Open Access Article
Open Access ArticleFirst synthesis of heterocyclic allenes – benzazecine derivatives†
Leonid G.
Voskressensky
 *a,
Alexander A.
Titov
a,
Maksad S.
Dzhankaziev
a,
Tatiana N.
Borisova
a,
Maxim S.
Kobzev
a,
Pavel V.
Dorovatovskii
b,
Victor N.
Khrustalev
ac,
Alexander V.
Aksenov
*a,
Alexander A.
Titov
a,
Maksad S.
Dzhankaziev
a,
Tatiana N.
Borisova
a,
Maxim S.
Kobzev
a,
Pavel V.
Dorovatovskii
b,
Victor N.
Khrustalev
ac,
Alexander V.
Aksenov
 d and
Alexey V.
Varlamov
a
d and
Alexey V.
Varlamov
a
aOrganic Chemistry Department, Peoples' Friendship University of Russia, 6 Miklukho-Maklaya St., Moscow 117198, Russia. E-mail: lvoskressensky@sci.pfu.edu.ru
bNational Research Center “Kurchatov Institute”, 1, Akademika Kurchatova pl., Moscow 123182, Russia
cNesmeyanov Institute of Organoelement Compounds of RAS, 28, Vavilova St., GSP-1, Moscow 119991, V-334, Russia
dNorth-Caucasus Federal University, 1, Pushkin Street, Stavropol 355009, Russia
First published on 16th January 2017
Abstract
Benzazecines with an allene fragment were prepared for the first time and in high yields via tandem reaction of 1-phenylethynyl-1-methyl(benzyl)-1,2,3,4-tetrahydroisoquinolines with activated alkynes in trifluoroethanol.
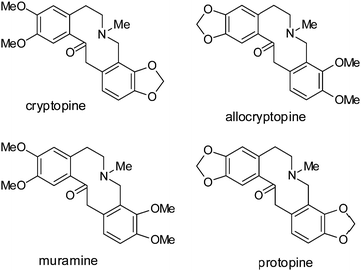
Azecine fragments have been found in a number of natural products. The well-known alkaloids protopine and allocryptopine1 (Fig. 1) were isolated from plants of the families Berberidaceae, Papaveraceae, Fumariaceae, and Rutaceae. Both compounds play important roles in protecting plants from biotic stress and also exhibit several biological effects in mammals. These compounds expand capillaries, cause analgesia, and disrupt cell division. They are used to treat gout and skin tumors. Protopine was isolated from celandine. It desensitizes the autonomous nervous system and enhances smooth-muscle tone. Muramine1c is found in several leafy vegetables and is also used for therapeutic purposes (Fig. 1).
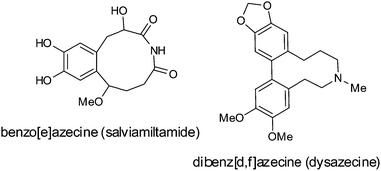
Alkaloids containing an azecine ring, salviamiltamide2 and dysazecine,3 were isolated from the plants Salvia miltiorrhiza Bunge (Lamiaceae) and Dysoxylum lenticellarei, respectively. Benzazecines are dopamine 5-HT2A antagonists, could be potential neuroleptics,4 and inhibit acetyl- and butyrylcholine esterases.5
Only a few synthetic methods for condensed azecines have been published.6–8 They all are based on difficult-to-access starting materials and can hardly be considered common. Heterocyclic allenes represent an even less known class of compounds. Stable S-containing cyclic allenes were obtained in moderate yields from a [2,3] sigmatropic rearrangement of 1-ethynylisothiochromenium salts.9
Only two examples10,11 of the preparation of a cyclic allene with a N atom in the ring have been reported. 10- and 11-membered lactams 3-chloro-4,5-allenyllactams11 were prepared by the reactions of 2-phenylethynylpiperidone and 2-phenylethynylazepine with chloroacetylfluroide through [3,3]-sigmatropic rearrangement. These results motivated our interest towards exploring the domino reaction of 1-phenylethynylisoquinolines with activated alkynes.
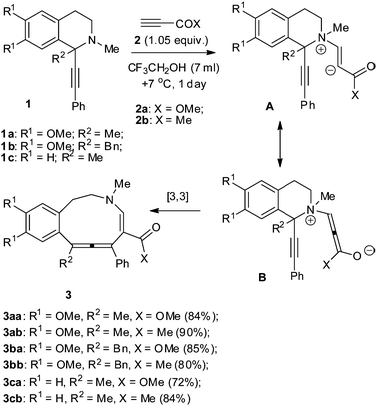
Our team has worked for many years on domino transformations of tetrahydropyridines with [c]-condensed aromatic fragments with activated alkynes. As a result, preparative synthetic methods for a series of azocines annelated with pyrrole,12 chromene,13 benzothiophene,14 thiophene15 and indole16 were developed. It was shown that tetrahydroisoquinolines with 1-phenyl substituents reacted with methyl propiolate and acetylacetylene to give the expected benzazocines in good yields.17–19 As it turned out, 1,1-disubstituted tetrahydroisoquinolines, 1-methyl- and 1-benzyl-1-phenylethynyl-1,2,3,4-tetrahydroisoquinolines 1a–c reacted differently with activated terminal alkynes 2a,b in trifluoroethanol (Scheme 1). The reaction occurred rather quickly in this solvent at +7 °C. The major reaction products benzazecines 3 with allene systems were obtained in high yields.
We suppose that the transformation of 3 began with Michael addition of the tetrahydropyridine N atom to the alkynes 2a,b to form zwitterion A, which converted into intermediate B. [3,3] Sigmatropic rearrangement resulted in the formation of the azecine ring.
The structures of 3 were elucidated by 1H and 13C NMR spectroscopy, mass spectrometry, and IR spectroscopy. The IR spectra lacked ethynyl stretching bands and exhibited bands at 1934–1937 cm−1 and 1648–1682 cm−1, confirming that the molecules contained an allene system and carbonyls. The 1H NMR spectra of azecines 3 showed a characteristic singlet for the enamine proton at δ 7.53–7.59 ppm.
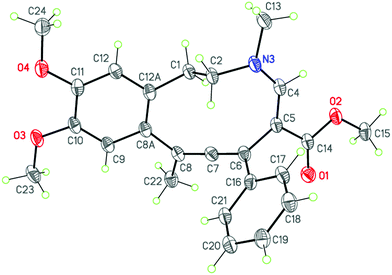
The structure of product 3aa was unambiguously established by an X-ray diffraction study and is shown in Fig. 2 along with the atomic numbering scheme.
Compound 3aa comprises the benzazecine system containing the C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C7
C7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C8 allene fragment. The ten-membered azecine ring adopts a chair conformation. As expected, the dihedral angle between the C6
C8 allene fragment. The ten-membered azecine ring adopts a chair conformation. As expected, the dihedral angle between the C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C7
C7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C8(C22)–C8A and C5–C6(C16)
C8(C22)–C8A and C5–C6(C16)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C7
C7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C8 planes is close to 90° [89.01(6)°]. The molecule has the E-configuration of substituents at the C4
C8 planes is close to 90° [89.01(6)°]. The molecule has the E-configuration of substituents at the C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5 double bond. The carboxylate group is almost coplanar with the C4
C5 double bond. The carboxylate group is almost coplanar with the C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5 double bond (rms deviation is 0.027 Å) due to the presence of bond conjugation. The N3 nitrogen atom is in a slightly pyramidal configuration [the sum of the bond angles is 356.9(4)°]. The methoxy substituents lie within the benzene plane (rms deviation is 0.028 Å).
C5 double bond (rms deviation is 0.027 Å) due to the presence of bond conjugation. The N3 nitrogen atom is in a slightly pyramidal configuration [the sum of the bond angles is 356.9(4)°]. The methoxy substituents lie within the benzene plane (rms deviation is 0.028 Å).
The crystal packing of the molecules includes stacks along the crystallographic a axis. The molecules are arranged at van der Waals distances.
Thus, we developed an original synthesis of benzo[d]-3-aza-deca-4,6,7-triens with an enamine fragment in the α-position relative to the allene system from readily available 1-methyl- and 1-benzyl-1-phenylethynyl substituted tetrahydroisoquinolines and activated terminal alkynes.
Acknowledgements
This work was sponsored by the RF Ministry of Education and Science under the program for improving the competitiveness of RUDN among leading global science-education centers for 2016-2020, and the Russian Foundation for Basic Research (grants 15-33-20187-mol_a_ved, 17-03-00605, 17-53-540001).Notes and references
- (a) M. Kubala, J. Vacek, I. Popa, M. Janovská, P. Kosina, J. Ulrichová, Z. Trávníček and V. Šimánek, J. Lumin., 2011, 131, 1340 CrossRef CAS; (b) L. Skarydova, J. Hofman, J. Chlebek, J. Havrankova, K. Kosanova, A. Skarka, A. Hostalkova, T. Plucha, L. Cahlikova and V. Wsol, J. Steroid Biochem., 2014, 143, 250 CrossRef CAS PubMed; (c) Y. Wada, H. Kaga, S. Uchiito, E. Kumazawa, M. Tomiki, Y. Onozaki, N. Kurono, M. Tokuda, T. Ohkuma and K. Orito, J. Org. Chem., 2007, 72, 7301 CrossRef CAS PubMed.
- J. S. Choi, H. S. Kang, H. A. Jung, J. H. Jung and S. S. Kang, Fitoterapia, 2001, 72, 30 CrossRef CAS PubMed.
- (a) A. J. Aladesanmi, C. J. Kelley and J. D. Leary, J. Nat. Prod., 1983, 46, 127 CrossRef CAS; (b) P. Dunkel, G. Túrós, A. Bényei, K. Ludányi and P. Mátyus, Tetrahedron, 2010, 66, 2331 CrossRef CAS.
