS PubMed.
- X.-n. Han, C.-y. Liu, Y.-l. Liu, Q.-m. Xu, X.-r. Li and S.-l. Yang, J. Agric. Food Chem., 2013, 61, 12692–12699 CrossRef CAS PubMed.
- B. Pereira da Silva and J. P. Parente, Phytochem. Lett., 2013, 6, 633–639 CrossRef CAS.
- X. Tian, J. Feng, H. Tang, M. Zhao, Y. Li, W. Hai and X. Zhang, Fitoterapia, 2013, 90, 233–239 CrossRef CAS PubMed.
- W. Zhang, X. Wang, H. Tang, M. Wang, L. Ji, A. Wen and J. Wang, Fitoterapia, 2013, 84, 326–331 CrossRef CAS PubMed.
- I. Krasteva, M. Yotova, K. Jenett-Siems, P. Zdraveva and S. Nikolov, Nat. Prod. Commun., 2013, 8, 765–766 CAS.
- L. Voutquenne-Nazabadioko, R. Gevrenova, N. Borie, D. Harakat, C. Sayagh, A. Weng, M. Thakur, M. Zaharieva and M. Henry, Phytochemistry, 2013, 90, 114–127 CrossRef CAS PubMed.
- L. Liao, X. Zhou, Y.-l. Liu, Q.-m. Xu, X.-r. Li and S.-l. Yang, Phytochem. Lett., 2013, 6, 429–434 CrossRef CAS.
- M. J. Manase, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, S. Delemasure, P. Dutartre and M.-A. Lacaille-Dubois, Fitoterapia, 2013, 91, 231–235 CrossRef CAS PubMed.
- Z. Shu, Z. Chen, Y.-l. Liu, W.-f. Zhu, Y.-l. Feng, Q.-m. Xu, X.-r. Li and S.-l. Yang, Nat. Prod. Res., 2013, 27, 2196–2201 CrossRef CAS PubMed.
- C. Acharya and N. A. Khan, Chem. Nat. Compd., 2013, 49, 54–57 CrossRef CAS.
- A. Sharma, S. C. Sati, O. P. Sati, M. D. Sati and S. K. Kothiyal, E-J. Chem., 2013, 613190 Search PubMed.
- A. Sharma, S. C. Sati, O. P. Sati, M. D. Sati, S. K. Kothiyal, D. K. Semwal and A. Mehta, J. Chem, 2013, 218510 Search PubMed.
- B. Moniuszko-Szajwaj, L. Pecio, M. Kowalczyk, A. M. Simonet, F. A. Macias, M. Szumacher-Strabel, A. Cieslak, W. Oleszek and A. Stochmal, Nat. Prod. Commun., 2013, 8, 1687–1690 CAS.
- W. Yuan, P. Wang, Z. Zhang, Z. Su and S. Li, Phytochem. Lett., 2013, 6, 106–109 CrossRef CAS.
- M. F. de Araujo, I. J. Curcino Vieira, C. M. R. Sant'Anna, D. Rosa da Silva, A. I. V. Maia, R. Braz-Filho, O. Vieira-da-Motta and L. Mathias, Nat. Prod. Res., 2013, 27, 1888–1895 CrossRef CAS PubMed.
- T.-W. Cao, C.-A. Geng, F.-Q. Jiang, Y.-B. Ma, K. He, N.-J. Zhou, X.-M. Zhang, J. Zhou and J.-J. Chen, Fitoterapia, 2013, 89, 175–182 CrossRef CAS PubMed.
- A. J. Perez, M. Kowalczyk, A. M. Simonet, F. A. Macias, W. Oleszek and A. Stochmal, J. Agric. Food Chem., 2013, 61, 2631–2637 CrossRef CAS PubMed.
- W. Xiao, Y. Wang, P. Zhang, N. Li, S. Jiang, J.-h. Wang, J. Huang and X. Li, Eur. J. Med. Chem., 2013, 60, 263–270 CrossRef CAS PubMed.
- F. Machumi, J. O. Midiwo, M. R. Jacob, S. I. Khan, B. L. Tekwani, J. Zhang, L. A. Walker and I. Muhammad, Nat. Prod. Commun., 2013, 8, 761–764 CAS.
- C. E. Fingolo, T. d. S. Santos, M. D. M. Vianna Filho and M. A. C. Kaplan, Molecules, 2013, 18, 4247–4256 CrossRef CAS PubMed.
- S.-L. Wang, Z. Chen, X.-J. Tong, Y.-L. Liu, X. Li, Q.-M. Xu, X.-R. Li and S.-L. Yang, Helv. Chim. Acta, 2013, 96, 1126–1133 CrossRef CAS.
- N. Boutaghane, L. Voutquenne-Nazabadioko, A. Simon, D. Harakat, K. Benlabed and Z. Kabouche, Phytochem. Lett., 2013, 6, 519–525 CrossRef CAS.
- J. Xiong, Y. Huang, Y. Tang, M. You and J. Hu, Youji Huaxue, 2013, 33, 1304–1308 CrossRef CAS.
- A. S. Wanas, M. A. Fouad, M. S. Kamel, K. Matsunami and H. Otsuka, Nat. Prod. Commun., 2013, 8, 767–769 CAS.
- I. J. Momo, T.-H. Dufat, J. Wandji, S. Michel and D. D. Chiozem, Nat. Prod. Res., 2013, 27, 137–145 CrossRef CAS PubMed.
- M.-Y. Huang, L.-J. Zhong, J.-M. Xie, F. Wang and Y.-H. Zhang, Helv. Chim. Acta, 2013, 96, 2040–2045 CrossRef CAS.
- S. Kaennakam, J. Sichaem, S. Khumkratok, P. Siripong and S. Tip-pyang, Nat. Prod. Commun., 2013, 8, 1371–1372 CAS.
- R. Tanaka, T. Kikuchi, S. Nakasuji, Y. Ue, D. Shuto, K. Igarashi, R. Okada and T. Yamada, Molecules, 2013, 18, 7448–7459 CrossRef CAS PubMed.
- T. Kikuchi, M. Takebayashi, M. Shinto, T. Yamada and R. Tanaka, Molecules, 2013, 18, 5568–5579 CrossRef CAS PubMed.
- C.-M. Cao, Y.-B. Wu, R.-X. Guo, M. Dong, F. Sauriol, C.-H. Huo, Q.-W. Shi, T. Yamada, H. Kiyota, Y.-C. Gu and B. Cong, Helv. Chim. Acta, 2013, 96, 375–378 CrossRef CAS.
- W.-G. Shan, L.-W. Zhang, J.-G. Xiang and Z.-J. Zhan, Chem. Biodiversity, 2013, 10, 1392–1434 CAS.
- A. M. Lannang, B. S. Noudou and N. Sewald, Phytochem. Lett., 2013, 6, 157–161 CrossRef CAS.
- M. C. Duarte, J. F. Tavares, S. A. L. Madeiro, V. C. O. Costa, J. M. B. Filho, M. de Fatima Agra, R. B. Filho and M. S. da Silva, J. Braz. Chem. Soc., 2013, 24, 1697–1700 CAS.
- Q. Zhao, R.-j. Liang, Y.-j. Zhang, A.-h. Hong, Y.-f. Wang and Y.-z. Cen, Zhongcaoyao, 2013, 44, 133–136 CAS.
- C. G. Magalhaes, G. D. de Fatima Silva, L. P. Duarte, I. L. Bazzocchi, A. J. Diaz, L. Moujir, M. R. Lopez, R. C. Figueiredo and S. A. Vieira Filho, Helv. Chim. Acta, 2013, 96, 1046–1054 CrossRef CAS.
- J.-Q. Liu, X.-R. Peng, X.-Y. Li, T.-Z. Li, W.-M. Zhang, L. Shi, J. Han and M.-H. Qiu, Org. Lett., 2013, 15, 1580–1583 CrossRef CAS PubMed.
- L. Yu, W.-j. Zuo, W.-l. Mei, Z.-k. Guo, X.-n. Li and H.-f. Dai, Phytochem. Lett., 2013, 6, 472–475 CrossRef CAS.
- I. S. Sarma and B. Dinda, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2013, 52, 1527–1530 Search PubMed.
- I. T. Babalola and F. O. Shode, J. Pharmacogn. Phytochem., 2013, 2, 214–222 CAS.
- M. K. Shanmugam, X. Dai, A. P. Kumar, B. K. H. Tan, G. Sethi and A. Bishayee, Biochem. Pharmacol., 2013, 85, 1579–1587 CrossRef CAS PubMed.
- K. Mazumder, K. Tanaka and K. Fukase, Molecules, 2013, 18, 8929–8944 CrossRef CAS PubMed.
- N. Akhtar, M. Saleem, N. Riaz, M. S. Ali, A. Yaqoob, F.-u.-H. Nasim and A. Jabbar, Phytochem. Lett., 2013, 6, 291–298 CrossRef CAS.
- Y.-J. Chen, H.-X. Jiang and K. Gao, Biochem. Syst. Ecol., 2013, 47, 1–4 CrossRef CAS.
- X.-D. Wu, J. He, X.-Y. Li, L.-B. Dong, X. Gong, X. Gao, L.-D. Song, Y. Li, L.-Y. Peng and Q.-S. Zhao, Planta Med., 2013, 79, 1356–1361 CrossRef CAS PubMed.
- M. Moridi Farimani, S. Nejad Ebrahimi, P. Salehi, M. B. Bahadori, A. Sonboli, H. R. Khavasi, S. Zimmermann, M. Kaiser and M. Hamburger, J. Nat. Prod., 2013, 76, 1806–1809 CrossRef CAS PubMed.
- Y.-L. Zhang, W.-S. Feng, X.-K. Zheng, Y.-G. Cao, Y.-Y. Lv, H. Chen and H.-X. Kuang, Fitoterapia, 2013, 89, 15–19 CrossRef CAS PubMed.
- K. Jiao, H.-Y. Li, P. Zhang, H.-F. Pi, H.-L. Ruan and J.-Z. Wu, J. Asian Nat. Prod. Res., 2013, 15, 962–968 CrossRef CAS PubMed.
- A. Choudhary, A. K. Mittal, M. Radhika, D. Tripathy, A. Chatterjee, U. C. Banerjee and I. P. Singh, Fitoterapia, 2013, 91, 290–297 CrossRef CAS PubMed.
- A. Al-Harrasi, L. Ali, N. Ur Rehman, J. Hussain, H. Hussain, A. Al-Rawahi and T. Shamim Rizvi, Chem. Biodiversity, 2013, 10, 1501–1506 CAS.
- P. Ghosh, A. Mandal and M. G. Rasul, J. Chem. Sci., 2013, 125, 359–364 CrossRef CAS.
- J.-C. Shu, J.-Q. Liu and G.-X. Chou, Nat. Prod. Res., 2013, 27, 1293–1297 CrossRef CAS PubMed.
- S. R. Ayinampudi, R. Merugu and A. Thirupathaiah, Int. J. ChemTech Res., 2013, 5, 342–346 CAS.
- Z. Zhou, X. Wei, H. Fu and Y. Luo, Fitoterapia, 2013, 88, 91–95 CrossRef CAS PubMed.
- L. Jia, J. Wang, C. Lv, T. Xu, L. He, Y. Dong and J. Lu, Nat. Prod. Res., 2013, 27, 1361–1365 CrossRef CAS PubMed.
- N. A. Siddiqui, M. Ali, A. Ahmad, T. H. Khan and A. Ahmad, Asian J. Chem., 2013, 25, 8557–8560 CrossRef CAS.
- K.-Y. Niu, L.-Y. Wang, S.-Z. Liu and W.-M. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 1–8 CrossRef CAS PubMed.
- Z.-X. Zhang, Q. Fu and K. Y.-Z. Zheng, J. Asian Nat. Prod. Res., 2013, 15, 453–458 CrossRef CAS PubMed.
- Z. Zhou, Z. Feng, W. Yin and H. Zhang, Chem. Nat. Compd., 2013, 49, 682–684 CrossRef CAS.
- Z.-L. Zhou, Z.-C. Feng, C.-Y. Fu and L. Zeng, Nat. Prod. Res., 2013, 27, 1343–1347 CrossRef CAS PubMed.
- Z.-f. Zhang, L.-y. Lu, P. Luo, L.-s. Qing and Y. Liu, Nat. Prod. Res., 2013, 27, 2256–2262 CrossRef CAS PubMed.
- Z.-X. Zhao, C.-Z. Lin, C.-C. Zhu and W.-J. He, Chin. J. Nat. Med., 2013, 11, 415–418 CrossRef CAS PubMed.
- L. Ali and F. Shaheen, Rec. Nat. Prod., 2013, 7, 80–85 CAS.
- S. Y. Lee, H. K. Kim and K. R. Lee, Can. J. Chem., 2013, 91, 382–386 CrossRef CAS.
- Y. Q. Xu, S. Pang, J. Y. Hu and H. Q. Duan, Chem. Nat. Compd., 2013, 49, 268–270 CrossRef CAS.
- X. Hao, J. Zhang, G. Xia, Y. Xue, Z. Luo, Y. Si, G. Yao and Y. Zhang, Molecules, 2013, 18, 1262–1269 CrossRef CAS PubMed.
- B. Guan, C.-C. Peng, Q. Zeng, X.-R. Cheng, S.-K. Yan, H.-Z. Jin and W.-D. Zhang, Planta Med., 2013, 79, 365–368 CrossRef CAS PubMed.
- N. A. Masevhe, M. D. Awouafack, A. S. Ahmed, L. J. McGaw and J. N. Eloff, Helv. Chim. Acta, 2013, 96, 1693–1703 CrossRef CAS.
- J. Zhao, Chem. Nat. Compd., 2013, 49, 467–469 CrossRef CAS.
- H. M. Abdallah, S. M. Ezzat, R. S. El Dine, E. Abdel-Sattar and A. B. Abdel-Naim, Phytochem. Lett., 2013, 6, 73–78 CrossRef CAS.
- M. D. T. Kongue, F. M. Talontsi, M. Lamshoft, T. J. N. Kenla, B. Dittrich, G. D. W. F. Kapche and M. Spiteller, Fitoterapia, 2013, 85, 176–180 CrossRef CAS PubMed.
- S. Begum, S. A. Syed, B. S. Siddiqui, S. A. Sattar and M. I. Choudhary, Phytochem. Lett., 2013, 6, 91–95 CrossRef CAS.
- L. Zhang, F. Wang, Z.-Y. Jiang, Y.-K. Zhu and Y.-Z. Cen, E-J. Chem., 2013, 727136 Search PubMed.
- D. Kumar, V. Shah, R. Ghosh and B. C. Pal, Nat. Prod. Res., 2013, 27, 624–629 CrossRef CAS PubMed.
- R.-m. Lu, M. Cao, P.-y. Liao and J.-h. Wei, Zhongcaoyao, 2013, 44, 2195–2199 CAS.
- S. Haider, C. Kharbanda, M. S. Alam, H. Hamid, M. Ali, M. Alam, S. Nazreen and Y. Ali, Nat. Prod. Res., 2013, 27, 2304–2310 CrossRef CAS PubMed.
- L.-S. Wan, T.-T. Liu, X.-J. Lin, Q.-X. Min and J.-C. Chen, Molecules, 2013, 18, 8518–8523 CrossRef CAS PubMed.
- W.-B. Zhou, G.-Z. Zeng, H.-M. Xu, W.-J. He and N.-H. Tan, Molecules, 2013, 18, 14585–14596 CrossRef PubMed.
- D.-Q. Xue, S.-C. Mao, X.-Q. Yu and Y.-W. Guo, Biochem. Syst. Ecol., 2013, 49, 101–106 CrossRef CAS.
- C.-Q. Liang, Y.-M. Shi, X.-Y. Li, R.-H. Luo, Y. Li, Y.-T. Zheng, H.-B. Zhang, W.-L. Xiao and H.-D. Sun, J. Nat. Prod., 2013, 76, 2350–2354 CrossRef CAS PubMed.
- P.-C. Wang, X.-H. Ran, H.-R. Luo, Q.-Y. Ma, Y.-Q. Liu, H.-F. Dai, J. Zhou and Y.-X. Zhao, Org. Lett., 2013, 15, 2898–2901 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |