Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The cumene/O2 system: a very simple tool for the radical chain oxidation of some functional groups†‡
A.
Malekafzali
ab,
K.
Malinovska
a and
F. W.
Patureau
 *a
*a
aFB Chemie, Technische Universiät Kaiserslautern, Erwin-Schrödinger Str. 52, 67663 Kaiserslautern, Germany. E-mail: patureau@chemie.uni-kl.de; Web: http://www.chemie.uni-kl.de/patureau
bChemistry Department, Tarbiat Modares University, Jalale-Ale-Ahmad Highway, 14117-13116 Tehran, Iran
First published on 30th June 2017
Abstract
Due to the relative stability of the cumyl radical, cumenes and α-methyl-styrenes are ideally structured to directly harvest the oxidizing reactivity of O2 and initiate radical chain reactions in catalyst-free conditions. In the absence of additional substrates, these processes can lead to acetophenones. In the presence of substrates, the cumene oxidation process can be intercepted in various chain reactions, affording very simple protocols for functional group oxidation.
Phenones are traditionally prepared by ozonolysis (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleaving oxidation of styrenes with O3),1 metal catalysed oxidation of styrenes with O2, such as in the Wacker process,2 or simply by oxidation of their alkyl benzene precursors, to name only a few methods.3 The chemistry of phenones, their preparation but also their very rich and versatile reactivity has been at the heart of organic synthesis for well over a century. Recently, Jianliang Xiao reported a C
C bond cleaving oxidation of styrenes with O3),1 metal catalysed oxidation of styrenes with O2, such as in the Wacker process,2 or simply by oxidation of their alkyl benzene precursors, to name only a few methods.3 The chemistry of phenones, their preparation but also their very rich and versatile reactivity has been at the heart of organic synthesis for well over a century. Recently, Jianliang Xiao reported a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleaving oxidation of styrenes towards the corresponding phenones while using an iron(III)-triflate catalyst bearing a tridentate amine ligand, with O2 as the terminal oxidant (Scheme 1).4 More recently, Xiao Wang reported an analogous protocol utilizing an aromatic disulfide organocatalyst under white LED, also under 1 atm of O2.5 The latter methods afford the corresponding phenones in good yields at mild temperatures (as low as 25 °C, Scheme 1). However to what extend exactly does one need a catalyst for this reaction to occur? While the activation of molecular O2 can be troublesome in mild reaction conditions without a catalyst, some early work conducted by Hayashi in 2002 did document the neat transformation of α-methyl-styrenes into acetophenones with O2 as the sole reagent.6
C bond cleaving oxidation of styrenes towards the corresponding phenones while using an iron(III)-triflate catalyst bearing a tridentate amine ligand, with O2 as the terminal oxidant (Scheme 1).4 More recently, Xiao Wang reported an analogous protocol utilizing an aromatic disulfide organocatalyst under white LED, also under 1 atm of O2.5 The latter methods afford the corresponding phenones in good yields at mild temperatures (as low as 25 °C, Scheme 1). However to what extend exactly does one need a catalyst for this reaction to occur? While the activation of molecular O2 can be troublesome in mild reaction conditions without a catalyst, some early work conducted by Hayashi in 2002 did document the neat transformation of α-methyl-styrenes into acetophenones with O2 as the sole reagent.6