- (a) M. Decker, K. J. Schleifer, M. Nieger and J. Lehmann, Eur. J. Med. Chem., 2004, 39, 481 CrossRef CAS PubMed; (b) C. Enzensperger, T. Görnemann, H. H. Pertz and J. Lehmann, Bioorg. Med. Chem. Lett., 2008, 18, 3809 CrossRef CAS PubMed.
- (a) M. Schulze, F. K. U. Müller, J. M. Mason, H. Görls, J. Lehmann and C. Enzensperger, Bioorg. Med. Chem., 2009, 17, 6898 CrossRef CAS PubMed; (b) M. Schulze, O. Siol, M. Decker and J. Lehmann, Bioorg. Med. Chem. Lett., 2010, 20, 2946 CrossRef CAS PubMed.
- (a) T. Witt, F. J. Hock and J. Lehmann, J. Med. Chem., 2000, 43, 2079 CrossRef CAS PubMed; (b) B. Hoefgen, M. Decker, P. Mohr, A. M. Schramm, S. A. F. Rostom, H. El-Subbagh, P. M. Schweikert, D. R. Rudolf, M. U. Kassack and J. Lehmann, J. Med. Chem., 2006, 49, 760 CrossRef CAS PubMed; (c) C. Enzensperger, S. Kilian, M. Ackermann, A. Koch, K. Kelch and J. Lehmann, Bioorg. Med. Chem. Lett., 2007, 17, 1399 CrossRef CAS PubMed; (d) L. G. Voskressensky, T. N. Borisova, A. A. Titov, A. V. Listratova, L. N. Kulikova, A. V. Varlamov, V. N. Khrustalev and G. G. Aleksandrov, Russ. Chem. Bull., 2012, 61, 1231 CrossRef CAS.
- A. M. Jones, M. M. Lorion, T. Lebl, A. M. Z. Slawin, D. Philp and N. J. Westwood, Tetrahedron, 2010, 66, 9667 CrossRef CAS.
- (a) M. H. Weston, K. Nakajima and T. G. Back, J. Org. Chem., 2008, 73, 4630 CrossRef CAS PubMed; (b) Á. González-Gómez, G. Domínguez, U. Amador and J. Pérez-Castells, Tetrahedron Lett., 2008, 49, 5467 CrossRef; (c) J. B. Bremner and D. F. Perkins, Tetrahedron, 2005, 61, 2659 CrossRef CAS.
- H. Sashida and T. Tsuchiya, Chem. Pharm. Bull., 1986, 34, 3644 CrossRef CAS.
- H. Sashida and T. Tsuchiya, Chem. Pharm. Bull., 1984, 32, 4600 CrossRef CAS.
- M. Perscheid, D. Schollmeyer and U. Nubbemeyer, Eur. J. Org. Chem., 2011, 5250 CrossRef CAS.
- A. V. Varlamov, T. N. Borisova, L. G. Voskressensky, T. A. Soklakova, L. N. Kulikova, A. I. Chernyshev and G. G. Alexandrov, Tetrahedron Lett., 2002, 43, 6767 CrossRef CAS.
- L. G. Voskressensky, L. N. Kulikova, S. V. Gozun, V. N. Khrustalev, T. N. Borisova, A. V. Listratova, M. V. Ovcharov and A. V. Varlamov, Tetrahedron Lett., 2011, 52, 4189 CrossRef CAS.
- L. G. Voskressensky, S. A. Kovaleva, T. N. Borisova, A. V. Listratova, A. B. Eresko, V. S. Tolkunov, S. V. Tolkunov and A. V. Varlamov, Tetrahedron, 2010, 66, 9421 CrossRef CAS.
- L. G. Voskressensky, A. V. Listratova, T. N. Borisova, S. A. Kovaleva, R. S. Borisov and A. V. Varlamov, Tetrahedron, 2008, 64, 10443 CrossRef CAS.
- A. Carotti, M. de Candia, M. Catto, T. N. Borisova, A. V. Varlamov, E. Méndez-Álvares, R. Soto-Ofero, L. G. Voskressensky and C. Altomare, Bioorg. Med. Chem., 2006, 14, 7205 CrossRef CAS PubMed.
- L. G. Voskressensky, T. N. Borisova, A. V. Listratova, L. N. Kulikova, A. A. Titov and A. V. Varlamov, Tetrahedron Lett., 2006, 47, 4585 CrossRef CAS.
- L. G. Voskressensky, A. V. Listratova, T. N. Borisova, G. G. Alexandrov and A. V. Varlamov, Eur. J. Org. Chem., 2007, 6106 CrossRef CAS.
- L. G. Voskressensky, A. A. Titov, R. Samavati, T. N. Borisova, E. A. Sorokina and A. V. Varlamov, Chem. Heterocycl. Compd., 2016, 52, 316 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of 1H and 13C spectra. CCDC 1507230. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6nj03403a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |