Usual and unusual reactions of cyclohexane-1,2-dione with aryl azides and amines: a structural corrigendum†‡
Abstract
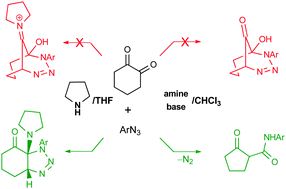
The reported syntheses of alleged functionalised 1,2,3-triazines from cyclohexane-1,2-dione and aryl azides, in the presence of pyrrolidine and other amines, were repeated. The products do not contain the bicyclic triazine and bicyclic ketone moieties; instead, cyclohexane-fused 4,5-dihydro-1,2,3-triazoles and monocyclic β-ketoamides were obtained, respectively. These corrections are well supported by careful analyses of NMR spectra, IR spectra, elemental analysis and comparison with data which were previously published in the literature. Suitable mechanisms are discussed for the synthesis of the observed compounds.


 Please wait while we load your content...
Please wait while we load your content...