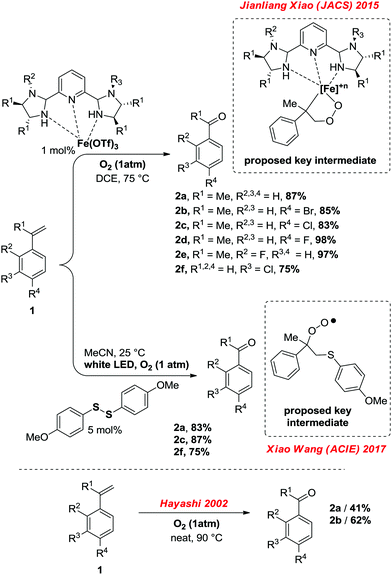 | ||
| Scheme 1 Oxygenolysis of (α-methyl)-styrenes according to recent reports of Xiao (top)4 Wang (middle),5 selected examples, in comparison to the catalyst-free method of Hayashi (beneath).6 | ||
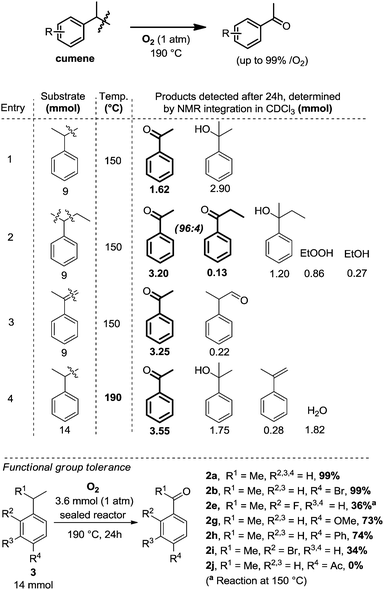
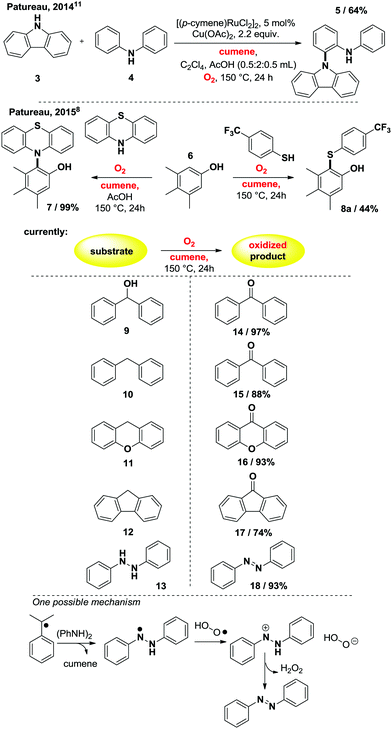
Beyond Hayashi's catalyst free method for α-methyl-styrenes,6 it seemed clear that based on the known chemistry of cumyl-hydroperoxide, pure cumene should also be a competent substrate in this reaction. This assumption is moreover supported by the decades-old decomposition of cumyl-hydroperoxide, which is known to yield acetophenone.7 It thus occurred to us that cumene,8 a precursor to α-methyl-styrene, would probably display a similar reactivity than Hayashi's oxygenolysis with O2, in completely additive free conditions.9 We thus conducted an initial reactivity and mechanistic survey, summarized in Table 1.
The following reaction conditions were eventually selected: cumene, 14 mmol, is placed in an 85 mL glass reactor, which is then flushed with O2 for approximatively 1 minute, and then sealed and heated up for 24 h at 190 °C. When cumene is submitted to those conditions, one molecule of O2 produces approximatively one molecule of acetophenone. The reaction is still operational at 150 °C but conversion is then lower (1.62 mmol, Table 1, entry 1). By increasing reaction temperature, dehydration of the dimethyl-phenyl-carbinol byproduct increases also, yielding the corresponding α-methyl styrene which can then re-engage itself in an oxidation event with O2 to produce the desired acetophenone. As opposed to the Xiao4 and Wang5 systems, in which the position of the olefin starting material is already defined in the substrate, starting directly from unsymmetrical alkyl-benzene precursors can offer competing C–C bond cleaving pathways. Interestingly, utilizing 2-phenyl-butane instead of cumene is more effective at 150 °C than cumene, with a yield of acetophenone of 3.20 mmol (entry 2). Notably, C–C bond cleavage occurs with a very large preference on the side of the longer alkyl chain, as the acetophenone product largely exceeds the propiophenone product by a ratio of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
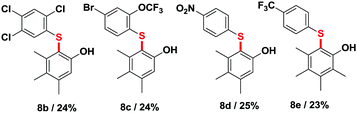
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (entry 2). This suggests the intermediacy of the corresponding styrene as a significant intermediate, similarly to the Xiao,4 Wang5 and Hayashi6 systems, the internal alkene being largely favored over the external one in the case of an in situ dehydrogenative event. This is confirmed by entry 3, in which neat α-methylstyrene is also converted to acetophenone (3.25 mmol).9 We also tried to run the reaction under air at cumene's boiling point. Only 2.6% of the cumene converted to acetophenone in those open-flask conditions. With those initial conditions in hand, we then evaluated the effects of some simple electron donating and withdrawing substitution patterns (Table 1). We found that strongly withdrawing groups (R4 = Ac, 2j), make the cumenes unreactive, likely by preventing the first oxidation step: the cumyl H-atom abstraction. For halides, methoxy, and phenyl functional groups, the tolerance is generally acceptable. A mechanism is proposed in Scheme 2 consisting in a stepwise radical10 dehydrogenation, producing α-methyl-styrene which would then be further oxidized with O2 in a Hayashi-like mechanism.6 Alternatively, a classical cumyl-hydroperoxide route can be considered, as documented notably by Di Somma,7 which is also reasonable in view of some of the byproducts observed in Table 1. In any case, 2 mmol of 2,4,6-tri-tert-butylphenol added to the reactor as a radical inhibitor suppresses acetophenone formation (Scheme 2). This result thus confirms the radical chain character of this transformation. Because cumene is so readily oxidized by O2, it may potentially be a good solvent to carry out simple functional group oxidation and/or oxidative coupling reactions through radical chain oxidations. In two recent cross-dehydrogenative coupling methods, the cumene/O2 couple was utilized as an enabling (re-)oxidizing strategy (Scheme 3). One method relies on a Ru/Cu catalysed C–H/N–H bond activation system,11 while the other method is entirely additive-free.8 The cumene/O2 system notably enables the direct and additive-free cross dehydrogenative C–N coupling of phenols with phenothiazines in excellent yields, a reaction which we previously reported.8 A similar system also allows the C–S cross dehydrogenative coupling between phenols and thiophenols, although with moderate yields (product 8a, 44%).12 We thus wondered whether the cumene/O2 system would also be competent for simple functional group oxidation. We therefore considered the oxidation of a series of organic substrates under those conditions. For example, benzhydrol 9 yields benzophenone (entry 14, 97% isolated yield). Diphenylmethane 10 also yields benzophenone (entry 15, 88%), while xanthene (11) and fluorene (12) produce xanthone 16 and fluorenone 17 with 93% and 74% yield respectively. Finally 1,2-diphenylhydrazine (13) yields azobenzene 18 (93%). All were efficiently transformed while simply being heated up in cumene under 1 atm of O2 at 150 °C, without any further additive. These trivial oxidation processes are among the most studied reactions in the literature.13–16 However the vast majority of these oxidative methods rely on metal additives, sometimes also on externally added oxidants, which arguably makes our method an interesting alternative.
4 (entry 2). This suggests the intermediacy of the corresponding styrene as a significant intermediate, similarly to the Xiao,4 Wang5 and Hayashi6 systems, the internal alkene being largely favored over the external one in the case of an in situ dehydrogenative event. This is confirmed by entry 3, in which neat α-methylstyrene is also converted to acetophenone (3.25 mmol).9 We also tried to run the reaction under air at cumene's boiling point. Only 2.6% of the cumene converted to acetophenone in those open-flask conditions. With those initial conditions in hand, we then evaluated the effects of some simple electron donating and withdrawing substitution patterns (Table 1). We found that strongly withdrawing groups (R4 = Ac, 2j), make the cumenes unreactive, likely by preventing the first oxidation step: the cumyl H-atom abstraction. For halides, methoxy, and phenyl functional groups, the tolerance is generally acceptable. A mechanism is proposed in Scheme 2 consisting in a stepwise radical10 dehydrogenation, producing α-methyl-styrene which would then be further oxidized with O2 in a Hayashi-like mechanism.6 Alternatively, a classical cumyl-hydroperoxide route can be considered, as documented notably by Di Somma,7 which is also reasonable in view of some of the byproducts observed in Table 1. In any case, 2 mmol of 2,4,6-tri-tert-butylphenol added to the reactor as a radical inhibitor suppresses acetophenone formation (Scheme 2). This result thus confirms the radical chain character of this transformation. Because cumene is so readily oxidized by O2, it may potentially be a good solvent to carry out simple functional group oxidation and/or oxidative coupling reactions through radical chain oxidations. In two recent cross-dehydrogenative coupling methods, the cumene/O2 couple was utilized as an enabling (re-)oxidizing strategy (Scheme 3). One method relies on a Ru/Cu catalysed C–H/N–H bond activation system,11 while the other method is entirely additive-free.8 The cumene/O2 system notably enables the direct and additive-free cross dehydrogenative C–N coupling of phenols with phenothiazines in excellent yields, a reaction which we previously reported.8 A similar system also allows the C–S cross dehydrogenative coupling between phenols and thiophenols, although with moderate yields (product 8a, 44%).12 We thus wondered whether the cumene/O2 system would also be competent for simple functional group oxidation. We therefore considered the oxidation of a series of organic substrates under those conditions. For example, benzhydrol 9 yields benzophenone (entry 14, 97% isolated yield). Diphenylmethane 10 also yields benzophenone (entry 15, 88%), while xanthene (11) and fluorene (12) produce xanthone 16 and fluorenone 17 with 93% and 74% yield respectively. Finally 1,2-diphenylhydrazine (13) yields azobenzene 18 (93%). All were efficiently transformed while simply being heated up in cumene under 1 atm of O2 at 150 °C, without any further additive. These trivial oxidation processes are among the most studied reactions in the literature.13–16 However the vast majority of these oxidative methods rely on metal additives, sometimes also on externally added oxidants, which arguably makes our method an interesting alternative.
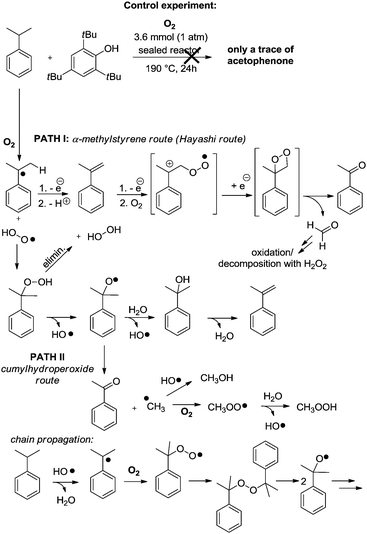 | ||
| Scheme 2 Possible chain propagation mechanisms taking into account the observed by-products of Table 1, PATH I through α-methyl-styrene, notably proposed by Hayashi,6 and PATH II through the cumyl-hydroperoxide route, notably documented by Di Somma.7 | ||
In conclusion, the oxidative C–C bond cleaving oxidation of cumenes or α-methyl-styrenes with O2 towards the corresponding phenones can proceed efficiently without a catalyst. These typically require significantly higher temperatures however than under catalytic conditions.17 Importantly, the O2 mediated oxidation of cumene can be intercepted in the frame of cross dehydrogenative couplings, or the simple oxidation of diverse functional groups. Clearly, cumene and its congeners are well suited for the organic activation of O2 towards oxidative applications.
Acknowledgements
The research of FWP is generously supported by the DFG-funded transregional collaborative research center SFB/TRR 88 “Cooperative effects in homo and heterometallic complexes” (http://3MET.de), DFG funded project PA 2395/2-1, COST Action CA15106 (CHAOS), and since March 2017: by ERC project 716136: “2O2ACTIVATION”. A. M. also acknowledges a fellowship from the Iranian ministry of science, research and technology. Mr Alexander Jones is acknowledged for teaching the laboratory techniques to K. M.Notes and references
- A selection: (a) R. W. Murray, Acc. Chem. Res., 1968, 1, 313 CrossRef CAS; (b) R. Criegee, Angew. Chem., Int. Ed. Engl., 1975, 14, 745 ( Angew. Chem. , 1975 , 87 , 765 ) CrossRef; (c) R. L. Kuczkowski, Chem. Soc. Rev., 1992, 21, 79 RSC; (d) S. G. Van Ornum, R. M. Champeau and R. Pariza, Chem. Rev., 2006, 106, 2990 CrossRef CAS PubMed.
- A selection: (a) T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 232 CrossRef PubMed; (b) C. N. Cornell and M. S. Sigman, Inorg. Chem., 2007, 46, 1903 CrossRef CAS PubMed; (c) J. A. Keith and P. M. Henry, Angew. Chem., Int. Ed., 2009, 48, 9038 CrossRef CAS PubMed; (d) J. J. Dong, W. R. Browne and B. L. Feringa, Angew. Chem. Int. Ed., 2015, 54, 734 CrossRef CAS PubMed.
- Selected references: (a) K. Suga, K. Ohkubo and S. Fukuzumi, J. Phys. Chem. A, 2003, 107, 4339 CrossRef CAS; (b) A. Rajagopalan, M. Lara and W. Kroutil, Adv. Synth. Catal., 2013, 355, 3321 CrossRef CAS; (c) A. Rubinstein, P. Jimenez-Lozanao, J. J. Carbo, J. M. Poblet and R. Neumann, J. Am. Chem. Soc., 2014, 136, 10941 CrossRef CAS PubMed; (d) J.-P. Wan, Y. Gao and L. Wei, Chem. – Asian J., 2016, 11, 2092 CrossRef CAS PubMed.
- A. Gonzalez-de-Castro and J. Xiao, J. Am. Chem. Soc., 2015, 137, 8206 CrossRef CAS PubMed.
- (a) Y. Deng, X.-J. Wei, H. Wang, Y. Sun, T. Noël and X. Wang, Angew. Chem., Int. Ed., 2017, 56, 832 CrossRef CAS PubMed , see also; (b) X. Baucherel, J. Uziel and S. Jugé, J. Org. Chem., 2001, 66, 4504 CrossRef CAS PubMed.
- See also: (a) Y. Hayashi, M. Takeda, Y. Miyamoto and M. Shoji, Chem. Lett., 2002, 414 CrossRef CAS; (b) R. Lin, F. Chen and N. Jiao, Org. Lett., 2012, 14, 4158 CrossRef CAS PubMed.
- A selection: (a) M. S. Kharasch, A. Fono and W. Nudenberg, J. Org. Chem., 1951, 16, 113 CrossRef CAS; (b) A. Baignee, J. A. Howard, J. C. Scaiano and L. C. Stewart, J. Am. Chem. Soc., 1983, 105, 6120 CrossRef CAS; (c) I. Di Somma, R. Andreozzi, M. Canterino and V. Caprio, AIChE J., 2008, 54, 1579 CrossRef CAS; (d) I. Di Somma, R. Marotta, R. Andreozzi and V. Caprio, Process Saf. Environ. Prot., 2013, 91, 262 CrossRef CAS; (e) Y.-S. Duh, Thermochim. Acta, 2016, 637, 102 CrossRef CAS.
- (a) M.-L. Louillat-Habermeyer, R. Jin and F. W. Patureau, Angew. Chem., Int. Ed., 2015, 54, 4102 CrossRef CAS PubMed , see also: ; (b) Y. Zhao, B. Huang, C. Yang and W. Xia, Org. Lett., 2016, 18, 3326 CrossRef CAS PubMed.
- Cumene is a better starting material than neat α-methyl-styrene. In the latter case, a white precipitate also appears, presumably oligomeric by-products.
- A recent review on radical mechanisms: A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2016, 55, 58 CrossRef CAS PubMed.
- M.-L. Louillat, A. Biafora, F. Legros and F. W. Patureau, Angew. Chem., Int. Ed., 2014, 53, 3505 CrossRef CAS PubMed.
- See ESI‡ for details. Other examples, with unfortunately
lower yields.
. - For the oxidation of 9, 10, 11, and 12, see these selected recent methods: (a) H. Wang, Z. Wang, H. Huang, J. Tan and K. Xu, Org. Lett., 2016, 18, 5680 CrossRef CAS PubMed; (b) F. Rusch, J.-C. Schober and M. Brasholz, ChemCatChem, 2016, 8, 2881 CrossRef CAS; (c) A. Alanthadka, E. S. Devi, S. Nagarajan, V. Sridharan, A. Suvitha and C. U. Maheswari, Eur. J. Org. Chem., 2016, 4872 CrossRef CAS; (d) G. Pandey, R. Laha and D. Singh, J. Org. Chem., 2016, 81, 7161 CrossRef CAS PubMed; (e) B. Majumdar, T. Bhattacharya and T. K. Sarma, ChemCatChem, 2016, 8, 1825 CrossRef CAS; (f) R. Xie, G. Fan, L. Yang and F. Li, ChemCatChem, 2016, 8, 363 CrossRef CAS; (g) Z. Ma, H. Zhang, Z. Yang, G. Ji, B. Yu, X. Liu and Z. Liu, Green Chem., 2016, 18, 1976 RSC; (h) M. Zarghani and B. Akhlaghinia, RSC Adv., 2016, 6, 38592 RSC; (i) S. Verma, R. B. N. Baig, M. N. Nadagouda and R. S. Varma, ACS Sustainable Chem. Eng., 2016, 4, 2333 CrossRef CAS; (j) S. Yang, L. Peng, P. Huang, X. Wang, Y. Sun, Ch. Cao and W. Song, Angew. Chem., Int. Ed., 2016, 55, 4016 CrossRef CAS PubMed; (k) A. Al-hunaiti, M. Raisanen and T. Repo, Chem. Commun., 2016, 52, 2043 RSC; (l) C. Miao, H. Zhao, Q. Zhao, C. Xiaa and W. Sun, Catal. Sci. Technol., 2016, 6, 1378 RSC; (m) Y. Kuwahara, Y. Yoshimuraa and H. Yamashita, Catal. Sci. Technol., 2016, 6, 442 RSC; (n) T. Uematsu, Y. Miyamoto, Y. Ogasawara, K. Suzuki, K. Yamaguchi and N. Mizuno, Catal. Sci. Technol., 2016, 6, 222 RSC; (o) A. Shaabani, Z. Hezarkhani and E. Badali, RSC Adv., 2015, 5, 61759 RSC; (p) Y. Chen, X. Feng, X. Huang, Z. Lin, X. Pei, S. Li, J. Li, S. Wang, R. Li and B. Wang, Chem. Eur. J., 2015, 21, 13894 CrossRef CAS PubMed; (q) R. Chebolu, A. Bahuguna, R. Sharma, V. K. Mishra and P. C. Ravikumar, Chem. Commun., 2015, 51, 15438 RSC; (r) H. Yi, C. Bian, X. Hu, L. Niu and A. Lei, Chem. Commun., 2015, 51, 14046 RSC; (s) O. Martínez-Ferraté, G. J. P. Britovsek, C. Claver and P. W. N. M. van Leeuwen, Inorg. Chim. Acta, 2015, 431, 156 CrossRef; (t) S. K. Pahari, P. Pal, A. Sinhamahapatra, A. Saha, C. Santra, S. C. Ghosh, B. Chowdhury and A. B. Panda, RSC Adv., 2015, 5, 45144 RSC; (u) S. K. Pahari, P. Pal, D. N. Srivastava, S. Ch. Ghosh and A. B. Panda, Chem. Commun., 2015, 51, 10322 RSC; (v) G. Urgoitia, R. SanMartin, M. T. Herrero and E. Domınguez, Chem. Commun., 2015, 51, 4799 RSC; (w) B. Muehldorf and R. Wolf, Chem. Commun., 2015, 51, 8425 RSC; (x) F. Napoly, R. Kieffer, L. Jean-Gérard, C. Goux-Henry, M. Draye and B. Andrioletti, Tetrahedron Lett., 2015, 56, 2517 CrossRef CAS; (y) Y. Wang, Y. Kuang and Y. Wang, Chem. Commun., 2015, 51, 5852 RSC; (z) J.-K. Li, X.-Q. Huang, S. Yang, H.-W. Ma, Y.-N. Chi and C.-W. Hu, Inorg. Chem., 2015, 54, 1454 CrossRef CAS PubMed.
- Selected recent methods for the oxidation of hydrazines: (a) L. Wang, A. Ishida, Y. Hashidoko and M. Hashimoto, Angew. Chem., Int. Ed., 2017, 56, 870 CrossRef CAS PubMed; (b) M. Lin, Z. Wang, H. Fang, L. Liu, H. Yin, C.-H. Yan and X. Fu, RSC Adv., 2016, 6, 10861 RSC; (c) B. Dutta, S. Biswas, V. Sharma, N. O. Savage, S. P. Alpay and S. L. Suib, Angew. Chem. Int. Ed., 2016, 55, 2171 CrossRef CAS PubMed; (d) S. Singh, P. Chauhan, M. Ravi, I. Taneja, Wahajuddinb and P. P. Yadav, RSC Adv., 2015, 5, 61876 RSC; (e) S. Donck, E. Gravel, A. Li, P. Prakash, N. Shah, J. Leroy, H. Li, I. N. N. Namboothiri and E. Doris, Catal. Sci. Technol., 2015, 5, 4542 RSC; (f) T. Hashimoto, D. Hirose and T. Taniguchia, Adv. Synth. Catal., 2015, 357, 3346 CrossRef CAS; (g) K. Monir, M. Ghosh, S. Mishra, A. Majee and A. Hajra, Eur. J. Org. Chem., 2014, 1096 CrossRef CAS; (h) S. Okumura, C.-H. Lin, Y. Takeda and S. Minakata, J. Org. Chem., 2013, 78, 12090 CrossRef CAS PubMed; (i) W. Gao, Z. He, Y. Qian, J. Zhao and Y. Huang, Chem. Sci., 2012, 3, 883 RSC.
- For other selected related systems, see: (a) M. Mitra, H. Nimir, S. Demeshko, S. S. Bhat, S. O. Malinkin, M. Haukka, J. Lloret-Fillol, G. C. Lisensky, F. Meyer, A. A. Shteinman, W. R. Browne, D. A. Hrovat, M. G. Richmond, M. Costas and E. Nordlander, Inorg. Chem., 2015, 54, 7152 CrossRef CAS PubMed; (b) C. Jin, L. Zhang and W. Su, Synlett, 2011, 1435 CAS; (c) B. Xu, E. M. Hartigan, G. Feula, Z. Huang, J.-P. Lumb and B. A. Arndtsen, Angew. Chem., Int. Ed., 2016, 55, 15802 CrossRef CAS PubMed.
- See also: (a) G. Franz and J. H. Sheldon, Oxidation, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) C. Aellig, D. Scholz and I. Hermans, ChemSusChem, 2012, 5, 1732 CrossRef CAS PubMed; (c) I. Hermans, E. S. Spier, U. Neuenschwander, N. Turra and A. Baiker, Top. Catal., 2009, 52, 1162 CrossRef CAS.
- Proper safety precautions should be utilized for these reactions, suitable protective shields, etc. Upscaling in batch conditions is not advised. In the acetophenone process (Table 1), utilizing O2 as limiting reagent is designed at making the reaction safer for the operator. This approach has the merit of avoiding cumyl-hydroperoxide isolation, as it is usually done otherwise, potentially leading to explosions.7 The amount of O2 is calculated utilizing the gas equation PV = Z·n·R·T in which Z, the compressibility factor, is approximated to 1 for O2 gas at 20 °C and atmospheric pressure. In those conditions, which prevail when the reactor is filled and closed, 85 mL of O2 gas represents approximatively 3.5 mmol of O2, ±0.2 mmol estimated precision because of small differences in the handmade glass reactors, small variations of room temperature, and accuracy of the O2-flushing technique.
Footnotes |
| † All the work presented here was conducted in Kaiserslautern. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and analytical data. See DOI: 10.1039/c7nj01666b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |